Abstract
Osteogenesis Imperfecta (OI) is the most common skeletal dysplasia that predisposes to recurrent fractures and bone deformities. In spite of significant advances in understanding the genetic basis of OI, there have been no large-scale natural history studies. To better understand the natural history and improve the care of patients, a network of Linked Clinical Research Centers (LCRC) was established. Subjects with OI were enrolled in a longitudinal study, and in this report, we present cross-sectional data on the largest cohort of OI subjects (n=544). OI type III subjects had higher prevalence of dentinogenesis imperfecta, severe scoliosis, and long bone deformities as compared to those with OI types I and IV. Whereas the mean LS aBMD was low across all OI subtypes, those with more severe forms had lower bone mass. Molecular testing may help predict the subtype in type I collagen-related OI. Analysis of such well-collected and unbiased data in OI can not only help answer questions that are relevant to patient care but also foster hypothesis-driven research, especially in the context of “phenotypic expansion” driven by next-generation sequencing.
Keywords: Osteogenesis Imperfecta, longitudinal study, natural history study, bone mineral density in osteogenesis imperfecta
Osteogenesis imperfecta (OI) refers to a group of heritable disorders of connective tissue that are characterized by low bone mass, increased bone fragility, recurrent fractures, skeletal deformities, and extraskeletal manifestations (1). The spectrum of manifestations in OI varies widely and ranges from those with mild disease and few fractures to severe forms with intrauterine fractures and perinatal mortality (2). The majority of patients with OI have mutations in either COL1A1 or COL1A2 which encode for α1(I) and α2(I) chains of type I collagen, respectively. More recently, mutations in genes involved in post-translational modification of type I collagen, chaperone proteins, signaling molecules, and others with yet undetermined role in bone homeostasis have been discovered to cause OI (3–21). These advances in understanding the molecular bases of OI have led to a better appreciation of clinical heterogeneity and an expansion of the phenotypic classification of this disorder.
With a prevalence of 1 in 15,000–20,000 births and an estimated patient population of ~ 25,000 in the United States, OI is a prototype rare disease (22). Due to the rarity and the pan-ethnicity of OI, there is no geographic clustering of affected individuals and hence typically, few centers have sufficient patient populations to perform adequately powered studies. To our knowledge, there are no large-scale, multi-center studies that have been published on the natural history of OI. There are smaller, mostly single-site natural history studies of OI; however, the significant changes in the treatment of OI over the past few years, including the widespread use of bisphosphonates and new orthopedic devices, decrease the generalizability of results from these studies. Moreover the expansion of phenotype in OI fostered by new gene discoveries underscores the need for natural history studies that can prospectively describe differences among different genotypes.
Collaborative clinical research network sites have been effective in understanding the natural history, identifying biomarkers of clinical utility, implementation of therapies, and even developing effective therapies for rare disorders (23–28). To foster clinical research and advance the care of patients with OI across North America, the Linked Clinical Research Centers (LCRC), a network of five centers, was established by a joint initiative from the OI Foundation and the Children’s Brittle Bone Foundation. As an undertaking of the LCRC, the “Longitudinal Study of Osteogenesis Imperfecta” was initiated to model and power the development of a broader multidisciplinary network that will conduct longitudinal, multidisciplinary investigations of the natural history, therapeutic interventions, morbidity, and mortality in individuals with OI. In this report, we describe the cross-sectional clinical and molecular characteristics of the largest cohort of subjects with OI accrued to date.
Material and Methods
The Linked Clinical Research Centers (LCRC) is comprised of five clinical sites across North America, a data collection and analysis center, and a center for molecular and biochemical analysis. The first year of funding in 2009 supported three clinical sites: Baylor College of Medicine (Houston, TX), Kennedy Krieger Institute (Baltimore, MD) in collaboration with Nemours/Alfred I. DuPont Hospital for Children (Wilmington, DE), and Oregon Health & Science University (Portland, OR); two additional centers, Shriners Hospital for Children (Chicago, IL) and Shriners Hospital for Children (Montreal, Canada), were included in 2010. The Data Collection and Analysis Center at the University of South Florida College of Medicine (Tampa, FL) served as the center for data collection and analyses. The Collagen Diagnostic Laboratory at the University of Washington (Seattle, WA) performed biochemical and molecular diagnostics.
Individuals with a clinical, molecular, or biochemical diagnosis of OI were enrolled. For those without a molecular or biochemical diagnosis, the site PI and one of the two project PIs were required to be in agreement about the clinical diagnosis and subtype of OI based upon specific criteria outlined in Supplementary Tables 1 and 2. As there is significant overlap in the clinical phenotype between OI types III and IV and the recessive forms, genotypic information was used to appropriately reclassify patients when available. Ten subjects initially classified as OI types III or IV were reclassified appropriately into types V, VII, or ‘others and unclassified’ categories. Subjects with Type II OI were not enrolled due to the severe nature and perinatal lethality of the disease. Given the rarity of some of the recessive forms of OI, subjects with OI subtypes I–VII (excepting type II) were enrolled; the OI subtype in 12 subjects could not be characterized. By the end of June 2012, adequate number of OI type I subjects had been enrolled and hence further recruitment of individuals with this subtype was stopped. Subjects were recruited through various sources including the OI Foundation, Children’s Brittle Bone Foundation (CBBF), the OI registry, medical care providers, prenatal diagnostic centers, and local clinics. The recruitment procedures were conducted with strict adherence to standards for ethical conduct of research in compliance with Health Insurance Portability and Accountability Act (HIPAA). The Institutional Review Boards of participating sites approved the research protocol. Informed consent was obtained from subjects or their legal guardians.
The clinical diagnosis, imaging, and molecular or biochemical testing were reviewed at the screening visit. Demographic, clinical, and diagnostic data were obtained as outlined and follow-up assessments were made on a yearly basis (Supplementary Table 3). Data was collected uniformly at each site according to the detailed instructions in the Manual of Operations and the quality of data was assessed at the entry point using on-line case report forms. In addition, the Data Collection and Analysis Center identified missing or unclear data and generated data queries to the enrolling centers in addition to monitoring data delinquency to generate good-quality data.
Data analysis
Demographics and baseline clinical features were summarized by OI subtype. Although participants were followed over time and measurements of lumbar spine areal bone mineral density (LS aBMD) Z-scores were recorded annually, only baseline BMD results are described in this report. The baseline LS aBMD Z-scores were compared by OI subtypes using Analysis of Variance. The average LS aBMD Z-score was calculated for each participant and multivariate ANOVA was performed comparing the averages by OI subtypes for categorizations of age. The number of fractures in the year preceding enrollment was recorded. General Linear Models were constructed to compare this fracture count versus the LS aBMD Z-score. Outliers identified via studentized residues and Cook’s D statistics were excluded. All tests of significance were two-sided based on α level of 0.05. Tukey’s method was utilized to account for multiple comparisons. All analyses were performed using the SAS software system.
Results
This study includes 544 subjects with OI types I–VII (except type II). The demographic characteristics are summarized in Table 1. OI type I was the most prevalent form, followed by types IV and III. These dominant forms of OI accounted for more than 90% of the enrollees. The median age of the subjects was 12.6 years (range 0–67 years). A family history of OI was more prevalent in type I OI subjects as compared to those with types III and IV. Family history of disease was present in 25% of subjects with recessive forms of OI (types VI and VII). Genetic testing was performed on nearly 70% of subjects.
Table 1. Demographic characteristics of patients at enrollment.
Note that the percentages in parentheses depict proportion within OI subtype. Percentages for gene mutations (COL1A1, COL1A2, etc.) indicate proportion of those with genetic testing.
| OI I | OI III | OI IV | OI V | OI VI | OI VII | Unclassified & others |
All | |
|---|---|---|---|---|---|---|---|---|
| Enrollment number | 244 | 100 | 147 | 16 | 11 | 5 | 21 | 544 |
| Male, n (%) | 113 (46.3) | 42 (42.0) | 65 (44.2) | 5 (31.3) | 6 (54.5) | 0 | 9 (42.9) | 240 (44.1) |
| Female, n (%) | 131 (53.7) | 58 (58.0) | 82 (55.8) | 11 (68.8) | 5 (45.5) | 5 (100) | 12 (57.1) | 304 (55.9) |
| Median age, yrs (range) | 13.6 (0–67) | 11.4 (0–54) | 12 (0–63) | 13.6 (0–35) | 11.3 (2–32) | 4.3 (4–20) | 7.4 (1–33) | 12.6 (0–67) |
| Race, n (%) | ||||||||
| White | 228 (93.4) | 79 (79.0) | 125 (85.0) | 11 (68.8) | 7 (63.6) | 3 (60.0) | 15 (71.4) | 468 (86) |
| Black | 4 (1.6) | 7 (7.0) | 12 (8.2) | 2 (12.5) | 0 | 0 | 3 (14.3) | 28 (5.1) |
| Other | 12 (4.9) | 14 (14.0) | 10 (6.8) | 3 (18.8) | 4 (36.4) | 2 (40.0) | 3 (14.3) | 48 (8.8) |
| Family history, n (%) | 153 (62.7) | 7 (7.0) | 42 (28.6) | 5 (31.3) | 1 (9.1) | 3 (60.0) | 8 (38.1) | 219 (40.3) |
| Genetic testing, n (%) | 158 (64.8) | 64 (64.0) | 108 (73.5) | 14* (87.5) | 5 (45.5) | 5 (100) | 16 (76.2) | 370 (68.0) |
| COL1A1, n (%) | 140 (88.6) | 39 (60.9) | 49 (45.4) | 0 | 0 | 0 | NA | 228 (62.2) |
| COL1A2, n (%) | 14 (8.9) | 25 (39.1) | 59 (54.6) | 0 | 0 | 0 | NA | 99 (26.4) |
| SERPINF1, n (%) | 0 | 0 | 0 | 0 | 4 (80.0) | 0 | 0 | 4 (1.1) |
| IFITM5, n (%) | 0 | 0 | 0 | 7 (50.0) | 0 | 0 | 0 | 7 (1.9) |
| CRTAP, n (%) | 0 | 0 | 0 | 0 | 0 | 5 (100) | 0 | 5 (1.4) |
Genetic testing done prior to the discovery of IFITM5 as the cause for OI type V could be responsible for "no mutations" being detected in 7 patients with OI type V.
Clinical Characteristics
The prevalence of clinical features was categorized by OI subtype and is summarized in Table 2. The three most common characteristics in the pooled patient population were blue sclerae, joint hypermobility, and dentinogenesis imperfecta (DI). While blue sclerae were observed in all types of OI, it was more common in dominant as compared with recessive forms. DI was relatively uncommon in those with types I, V, VI, and VII. In those with dominant forms of type I collagen-related OI, hearing loss was present in ~10–20% of subjects. Type III OI subjects had a higher prevalence of DI, moderate-to-severe scoliosis (>30 degrees), and long bone deformities compared to those with types I and IV. Interestingly, the prevalence of mild scoliosis (<30 degrees) was similar between OI subtypes I, III, and IV (14–20%). Subjects with type III OI had significantly more fractures per year compared to those with type I and IV (p<0.05). As expected, use of bisphosphonate therapy was more prevalent in those with severe disease whereas less than 45% subjects with type I OI were receiving such therapy. The analysis of fracture data in types V, VI, VII, and those categorized to ‘unclassified and others’ category was limited by small number of subjects.
Table 2. Clinical characteristics by OI subtype.
The percentages in parentheses refer to the proportion within each OI subtype.
| OI I | OI III | OI IV | OI V | OI VI | OI VII | Unclassified & others |
All | |
|---|---|---|---|---|---|---|---|---|
| Blue sclerae, n (%) | 222 (91.0) | 77 (77.0) | 108 (73.5) | 3 (18.8) | 3 (27.3) | 2 (40.0) | 10 (47.6) | 425 (78.1) |
| Hypermobility, n (%) | 131 (53.7) | 66 (66.0) | 92 (62.6) | 5 (31.3) | 6 (54.5) | 2 (40.0) | 8 (38.1) | 310 (57.0) |
| DI, n (%) | 22 (9.0) | 77 (77.0) | 80 (54.4) | 2 (12.5) | 1 (9.1) | 1 (20.0) | 5 (23.8) | 188 (34.6) |
| Hearing loss, n (%) | 32 (13.1) | 19 (19.0) | 16 (10.9) | 0 (0.0) | 1 (9.1) | 0 (0.0) | 5 (23.8) | 73 (13.4) |
| Scoliosis, n (%) | ||||||||
| < 30 degrees | 39 (16.0) | 14 (14.0) | 29 (19.7) | 3 (18.8) | 0 (0.0) | 1 (20.0) | 1 (4.8) | 87 (16.0) |
| > 30 degrees | 4 (1.6) | 33 (33.0) | 17 (11.6) | 2 (12.5) | 3 (27.3) | 1 (20.0) | 2 (9.5) | 62 (11.4) |
| Deformities, n (%) | ||||||||
| Humerus | 8 (3.3) | 39 (39.0) | 22 (15.0) | 2 (12.5) | 1 (9.1) | 2 (40.0) | 3 (14.3) | 77 (14.2) |
| Femur | 7 (2.9) | 37 (37.0) | 26 (17.7) | 4 (25.0) | 1 (9.1) | 1 (20.0) | 3 (14.3) | 79 (14.5) |
| Tibia | 6 (2.5) | 36 (36.0) | 26 (17.7) | 3 (18.8) | 1 (9.1) | 2 (40.0) | 3 (14.3) | 77 (14.2) |
| Average* no. of fractures (SD) | 0.45 (0.71) | 1.56 (2.22)a | 0.75 (1.38) | 1.58 (2.68) | 1.84 (1.62) | 0.38 (0.75) | 11.80 (14.51) | 1.03 (2.89) |
| Bisphosphonate use, n (%) | 109 (44.7) | 88 (88.0) | 119 (81.0) | 11 (68.8) | 11 (100.0) | 5 (100.0) | 18 (85.7) | 361 (66.4) |
The average number of fractures was calculated as the number of fractures per subject in the year prior to enrollment.
P< 0.05 compared to OI types I and IV.
Type I collagen mutations can help predict OI subtype
Whereas OI types V, VI, and VII can be distinguished based on distinct clinical or molecular characteristics, the significant overlap between the phenotypic features of the type I collagen-related OI subtypes often makes categorization of patients challenging. Hence, we assessed whether specific classes of mutations could predict the subtypes of type I collagen-related OI. Pathogenic changes in COL1A1 and COL1A2 were categorized into glycine substitution mutations in the α-helical domain, non-glycine missense mutations, insertions and deletions that do not alter the reading frame, mutations affecting splicing, and loss-of-function alleles including nonsense mutations or whole gene deletions (Table 3). Logistic regression analysis with Type 1 error rate adjusted with the Bonferroni method was performed. To adjust for multiple comparisons, a p-value of 0.0167 (0.05/3) was used to determine statistical significance. The analyses revealed that splice site mutations, truncating mutations, and whole gene deletions in COL1A1 were strong predictors of type I OI (p<0.0001). Non-glycine missense substitutions and non-frameshift insertions or deletions in COL1A2 were predictive of OI type III (p<0.0001). Similarly, COL1A2 glycine substitutions in the helical domain almost reached statistical significance for being predictive of OI type IV (p=0.0412) accounting for 48.1% of all mutations in this subtype. All other mutations could not be used to predict the phenotype in our cohort.
Table 3. Prevalence of COL1A1 and COL1A2 mutations in autosomal dominant forms of OI.
Note that the numbers in the parentheses refer to the proportion of particular mutation class within each OI subtype in COL1A1 or COL1A2 and total type 1 collagen mutations, respectively.
| OI I | OI III | OI IV | All | |
|---|---|---|---|---|
| COL1A1 | 139 | 39 | 49 | 227 |
| Glycine substitutions in alpha-helical domain | 12 (8.6; 7.9) | 30 (76.9; 46.9) | 32 (65.3; 29.6) | 74 |
| Other missense substitutions or non-frameshift insertions or deletions | 8 (5.8; 5.2) | 5 (12.8; 7.8) | 5 (10.2; 4.6) | 18 |
| Splicing mutations | 44 (31.7; 20.9) | 4 (10.3; 6.3) | 9 (18.4; 8.3) | 57 |
| Truncating mutations or whole gene deletions | 75 (54; 49.7) | 0 | 3 (6.1; 2.8) | 78 |
| COL1A2 | 12 | 25 | 59 | 96 |
| Glycine substitutions in alpha-helical domain | 11 (91.7; 7.3) | 22 (88.0; 34.4) | 52 (88.1; 48.1) | 85 |
| Other missense substitutions or non-frameshift insertions or deletions | 1 (8.3; 0.7) | 3 (12.0; 4.7) | 2 (3.4; 1.9) | 6 |
| Splicing mutations | 0 | 0 | 5 (8.5; 4.6) | 5 |
Areal bone mineral density
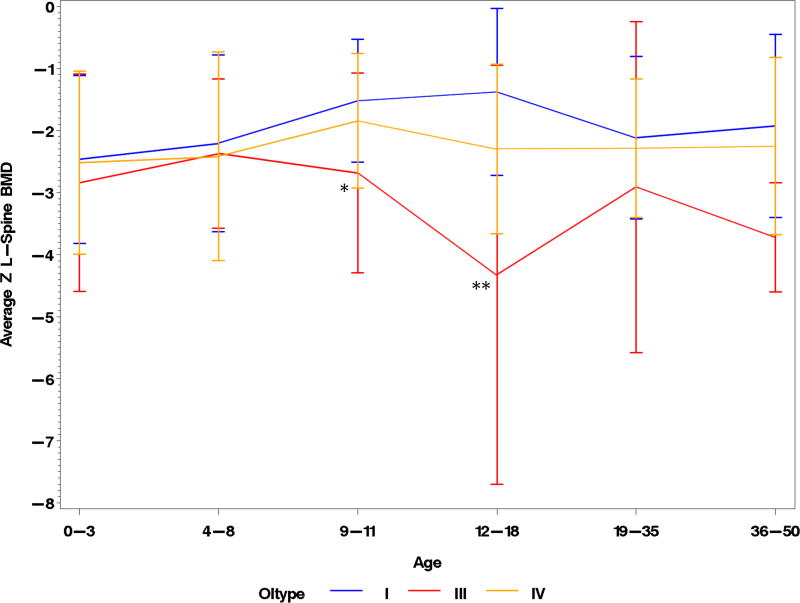
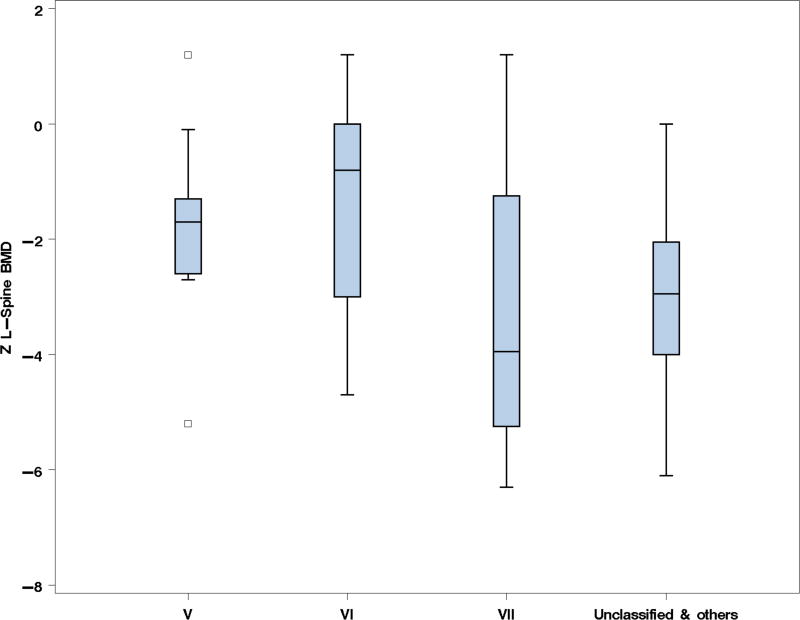
aBMD measured at the lumbar spine, hip, or forearm has been used as a surrogate marker of bone strength and a predictor of fracture risk in adults with osteoporosis (29–33). Though the correlation between aBMD and fracture risk in OI is not well established, it is a clinical parameter that is often used to guide therapy and has been utilized as the primary endpoint in multiple treatment trials (34–37). To explore the trends in aBMD, we compared the enrollment LS aBMD at various ages. In those with type I collagen-related OI, LS aBMD tended to be lower in the more severe clinical forms (Fig. 1). LS aBMD was relatively similar in age groups 0–3 and 4–8, which could be secondary to early and widespread use of bisphosphonates, which increases and/or preserves BMD, in types III and IV OI. Beyond age 8 years the LS aBMD Z-scores were significantly lower in type III OI as compared to types I and IV; in the age-group 9–11 years, the mean LS aBMD Z-scores (SD) in types I, III and IV were −1.52 (0.99), −2.79 (1.67), and −1.84 (1.09), respectively. Between the ages 12 and 18 years, there was an increase aBMD in those with type I OI during the pubertal ages whereas those with type III had a decline. The mean LS aBMD Z-scores (SD) in this age group for types I, III, and IV OI were −1.4 (1.35), −4.33 (3.38), and −2.29 (1.37), respectively. The aBMD in the rarer forms of OI did not merit analysis based on age, due to the limited number of subjects. The mean LS aBMD Z-scores in OI types V (−1.834; n=11), VI (−1.314; n=7), and VII (−3.250; n=5) showed no statistically significant differences between the groups (Fig. 2)
Fig. 1. Lumbar spine aBMD Z-scores in OI types I, III, and IV.
A graphic display of the variation in LS aBMD Z-scores among different age groups in OI. *p<0.05, compared to OI I; **p<0.05, compared to OI I & IV. Error bars indicate +/− 1 SD.
Fig. 2. Lumbar spine aBMD Z-scores in OI types V, VI, VII, and other rare forms.
Box plots comparing the LS aBMD Z-scores in types V, VI, VII, and unclassified & other OI types. The small sample size precluded further categorization by age. There were no differences in aBMD between the groups (p=0.1567).
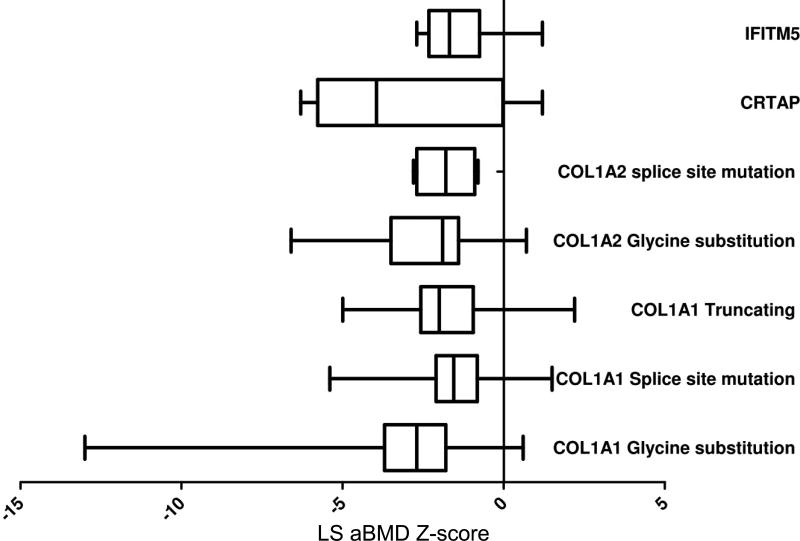
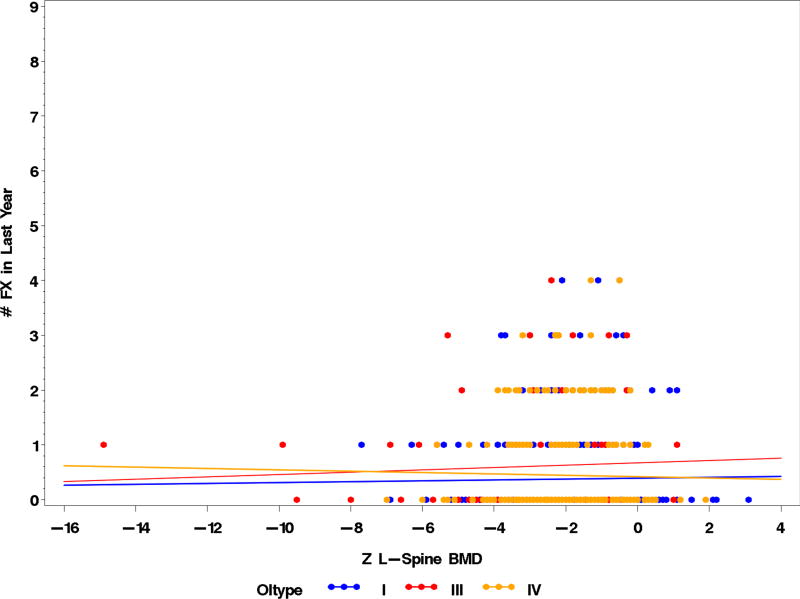
As a descriptive endpoint, we assessed the relationship between various genotypes and LS aBMD but no correlation was observed (Fig. 3). An important issue was to assess if aBMD could predict fracture risk. We performed logistic regression analysis to address whether LS aBMD Z-scores had an impact on the fracture in the preceding 12 months. The analysis was limited by the significant clinical heterogeneity observed in OI. The difficulty in estimating fracture risk in OI was underscored by the fact that many had fractures with near-normal aBMD whereas some with very low aBMD did not suffer any fractures. Overall there were no significant correlations between fracture risk in the preceding 12 months and LS aBMD (Fig. 4). Although data regarding mobility was collected, it was not analyzed as part of this report and may account in part for the lack of correlation as those with higher BMD are more likely to be ambulatory and active and thus at a higher risk for fracture. Future analyses will look at other clinical variables that would aid in the prediction of fracture risk.
Fig. 3. LS aBMD distribution by genotype.
Box plots showing the interquartile ranges and the 5th and 95th centiles (error bars) for LS aBMD Z-scores for various OI subtypes. The type I collagen-related OI have been further categorized based on the type of mutations. Note that the mean aBMD is low across all subtypes.
Fig. 4. Correlation between aBMD and fractures in OI types I, III, and IV.
The correlation between LS aBMD Z-scores and number of fractures in the preceding year show no statistically significant correlations in OI types I (r=0.01578; p=0.7554; n=392), III (r=0.05123; p=0.5800; n=119), and IV (r=−0.01979; p=0.7522; n=257). The number of fractures was calculated per subject. Data points may not represent the actual number of subjects due to graphic overlap of common points.
Discussion
We present cross-sectional data from the largest multicenter natural history study of OI conducted to date. This study was initiated and funded by the OI Foundation and the Children’s Brittle Bone Foundation, recognizing the need to foster collaborative research into this rare disease.
The term “rare disease” is used to define conditions with a prevalence of less than 200,000 in the US (38). This group comprising of Mendelian, polygenic, and complex disorders encompasses over 6,000 conditions that affect over 25 million Americans (http://rarediseases.info.nih.gov/). Whereas the conduct of clinical studies in rare diseases have been uncommon, the improved awareness due to activities by patient advocacy groups, increased funding from the National Institutes of Health (NIH), and escalating interest of the pharmaceutical industry in smaller niche markets have led to a significant increase in rare disease research over the recent years. It is now well-recognized that collaborative multicenter studies in rare diseases can lead to a better understanding of the natural history of disease, generate hypothesis for further research, identify biomarkers for disease monitoring, and provide better therapeutic options for patients (23–28, 39, 40). The Rare Disease Clinical Research Network established under the Office of Rare Disease Research of the NIH is an outstanding example for such collaborative multicenter clinical studies (http://rarediseasesnetwork.epi.usf.edu/). The OI LCRC was established to foster collaborative research and optimize care of patients with OI. The partnership of the OI Foundation and Children’s Brittle Bone Foundation with academic centers that have expertise in management of OI has been critical in highlighting the research areas and studies that are relevant to the patient population. The Longitudinal Study of Osteogenesis Imperfecta continues to enroll individuals with severe and recessive forms of OI and the data analyses on radiographic features, changes in aBMD in each subject over time, pain control and its relationship to functional and mobility, trends in growth and development, and manifestations such as hearing loss and pulmonary functions are ongoing. In this report, we present a cross sectional view of data that are clinically relevant to practitioners and health care providers.
The enrollment numbers in this study reflect the relative prevalence of the various subtypes with ~92% of subjects having dominant forms of type I collagen-related OI. Bluish discoloration of sclera, DI, and joint hypermobility were the three most common features. Consistent with previous reports, subjects with types III and IV have a higher prevalence of DI; however DI can be observed in the rarer forms of OI as well (41–44). The proportion of subjects with hearing loss in our cohort was lower than previously reported figures, likely due to the fact that the median age of the subjects in the cohort was relatively young (12.6 years) and the mixed conductive and sensorineural hearing loss in OI typically progresses with advancing age (45, 46). Consistent with the clinical classification, over a third of the subjects with type III OI had significant bony deformities while less than 5% of subjects with type I OI shared this feature. Notably, the prevalence of mild scoliosis did not differ between the mild and severe forms of OI, demonstrating that mild scoliosis is a problem for individuals with all subtypes of OI. Given the different ages of subjects, total number of fractures over a period of years could not be compared between the OI subtypes. We therefore evaluated fractures in the first year of enrollment and as would be expected, subjects with type III OI had significantly more number of fractures as compared to OI type I. Fracture rates tended to be higher in OI types V, VI, VII, and unclassified types but the small numbers precluded the conduct of formal statistical tests.
We have demonstrated that the type of mutation in COL1A1 and COL1A2 has predictive value in classification of the dominant forms of type I collagen-related OI. COL1A1 splice site, truncating and whole gene deletion mutations were highly predictive of type I OI; COL1A2 missense substitutions that did not involve an alpha-helical domain glycine as well as non-frameshift insertion/deletion mutations were highly predictive of type III OI; and COL1A2 glycine substitutions in the alpha-helical domain tended to be seen more commonly in type IV OI.
The aBMD in subjects with OI was low irrespective of the genotype or the clinical subtype. Generally, the average LS aBMD Z-scores were at least two standard deviations away from the mean as compared to age- and sex-matched controls. Subjects with type III OI tended to have lower bone mass than those with the milder forms. These results are consistent with observations from previous studies that have shown that subjects with glycine substitution mutations have average aBMD that is 0.6 standard deviations lower than those with haploinsufficiency mutations (47). LS aBMD was relatively similar in age groups 0–3 and 4–8, which could be secondary to early and widespread use of bisphosphonates in types III and IV OI. Subjects with type I OI were observed to have higher aBMD during the pubertal and post-pubertal years whereas those with type III had declining levels in the same period. However, these data reflect cross-sectional aBMD in different individuals and not longitudinal data on individual subjects and thus should not be interpreted as subjects with OI type III fail to increase BMD during the pubertal years. There are two distinct limitations in the interpretation of the aBMD data in our cohort: firstly, the bone mass was analyzed at the individual sites using different Dual-Energy X-ray Absorptiometry machines, secondly, the short stature accompanying the more severe forms of OI may have led to an underestimation of the Z-scores especially in older children and adults. The results of LS aBMD not correlating with fracture risk in the preceding year have to be interpreted with caution as we suspect that mobility, deformities, and the presence of intramedullary rods could affect fracture rate significantly. We therefore plan future studies to assess the impact of mobility and rodding surgeries on fracture rates in the various types of OI. However, this lack of observed correlation and the frequent lack of statistically significant decrease in fractures in bisphosphonate treatment studies suggests caution in interpretation of the clinical efficacy of bisphosphonates in treatment of OI.
In summary this largest-ever, multicenter cross-sectional study of OI allows us to summarize the clinically relevant baseline features to develop hypotheses for more detailed analyses of the longitudinal data to assess disease burden and outcomes for skeletal and extra-skeletal manifestations of OI. Moreover, this preliminary data will inform the design of both therapeutic and non-therapeutic studies that can be powered to answer relevant clinical questions related to genotypic correlations, testing of therapeutic intervention in phase I and II studies, and development of biomarkers that may be used to predict clinical severity.
Supplementary Material
Acknowledgments
VRS receives salary support from the Osteogenesis Imperfecta Foundation (OIF).
The respective LCRC sites would acknowledge the OI Foundation and Children’s Brittle Bone foundation for their support in the development and implementation of this study. We would also like to recognize the contributions of clinical research teams at the respective sites: Mary Mullins, Alyssa Tran, Susan Carter (Baylor College of Medicine and Texas Children’s Hospital); Vonda Vensel, Jill Christie, and Abigail Hata (Oregon Health & Science University). This work was supported by the OI Foundation and Children’s Brittle Bone Foundation, the NIH (HD070394, HD024064), the Rolanette and Berdon Lawrence Bone Disease Program of Texas, and the Shriners of North America. SCSN was supported by a fellowship grant from the OI LCRC and the Clinical Scientist Development Award from the Doris Duke Charitable Foundation.
Footnotes
Conflicts of Interest
Other authors have no financial disclosures and report no conflicts of interest relevant to this article.
References
- 1.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet. 2004;363:1377–1385. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 2.Sillence DO, Senn A, Danks DM. Genetic heterogeneity in osteogenesis imperfecta. Journal of medical genetics. 1979;16:101–116. doi: 10.1136/jmg.16.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sykes B, Ogilvie D, Wordsworth P, et al. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986;2:69–72. doi: 10.1016/s0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- 4.Morello R, Bertin TK, Chen Y, et al. CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell. 2006;127:291–304. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 5.Barnes AM, Chang W, Morello R, et al. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. The New England journal of medicine. 2006;355:2757–2764. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cabral WA, Chang W, Barnes AM, et al. Prolyl 3-hydroxylase 1 deficiency causes a recessive metabolic bone disorder resembling lethal/severe osteogenesis imperfecta. Nature genetics. 2007;39:359–365. doi: 10.1038/ng1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Dijk FS, Nesbitt IM, Zwikstra EH, et al. PPIB mutations cause severe osteogenesis imperfecta. American journal of human genetics. 2009;85:521–527. doi: 10.1016/j.ajhg.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christiansen HE, Schwarze U, Pyott SM, et al. Homozygosity for a missense mutation in SERPINH1, which encodes the collagen chaperone protein HSP47, results in severe recessive osteogenesis imperfecta. American journal of human genetics. 2010;86:389–398. doi: 10.1016/j.ajhg.2010.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lapunzina P, Aglan M, Temtamy S, et al. Identification of a frameshift mutation in Osterix in a patient with recessive osteogenesis imperfecta. American journal of human genetics. 2010;87:110–114. doi: 10.1016/j.ajhg.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alanay Y, Avaygan H, Camacho N, et al. Mutations in the gene encoding the RER protein FKBP65 cause autosomal-recessive osteogenesis imperfecta. American journal of human genetics. 2010;86:551–559. doi: 10.1016/j.ajhg.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes AM, Carter EM, Cabral WA, et al. Lack of cyclophilin B in osteogenesis imperfecta with normal collagen folding. The New England journal of medicine. 2010;362:521–528. doi: 10.1056/NEJMoa0907705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Becker J, Semler O, Gilissen C, et al. Exome sequencing identifies truncating mutations in human SERPINF1 in autosomal-recessive osteogenesis imperfecta. American journal of human genetics. 2011;88:362–371. doi: 10.1016/j.ajhg.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martinez-Glez V, Valencia M, Caparros-Martin JA, et al. Identification of a mutation causing deficient BMP1/mTLD proteolytic activity in autosomal recessive osteogenesis imperfecta. Human mutation. 2012;33:343–350. doi: 10.1002/humu.21647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaheen R, Alazami AM, Alshammari MJ, et al. Study of autosomal recessive osteogenesis imperfecta in Arabia reveals a novel locus defined by TMEM38B mutation. Journal of medical genetics. 2012;49:630–635. doi: 10.1136/jmedgenet-2012-101142. [DOI] [PubMed] [Google Scholar]
- 15.Cho TJ, Lee KE, Lee SK, et al. A single recurrent mutation in the 5'-UTR of IFITM5 causes osteogenesis imperfecta type V. American journal of human genetics. 2012;91:343–348. doi: 10.1016/j.ajhg.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laine CM, Joeng KS, Campeau PM, et al. WNT1 mutations in early-onset osteoporosis and osteogenesis imperfecta. The New England journal of medicine. 2013;368:1809–1816. doi: 10.1056/NEJMoa1215458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi JW, Sutor SL, Lindquist L, et al. Severe osteogenesis imperfecta in cyclophilin B-deficient mice. PLoS genetics. 2009;5:e1000750. doi: 10.1371/journal.pgen.1000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fahiminiya S, Majewski J, Mort J, et al. Mutations in WNT1 are a cause of osteogenesis imperfecta. Journal of medical genetics. 2013;50:345–348. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 19.Keupp K, Beleggia F, Kayserili H, et al. Mutations in WNT1 cause different forms of bone fragility. American journal of human genetics. 2013;92:565–574. doi: 10.1016/j.ajhg.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pyott SM, Tran TT, Leistritz DF, et al. WNT1 mutations in families affected by moderately severe and progressive recessive osteogenesis imperfecta. American journal of human genetics. 2013;92:590–597. doi: 10.1016/j.ajhg.2013.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semler O, Garbes L, Keupp K, et al. A mutation in the 5'-UTR of IFITM5 creates an in-frame start codon and causes autosomal-dominant osteogenesis imperfecta type V with hyperplastic callus. American journal of human genetics. 2012;91:349–357. doi: 10.1016/j.ajhg.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marini JC. Philadelphia: Saunders; 2004. [Google Scholar]
- 23.O'Leary M, Krailo M, Anderson JR, et al. Progress in childhood cancer: 50 years of research collaboration, a report from the Children's Oncology Group. Seminars in oncology. 2008;35:484–493. doi: 10.1053/j.seminoncol.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burns J, Ouvrier R, Estilow T, et al. Validation of the Charcot-Marie-Tooth disease pediatric scale as an outcome measure of disability. Annals of neurology. 2012;71:642–652. doi: 10.1002/ana.23572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clowse ME, Richeson RL, Pieper C, et al. Pregnancy outcomes among patients with vasculitis. Arthritis care & research. 2013;65:1370–1374. doi: 10.1002/acr.21983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mistry PK. Rare disease clinical research network's urea cycle consortium delivers a successful clinical trial to improve alternate pathway therapy. Hepatology. 2013;57:2100–2102. doi: 10.1002/hep.26106. [DOI] [PubMed] [Google Scholar]
- 27.Richesson RL, Sutphen R, Shereff D, et al. The Rare Diseases Clinical Research Network Contact Registry update: features and functionality. Contemporary clinical trials. 2012;33:647–656. doi: 10.1016/j.cct.2012.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuchman M, Lee B, Lichter-Konecki U, et al. Cross-sectional multicenter study of patients with urea cycle disorders in the United States. Molecular genetics and metabolism. 2008;94:397–402. doi: 10.1016/j.ymgme.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone KL, Seeley DG, Lui LY, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003;18:1947–1954. doi: 10.1359/jbmr.2003.18.11.1947. [DOI] [PubMed] [Google Scholar]
- 30.Johnell O, Kanis JA, Oden A, et al. Predictive value of BMD for hip and other fractures. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20:1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 31.Leslie WD, Tsang JF, Caetano PA, et al. Effectiveness of bone density measurement for predicting osteoporotic fractures in clinical practice. The Journal of clinical endocrinology and metabolism. 2007;92:77–81. doi: 10.1210/jc.2006-1415. [DOI] [PubMed] [Google Scholar]
- 32.Black DM, Cummings SR, Genant HK, et al. Axial and appendicular bone density predict fractures in older women. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1992;7:633–638. doi: 10.1002/jbmr.5650070607. [DOI] [PubMed] [Google Scholar]
- 33.Cauley JA, Hochberg MC, Lui LY, et al. Long-term risk of incident vertebral fractures. JAMA : the journal of the American Medical Association. 2007;298:2761–2767. doi: 10.1001/jama.298.23.2761. [DOI] [PubMed] [Google Scholar]
- 34.Adami S, Gatti D, Colapietro F, et al. Intravenous neridronate in adults with osteogenesis imperfecta. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003;18:126–130. doi: 10.1359/jbmr.2003.18.1.126. [DOI] [PubMed] [Google Scholar]
- 35.Chevrel G, Schott AM, Fontanges E, et al. Effects of oral alendronate on BMD in adult patients with osteogenesis imperfecta: a 3-year randomized placebo-controlled trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2006;21:300–306. doi: 10.1359/JBMR.051015. [DOI] [PubMed] [Google Scholar]
- 36.Gatti D, Antoniazzi F, Prizzi R, et al. Intravenous neridronate in children with osteogenesis imperfecta: a randomized controlled study. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20:758–763. doi: 10.1359/JBMR.041232. [DOI] [PubMed] [Google Scholar]
- 37.Phillipi CA, Remmington T, Steiner RD. Bisphosphonate therapy for osteogenesis imperfecta. The Cochrane database of systematic reviews. 2008:CD005088. doi: 10.1002/14651858.CD005088.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Public Law 98–551. Health Promotion and Disease Prevention Amendments of 1984. 1984 Oct 30; [Google Scholar]
- 39.Diaz GA, Krivitzky LS, Mokhtarani M, et al. Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology. 2013;57:2171–2179. doi: 10.1002/hep.26058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagamani SC, Shchelochkov OA, Mullins MA, et al. A randomized controlled trial to evaluate the effects of high-dose versus low-dose of arginine therapy on hepatic function tests in argininosuccinic aciduria. Molecular genetics and metabolism. 2012;107:315–321. doi: 10.1016/j.ymgme.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Majorana A, Bardellini E, Brunelli PC, et al. Dentinogenesis imperfecta in children with osteogenesis imperfecta: a clinical and ultrastructural study. International journal of paediatric dentistry / the British Paedodontic Society [and] the International Association of Dentistry for Children. 2010;20:112–118. doi: 10.1111/j.1365-263X.2010.01033.x. [DOI] [PubMed] [Google Scholar]
- 42.Malmgren B, Norgren S. Dental aberrations in children and adolescents with osteogenesis imperfecta. Acta odontologica Scandinavica. 2002;60:65–71. doi: 10.1080/000163502753509446. [DOI] [PubMed] [Google Scholar]
- 43.O’Connell A, Marini J. Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 1999;87:189–196. doi: 10.1016/s1079-2104(99)70272-6. [DOI] [PubMed] [Google Scholar]
- 44.Shapiro JR, Lietman C, Grover M, et al. Phenotypic variability of osteogenesis imperfecta type V caused by an IFITM5 mutation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013;28:1523–1530. doi: 10.1002/jbmr.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuurila K, Kaitila I, Johansson R, et al. Hearing loss in Finnish adults with osteogenesis imperfecta: a nationwide survey. The Annals of otology, rhinology, and laryngology. 2002;111:939–946. doi: 10.1177/000348940211101014. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro JR, Pikus A, Weiss G, et al. Hearing and middle ear function in osteogenesis imperfecta. JAMA : the journal of the American Medical Association. 1982;247:2120–2126. [PubMed] [Google Scholar]
- 47.Rauch F, Lalic L, Roughley P, et al. Relationship between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfecta. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2010;25:1367–1374. doi: 10.1359/jbmr.091109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.