Abstract
Background
Hepatitis B (HBV) in sub-Saharan Africa is believed to be horizontally acquired. However, because of the high HBV prevalence in northern Uganda, no hepatitis B vaccination at birth and no access to HBV immunoglobulin, we hypothesize that vertical transmission also could also play an important role. We therefore investigated the incidence of HBV among babies presenting for their first HBV vaccine dose in Gulu, Uganda.
Methods
We recruited mothers and their babies (at least 6-week old) presenting for their postnatal care and first HBV vaccine dose respectively. Socio-demographic and risk factors for HBV transmission were recorded. Mothers were tested for Hepatitis B core antibody (anti-HBc-IgG) and hepatitis B surface antigen (HBsAg). HBsAg-positive sera were tested for hepatitis B e antigen (HBeAg) and HBV viral load (HBVDNA). Babies were tested for HBsAg at presentation and at the last immunization visit. A sample of HBsAg-negative babies were tested for HBVDNA. Incident HBV infection was defined by either a positive HBsAg or HBVDNA test. Chi-square or fisher's exact tests were utilized to investigate associations and t-tests or Wilcoxon rank-sum test for continuous differences.
Results
We recruited 612 mothers, median age 23 years (IQR 20–28). 53 (8.7%) were HBsAg-positive and 339 (61.5%) were anti-HBc-IgG-positive. Ten (18.9%) of the HBsAg-positive mothers were HBeAg-positive. Median HBVDNA levels of HBV-infected mothers was 5.7log (IQR 4.6–7.0) IU/mL with 9 (17.6%) having levels ≥105 IU/mL. Eighty (13.3%) mothers were HIV-infected of whom 9 (11.5%) were co-infected with HBV. No baby tested HBsAg or HBVDNA positive.
Conclusion
Vertical transmission does not seem to contribute substantially to the high HBV endemicity in northern Uganda. The current practice of administering the first HBV vaccine to babies in Uganda at six weeks of age may be adequate in control of HBV transmission.
Keywords: Hepatitis B, Transmission, Early childhood
1. Introduction
Globally, it is estimated that two billion people have been exposed to the hepatitis B virus (HBV) and 350–400 million are chronic carriers. Of latter, 15% are in sub-Saharan Africa (SSA) where disease prevalence ranges from 8 to over 25% [1,2]. Approximately 20–30% of adults who are chronically infected will develop cirrhosis and/or liver cancer [3]. HBV is highly endemic in Uganda with a national prevalence of chronic infection of 10%. However the chronic carrier prevalence varies widely across the country, being highest in the North and Northeast (18–24%) and lowest in the Southwestern region (4%) [4,5]. Relatedly, infection with human immune deficiency virus (HIV) is estimated to be 6.4% in Uganda and the prevalence of coinfection with HIV and HBV ranges from 7–17% [6,7]. Co-infection accelerates progression of liver disease to cirrhosis and its complications [8]. Co-infected individuals receiving tenofovir (TDF) and/or lamivudine (3TC) as part of their anti–retroviral therapy (ART) may on the other hand be able to supress HBV thereby lowering chances of disease transmission and progression.
HBV is the leading cause of liver cirrhosis, end stage liver disease, and liver cancer in Africa. It is a preventable disease but implementation of prevention methods is difficult since there is limited understanding about the modes of transmission. In view of the high prevalence of HBV from the above community studies, mother-to-infant transmission might be one of the important preventable routes of HBV in Uganda. This route of transmission is associated with chronic HBV infection, persistence of viral replication and severe liver disease [9]. An estimated 90% of neonatal, 20–50% of childhood and 5% of adulthood infections will progress from the acute to the chronic phase of the disease [3,10].
Similar to many SSA countries, Uganda's expanded program on immunization (UNEPI) integrated the HBV vaccination in its schedule in 2002 [11] with the first vaccine dose included in a pentavalent vaccine given at the age of six weeks and the second and third dose at 10 and 14 weeks respectively [12]. Currently, the HepB3 coverage in Uganda is about 78% [13]. Mother to infant transmission of HBV at birth can be efficiently prevented by the concurrent administration of the HBV vaccine and the hepatitis B immune globulin (HBIG) at birth [14,15]. Currently, it is not standard practice to screen pregnant mothers for HBV infection in Uganda and the monovalent HBV vaccine and the HBV immunoglobulin are too expensive and not readily available for the prevention of mother to child transmission of HBV in SSA. However, even in the absence of HBIG, the monovalent HBV vaccine can significantly prevent disease transmission if administered within the first 12–24 h of delivery [16,17]. This delay in administering the first dose of HBV vaccine may provide a window in which HBV may be transmitted from infected close contacts to babies in their early weeks of life. A maternal H BV-DNA level >199,999 IU/mL is predictive of failure of passive-active immune prophylaxis in infants born to HBsAg positive mothers [18].
Advocating for a change in the vaccination schedule to have the first HBV dose given at birth in resource poor settings requires data demonstrating the HBV incidence rate among babies. This study assessed the HBV incidence rate and its risk factors among babies till their last UNEPI visit in Gulu, rural Uganda.
2. Methods
2.1. Study participants
This study enrolled mothers and their babies attending the postnatal/immunization clinic of a rural University teaching hospital in Gulu, Northern Uganda. The babies are immunized following the Uganda Government's national expanded programme on immunization (UNEPI) schedule. Following this guide, the first dose of the pentavalent vaccine which contains the HBV vaccine is given at the age of 6 weeks, the second and third doses at 10 and 14 weeks respectively. In the UNEPI schedule, the last immunization which is for measles is given at 9 months (or at the first contact after these age cut off points) [12].
All mothers who presented their babies (≥6 weeks old) for their first HBV vaccine dose during the study period which ran from July 2012 to June 2014 were asked to participate in this study. Those that consented were recruited. A data collection tool was used to record data on socio- demographic, obstetric history, and HIV infection. Mothers whose HIV serostatus was not known underwent counseling and testing for HIV.
Laboratory testing: Mothers and their babies were all subjected to blood tests. Five mls of blood was drawn from the mothers for HBV, HIV and liver function tests. All mothers were screened for previous and current HBV infection using the hepatitis B core IgG antibody (anti-HBc IgG) and a single hepatitis B surface antigen (HBsAg) rapid test respectively. HBsAg positive sera were later tested for the hepatitis e antigen (HBeAg) and hepatitis B viral load (HBVDNA) test using a PCR assay (Roche TaqMan© with HBVDNA detection range of 20–170,000,000 IU/mL). Liver function tests were also performed. Further, all HIV infected mothers underwent CD4 T-cell count and HIV viral load testing using flow cytometry and PCR respectively.
Regardless of the mother's HBsAg status, babies were tested for HBsAg using a finger prick sample at the first visit (6 weeks) and again at 9 months. A random sample of HBsAg negative babies at both testing rounds was subjected to nucleic acid (HBVDNA) testing to exclude occult disease.
HBsAg testing was done on fresh serum samples using the Cypress Diagnostics (Cypress Diagnostics, Langdorp, Belgium kit lot number B8201402009, expiry date 10.2.2016 and code 142-050/S), a one-step, visual immunochromatographic test for the qualitative detection of HBsAg in human serum and plasma. The test kit is known to have a sensitivity and specificity of 98.84% and 98.94% respectively.
Throughout the study, blood samples were shipped from the study site to MBN, a commercial clinical laboratory in Kampala, Uganda where they were processed and the study tests performed. On receiving the first HBV vaccine dose, mothers were reminded to bring back their children for subsequent vaccine doses. This study was approved by the School of Medicine Ethics and Research Committee, Makerere University College of Health Sciences and the Uganda National Council of Science and Technology.
Outcomes and measurements: The main study outcome was incident HBV infection of the baby defined by either a positive HBsAg test or detectable HBVDNA at any of the above testing points. Maternal exposures evaluated as risk factors for HBV acquisition included socio-demographic and clinical factors such as the number of life sexual partners, body cuts, and blood transfusion. Infantile exposures evaluated were: body cuts, blood transfusion, breastfeeding, and mode of delivery were considered. Laboratory parameters assessed among mothers included HBeAg, HBV DNA and HIV sero-status. In case of HIV infection, we assessed the CD4 T-cell count, HIV viral load, and ART use.
2.2. Statistical analysis
Data were analyzed using STATA software package, version 12.0 (College Station, Texas, USA).
Key variables were summarized as proportions if they were categorical, while means (standard deviations) or median (interquartile range) were obtained for the continuous variables.
Associations between variables were assessed utilizing both parametric and non-parametric statistical techniques i.e. chi-square or Fisher's exact for categorical outcomes and unpaired t-test or Wilcoxon rank sum test for continuous outcomes. The 95% confidence intervals for proportions of binary variables were computed using the exact binomial formula which provided insight about statistical significance. Logistic regression was performed to identify factors that are associated with maternal HBV infection.
3. Results
3.1. Mothers
We recruited 630 mothers 18 of whom had incomplete data and were dropped from further analysis. The study population comprised 612 young mothers with median age 23 (IQR 20–28). Over 97% reported less than five lifetime sexual partners. Risk factors for being a HBV chronic carrier were not statistically significant among the HBV infected and the non-infected. Overall, 7.5% previously undergone surgery, 5.0% blood transfusion and 0.8% body cuts.
In this cohort, 53 (8.7%) tested positive for HBsAg and 339 (61.5%) for anti-HBc (Fig. 1). Of the HBsAg positive mothers, 10 (18.9%) were HBeAg positive. The median HBV viral load of infected mothers was 5.7 log10 (IQR: 4.6–7.0) IU/mL The majority, 32 (62.7%) had HBVDNA levels below 2 × 103 IU/mL and only 9 (17.6%) had levels ≥105 IU/mL. All liver transaminase enzyme levels were within the normal range (0–45 U/L). Alanine amino transferase (ALT) levels were higher among HBV infected (22 IU/Ml; 95%CI: 18.2–27.7) compared to their uninfected counterparts (15.2 IU/mL; 95%CI: 11.8–18.1) (p = 0.04). There was no significant difference in albumin levels between HBV infected (4.0 g/L; 95%CI: 4.0–4.2) and uninfected mothers (4.1 g/L; 95%CI: 4.0–4.2) (p = 0.86).
Fig. 1.
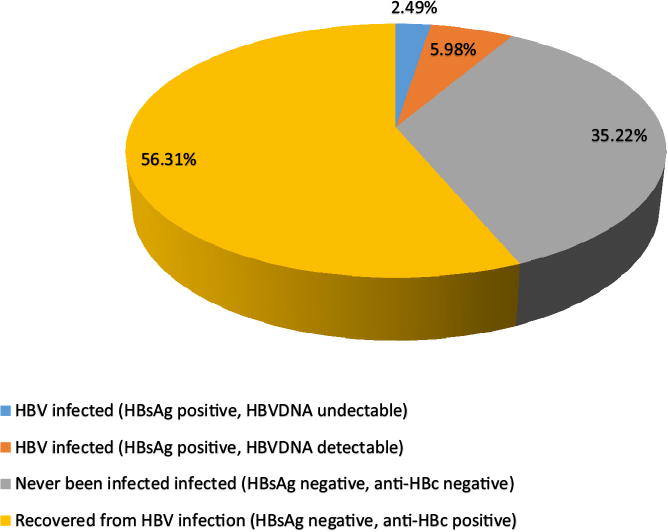
Participant status by hepatitis B infection.
Overall, HIV infection was prevalent in 80 (13.3%) of mothers while coinfection with HBV occurred in 9 (11.5%) of HIV infected individuals. There was a non-statistically significant difference in proportion of HBV infection among the HIV infected participants OR =1.3 (95%CI: 0.7–2.5) (p = 0.41). Five (55.6%) of the co-infected individuals were receiving ART that contained anti-HBV medications (three on a combination of TDF and 3TC, and two on 3TC only). During the peripartum period 65/80 (81.3%) mothers received a medication that could potentially prevent HIV transmission to their babies: 44/65 (67.7%) received ART (12 on a combination of TDF/3TC, 29 on a 3TC-based, and 3 on unspecified regimens); 18 and 3 ART-naïve mothers received single dose nevi-rapine (NVP) and zidovudine (AZT) respectively. None of the HBV mono-infected mothers were on either 3TC, TDF or both medications. Among mothers with detectable HBVDNA levels, all the 5 HIV/HBV co-infected mothers who were on ART had undetectable HBV viral load levels while the ART naïve had a HBV geometric mean of 7.8log10 (95% CI: 4.4–13.9) IU/mL. The HIV-uninfected participants had a HBVDNA geometric mean 8.3 log10 (95% CI: 6.9–10.1) IU/mL. The odds of having maternal HBV infection were 38% greater in the presence of HIV infection. The median CD4 T-cell count of the HIV-infected mothers was 540 cells/μL (IQR: 385–708) Table 1.
Table 1.
Maternal characteristics by hepatitis B surface antigen status.
| Total cohort n (%) or median (IQR) | HBsAg positive n (%) or median (IQR) | HBsAg negative n (%) or median (IQR) | p-value | |
|---|---|---|---|---|
| Pregnant women, number | 612 | 53 (8.7) | 559 (91.3) | |
| Age (years) | 23 (20–28) | 25 (21–29) | 23 (20–27) | 0.20 |
| Number of sexual partners* | ||||
| <5 | 593 | 52 (98.1) | 541 (96.8) | 1.00 |
| >5 | 13 | 1 (1.9) | 12 (2.2) | |
| Surgery | 46 | 3 (6.5) | 43 (93.5) | 0.79 |
| Blood transfusion | 31 | 3 (9.7) | 28 (90.3) | 0.74 |
| Body cuts | 5 | 0 (00.0) | 5 (100) | 1.00 |
| Anti-HBc IgG** | ||||
| Positive | 391 (64.7) | 52 (98.1) | 339 (61.5) | <0.0001 |
| Negative | 213 (35.3) | 1 (1.9) | 212 (38.5) | |
| HBeAg*** | ||||
| Positive | 10 (18.9) | |||
| Negative | 42 (79.3) | |||
| HBVDNA | ||||
| Detectable | 51(96.2) | |||
| Below detection limit | 15(28.3) | |||
| Missing | 2 (3.8) | |||
| HBVDNA log10 (IU/mL)a | 8.25 (6.93–9.82) | |||
| HBVDNA <105 | 42 (82.4) | |||
| HBVDNA >105 | 9 (17.6) | |||
| HBVDNA > 199,999 | 8 (15.7) | |||
| HBVDNA >2 × 103 | 32 (62.7) | |||
| Median ALT (IU/mL) | 18.0 (10.6–28.6) | 22.2 (15.1–37.0) | 15.2 (6.6–21.5) | 0.004 |
| Median Albumin (g/l) | 4.1 (3.7–4.4) | 4.0 (3.8–4.3) | 4.1 (3.7–4.5) | 0.86 |
| HIV serostatus**** | ||||
| Positive | 80(13.3) | 9 (17.0) | 71 (12.9) | 0.41 |
| Negative | 522(86.7) | 44 (83.0) | 478 (87.1) | |
| HIV positive on ART***** | ||||
| Yes | 44 (56.4) | 5 (55.6) | 39 (56.5) | 1.00 |
| No | 34 (43.6) | 4 (44.4) | 30 (43.5) | |
| Anti-viral therapy regimen | ||||
| Single dose nevirapine | 18 (27.7) | 2 (28.6) | 16 (27.6) | 1.00 |
| Single dose zidovudine | 3 (4.6) | 0 (0.0) | 3 (5.2) | |
| ART | 44 (66.7) | 5 (71.4) | 39 (67.2) | |
| HBV active ART | ||||
| 3TC based ART | 29 | 2 (6.9) | 27 (93.1) | |
| TDF/3TC based ART | 12 | 3 (25.0) | 9(75.0) | |
| HIVRNA log10 (IU/mL)b | 8.29 (7.54–9.12) | 9.78 (7.83–12.18) | 8.13 (7.33–9.02) | 0.31 |
| HIVRNA ≤40 (IU/mL)c | 14 | 3 (21.4) | 11 (78.6) | 0.13 |
| >40 (IU/mL) | 46 | 5 (10.9) | 41 (89.1) | |
| CD4 T-cell count/mm3 | 540 (385–708) | 540 (433–554) | 534 (377–711) | 0.99 |
AZT=3TC = lamivudine, TDF = tenofovir, TDF/3TC = combination of lamivudine and tenofovir, ALT = alanine aminotransferase.
Missing data on 6 participants.
Missing data on 8 participants.
Missing data on 1 participant.
Missing data on 10 participants.
Missing data on 2 participants.
Geometric mean and 95% confidence intervals of 36 hepatitis B-infected individuals.
Geometric mean and 95% confidence intervals of 46 HIV-infected individuals.
11/14 participants with undetectable HIVRNA (≤40 (IU/mL) were receiving ART.
3.2. Babies
619 babies were recruited, including 7 sets of twins. Similar to their mothers, few were exposed to known risk factors for HBV acquisition; 29 (4.5%) body cuts, 11 (1.8%) tooth extraction, and 10 (1.6%) blood transfusion (Table 2).
Table 2.
Mode of delivery and potential risk factors for acquisition of HBV by babies at 6 weeks.
| Babies | HBsAg negative n (%) |
|---|---|
| Babies, number (N = 619)a | 619 |
| Mode of delivery | |
| Normal | 561 (91.7) |
| Extraction | 7 (1.1) |
| Caesarian section | 44 (7.2) |
| Body cuts | 29 |
| Blood transfusion | 10 |
| False tooth extraction | 11 |
| Breast feeding | 607 |
None of the 619 babies was HBsAg positive.
From birth to presentation for the first HBV vaccine dose, 606 babies accrued a person-time of 80.4 years within which no HBV infection was registered. Similarly, follow up of 218 of these babies up to the last vaccination visit yielded an additional 142.1 person-years in which no HBV infection was diagnosed. HBVDNA was not detected in a randomly selected sample of 20 HBsAg negative babies from this visit.
4. Discussion
We found no transmission of HBV at the time of receiving the first HBV vaccine dose through the last childhood vaccination visit in a large cohort of over 600 babies born in a region where HBV is highly endemic. Our findings corroborate a recent study that evaluated the impact of the pentavalent HBV vaccine in northern Uganda in which the HBV prevalence was noted to increase steadily with age; from 0% among those below 5 years to 14.9% in the 15–19 year age group [19]. Our data are consistent with an earlier study in SSA where vertical HBV transmission was rare [20] but contrasts with the lower prevalence (3% and 6%) reported by similar SSA studies [21,22].
The high prevalence of a combination of HBsAg-positive HBeAg-negative infections recorded in our study is in line with data reported from other studies elsewhere in Africa [21,23]. Mothers with this pair of serological markers are less efficient at transmitting infection vertically to their off springs. Indeed, a recent study revealed that HBV genotypes A and D which are more efficiently transmitted by horizontal means [24] are the most predominant in Uganda [25]. Data indicate that individuals with HBV sub-genotype A1 and genotype D usually seroconvert to anti-HBe (HBeAg negative) status before their gestation age [26,27]. Thus, they are less likely to have significant ongoing HBV replication when they start conceiving. It is therefore not surprising that vertical HBV disease transmission was rare in our study cohort where also, the majority of mothers had a HBVDNA levels less than 105 IU/mL, a level that is considered to be the threshold below which no significant mother-to-child HBV disease transmission occurs [28]. The observed lack of disease transmission at viral load levels > 105 IU/mL could be attributed to the small number of mothers (n = 9) with such high HBVDNA levels. Unlike Southeast Asia, where vertical transmission is the predominant route of disease transmission, our findings support the hypothesis that majority of HBV infections in SSA are acquired through horizontal means. Horizontal transmission of HBV can be prevented through vaccination even during the 6 week first dose inoculation [26]. It is not clear why there is a high HBV prevalence in northern Uganda but we hypothesize that it is most probably a result of using unsterile equipment for cultural practices such as scarification.
Despite a high odds ratio, we found no relationship between HIV infection and HBV transmission. This finding should however be interpreted with caution since we had very few mothers who were co-infected with HIV and HBV. Most of the HIV-infected individuals were receiving ART so their CD4 T-cell count was preserved. HIV induced immunosuppression is associated with a high levels of HBV replication and thus high HBV levels [8]. Indeed, in a large randomized controlled trial that enrolled ART naïve expecting mothers in Malawi and used HBVDNA as a surrogate marker for infection, nearly 10% of the babies born to HIV/HBV co-infected mothers became infected by 48 weeks of age despite receiving the pentavalent HBV vaccine at 6, 10 and 14 weeks of age [29]. Similar findings were reported in South Africa [30].
In our study, none of all the randomly selected HBsAg negative babies that underwent HBVDNA testing had occult HBV.
This study has some limitations. We recruited a convenient sample of participants who presented to the hospital for postnatal care/immunization. Their health seeking behaviors might therefore not be representative of the entire population. Secondly, of the ten mothers who were both HBsAg and HBeAg positive, five were on tenofovir and/or lamivudine. The use of these anti-viral agents could have prevented disease transmission to children born to the five mothers who were using these medications. Further, HBV transmission could have been missed out at the 6-week vaccination since the HBsAg could take up to 9 weeks to be detected. We attempted to test at 6 weeks because, this is the time that we could easily get access to the mothers and their babies. At that age, there is limited contact of the babies with other persons apart from the mother and other very close relatives. Infection therefore could be presumed to be largely mother to child. We attempted to retest the babies at 9 months in case infection could not be picked at 6-weeks. We were however able to test only 35% of the babies as only a limited mothers present their children for measles vaccination at 9 months. Tracking the mothers became difficult and this may have affected the internal validity of the results. Despite these limitations, the study has strength: it prospectively evaluated a very large cohort of babies for incident HBV infection in a region where HBV is highly endemic. In addition, we performed nucleic acid testing on some samples to be sure that occult HBV infection was not missed.
In conclusion, we found no HBV transmission among babies born to HIV-infected as well as HIV-uninfected mothers in northern Uganda prior to the age of six weeks when the first HBV vaccine dose was administered. These findings suggest that the current practice of administering the first HBV vaccine to babies in Uganda at six weeks of age is adequate. Another study needs to be conducted to identify the factors fueling the high HBV prevalence in northern Uganda.
Acknowledgments
Funding: This research study was funded by the European & Developing Countries Clinical Trials Partnership (Grant No. TA.2011.40200.004). It was supported by VLIR-UOS via the HEFS Platform Harvest Call (ZIUS2016VOA0902) and by Grant No. D43TW010132 supported by Office Of The Director, National Institutes Of Health (OD), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Neurological Disorders And Stroke (NINDS), National Heart, Lung, And Blood Institute (NHLBI), Fogarty International Center (FIC), National Institute On Minority Health And Health Disparities (NIMHD). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the supporting offices.
The authors wish to thank the mothers, babies and the research staff whose hard work contributed to this study.
Footnotes
Conflict of interest: ES, JV, RS, CKO, JMK, JBS, RC and PO all have no conflicts of interest to declare.
References
- 1.Liaw YF, Chu CM. Hepatitis B virus infection. Lancet (London, England) 2009;373(9663):582–92. doi: 10.1016/S0140-6736(09)60207-5. [DOI] [PubMed] [Google Scholar]
- 2.Zampino R, Boemio A, Sagnelli C, Alessio L, Adinolfi LE, Sagnelli E, et al. Hepatitis B virus burden in developing countries. World J Gastroenterol. 2015;21(42):11941–53. doi: 10.3748/wjg.v21.i42.11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Hepatitis B fact sheet. Jul 2016; updated. [Google Scholar]
- 4.Bwogi J, Braka F, Makumbi I, Mishra V, Bakamutumaho B, Nanyunja M, et al. Hepatitis B infection is highly endemic in Uganda: findings from a national serosurvey. African Health Sci. 2009;9(2):98–108. [PMC free article] [PubMed] [Google Scholar]
- 5.Ochola E, Ocama P, Orach CG, Nankinga ZK, Kalyango JN, McFarland W, et al. High burden of hepatitis B infection in Northern Uganda: results of a population-based survey. BMC Public Health. 2013;13:727. doi: 10.1186/1471-2458-13-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ocama P, Seremba E, Apica B, Opio K. Hepatitis B and HIV co-infection is still treated using lamivudine-only antiretroviral therapy combination in Uganda. African Health Sci. 2015;15(2):328–33. doi: 10.4314/ahs.v15i2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baseke J, Musenero M, Mayanja-Kizza H. Prevalence of hepatitis B and C and relationship to liver damage in HIV infected patients attending Joint Clinical Research Centre Clinic (JCRC), Kampala, Uganda. African Health Sci. 2015;15(2):322–7. doi: 10.4314/ahs.v15i2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48(2):335–52. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 9.Shimakawa Y, Lemoine M, Njai HF, Bottomley C, Ndow G, Goldin RD, et al. Natural history of chronic HBV infection in West Africa: a longitudinal population-based study from The Gambia. Gut. 2016;65(12):2007–16. doi: 10.1136/gutjnl-2015-309892. [DOI] [PubMed] [Google Scholar]
- 10.Yim HJ, Lok AS. Natural history of chronic hepatitis B virus infection: what we knew in 1981 and what we know in 2005. Hepatology (Baltimore, MD) 2006;43(2 Suppl 1):S173–81. doi: 10.1002/hep.20956. [DOI] [PubMed] [Google Scholar]
- 11.Khamduang W, Gaudy-Graffin C, Ngo-Giang-Huong N, Jourdain G, Moreau A, Borkird T, et al. Analysis of residual perinatal transmission of hepatitis B virus (HBV) and of genetic variants in human immunodeficiency virus and HBV co-infected women and their offspring. J Clin Virol: Off Publ Pan Am Soc Clin Virol. 2013;58(2):415–21. doi: 10.1016/j.jcv.2013.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olaleye OA, Kuti O, Makinde NO, Ujah AO, Olaleye OA, Badejoko OO, et al. Perinatal transmission of hepatitis B virus infection in Ile-Ife, South Western, Nigeria. J Neonatal-perinatal Med. 2013;6(3):231–6. doi: 10.3233/NPM-1366412. [DOI] [PubMed] [Google Scholar]
- 13.http://apps.who.int/immunization_monitoring/globalsummary/estimates?c=UGA.
- 14.Hieu NT, Kim KH, Janowicz Z, Timmermans I. Comparative efficacy, safety and immunogenicity of Hepavax-Gene and Engerix-B, recombinant hepatitis B vaccines, in infants born to HBsAg and HBeAg positive mothers in Vietnam: an assessment at 2 years. Vaccine. 2002;20(13–14):1803–8. doi: 10.1016/s0264-410x(01)00518-7. [DOI] [PubMed] [Google Scholar]
- 15.Wong VC, Ip HM, Reesink HW, Lelie PN, Reerink-Brongers EE, Yeung CY, et al. Prevention of the HBsAg carrier state in newborn infants of mothers who are chronic carriers of HBsAg and HBeAg by administration of hepatitis-B vaccine and hepatitis-B immunoglobulin. Double-blind randomised placebo-controlled study Lancet (London, England) 1984;1(8383):921–6. doi: 10.1016/s0140-6736(84)92388-2. [DOI] [PubMed] [Google Scholar]
- 16.Milne A, West DJ, Chinh DV, Moyes CD, Poerschke G. Field evaluation of the efficacy and immunogenicity of recombinant hepatitis B vaccine without HBIG in newborn Vietnamese infants. J Med Virol. 2002;67(3):327–33. doi: 10.1002/jmv.10071. [DOI] [PubMed] [Google Scholar]
- 17.Poovorawan Y, Chongsrisawat V, Theamboonlers A, Srinivasa K, Hutagalung Y, Bock HL, et al. Long-term benefit of hepatitis B vaccination among children in Thailand with transient hepatitis B virus infection who were born to hepatitis B surface antigen-positive mothers. J Infect Dis. 2009;200(1):33–8. doi: 10.1086/599331. [DOI] [PubMed] [Google Scholar]
- 18.Pan CQ, Duan Z, Dai E, Zhang S, Han G, Wang Y, et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. New Engl J Med. 2016;374(24):2324–34. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 19.Teshale EH, Kamili S, Drobeniuc J, Denniston M, Bakamutamaho B, Downing R. Hepatitis B virus infection in northern Uganda: impact of pentavalent hepatitis B vaccination. Vaccine. 2015;33(46):6161–3. doi: 10.1016/j.vaccine.2015.09.058. [DOI] [PubMed] [Google Scholar]
- 20.Marinier E, Barrois V, Larouze B, London WT, Cofer A, Diakhate L, et al. Lack of perinatal transmission of hepatitis B virus infection in Senegal, West Africa. J Pediatr. 1985;106(5):843–9. doi: 10.1016/s0022-3476(85)80371-1. [DOI] [PubMed] [Google Scholar]
- 21.Roingeard P, Diouf A, Sankale JL, Boye C, Mboup S, Diadhiou F, et al. Perinatal transmission of hepatitis B virus in Senegal, west Africa. Viral Immunol. 1993;6(1):65–73. doi: 10.1089/vim.1993.6.65. [DOI] [PubMed] [Google Scholar]
- 22.Menendez C, Sanchez-Tapias JM, Kahigwa E, Mshinda H, Costa J, Vidal J, et al. Prevalence and mother-to-infant transmission of hepatitis viruses B, C, and E in Southern Tanzania. J Med Virol. 1999;58(3):215–20. doi: 10.1002/(sici)1096-9071(199907)58:3<215::aid-jmv5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 23.Noubiap JJ, Nansseu JR, Ndoula ST, Bigna JJ, Jingi AM, Fokom-Domgue J. Prevalence, infectivity and correlates of hepatitis B virus infection among pregnant women in a rural district of the Far North Region of Cameroon. BMC Public Health. 2015;15:454. doi: 10.1186/s12889-015-1806-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi YH. Correlation between hepatitis B virus genotypes and clinical outcomes. Japan J Infect Diseas. 2012;65(6):476–82. doi: 10.7883/yoken.65.476. [DOI] [PubMed] [Google Scholar]
- 25.Zirabamuzale JT, Opio CK, Bwanga F, Seremba E, Apica BS, Colebunders R, et al. Hepatitis B virus genotypes A and D in Uganda. J Virus Eradicat. 2016;2(1):19–21. doi: 10.1016/S2055-6640(20)30693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kramvis A. The clinical implications of hepatitis B virus genotypes and HBeAg in pediatrics. Rev Med Virol. 2016;26(4):285–303. doi: 10.1002/rmv.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Croagh CM, Desmond PV, Bell SJ. Genotypes and viral variants in chronic hepatitis B: a review of epidemiology and clinical relevance. World J Hepatol. 2015;7(3):289–303. doi: 10.4254/wjh.v7.i3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kumar M, Singh T, Sinha S. Chronic hepatitis B virus infection and pregnancy. J Clin Experiment Hepatol. 2012;2(4):366–81. doi: 10.1016/j.jceh.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chasela CS, Kourtis AP, Wall P, Drobeniuc J, King CC, Thai H, et al. Hepatitis B virus infection among HIV-infected pregnant women in Malawi and transmission to infants. J Hepatol. 2014;60(3):508–14. doi: 10.1016/j.jhep.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann CJ, Mashabela F, Cohn S, Hoffmann JD, Lala S, Martinson NA, et al. Maternal hepatitis B and infant infection among pregnant women living with HIV in South Africa. J Internat AIDS Soc. 2014;17:18871. doi: 10.7448/IAS.17.1.18871. [DOI] [PMC free article] [PubMed] [Google Scholar]


