Abstract
The objective of the present study was to identify altered pathways in breast cancer based on the individualized pathway aberrance score (iPAS) method combined with the normal reference (nRef). There were 4 steps to identify altered pathways using the iPAS method: Data preprocessing conducted by the robust multi-array average (RMA) algorithm; gene-level statistics based on average Z; pathway-level statistics according to iPAS; and a significance test dependent on 1 sample Wilcoxon test. The altered pathways were validated by calculating the changed percentage of each pathway in tumor samples and comparing them with pathways from differentially expressed genes (DEGs). A total of 688 altered pathways with P<0.01 were identified, including kinesin (KIF)- and polo-like kinase (PLK)-mediated events. When the percentage of change reached 50%, 310 pathways were involved in the total 688 altered pathways, which may validate the present results. In addition, there were 324 DEGs and 155 common genes between DEGs and pathway genes. DEGs and common genes were enriched in the same 9 significant terms, which also were members of altered pathways. The iPAS method was suitable for identifying altered pathways in breast cancer. Altered pathways (such as KIF and PLK mediated events) were important for understanding breast cancer mechanisms and for the future application of customized therapeutic decisions.
Keywords: breast cancer, average Z, individualized pathway aberrance score, altered pathways
Introduction
Breast cancer is characterized by a distinct metastatic pattern involving the regional lymph nodes, bone marrow, lungs and the liver (1). It is the most common type of cancer diagnosed among women and the second leading cause of cancer mortality among women following lung cancer (2). A family history of breast cancer and several other factors (including female sex, old age and exposure to ionizing radiation) increase the risk of developing breast cancer (3). In addition, 5–10% of breast cancer cases are caused by inherited gene mutations (4). Several gene markers have been identified to predict responses to therapeutic regimens, such as receptor tyrosine-protein kinase erbB-2 and Stearoyl-CoA desaturase-1 (5–7). However, development remains necessary to understanding the mechanisms of breast cancer, in order to customize anticancer therapies and to identify altered pathways in an individual with breast cancer.
Pathway analysis has become the first choice for gaining insight into the underlying biology of genes and proteins, as it reduces complexity and has increased explanatory power (8). Existing pathway analysis techniques are predominantly focused on discovering altered pathways between normal and cancer groups and are not suitable for identifying the pathway aberrance that may occur in an individual sample (9). A simple way to identify an individual's pathway aberrance is to compare normal and tumor data from the same individual. However, matched normal data from the same individual is often unavailable in clinical situations. Therefore, the present study applied a new approach for the personalized identification of altered pathways, making special use of accumulated normal data in cases when a patient's matched normal data were unavailable (10).
The present study identified altered pathways in breast cancer based on the individualized pathway aberrance score (iPAS) method which included data preprocessing, gene-level statistics, pathway-level statistics and a significant test. The altered pathways were validated by comparison with pathways based on differentially expressed genes (DEGs), and by calculating the percentage of changed pathways in breast cancer samples.
Materials and methods
Gene expression data
In the present study, the gene expression profile with accession number E-GEOD-10780 (11) was recruited from the ArrayExpress database (http://www.ebi.ac.uk/arrayexpress/). E-GEOD-10780, which was presented on the A-AFFY-44-Affymetrix GeneChip Human Genome U133 Plus 2.0 (Affymetrix, Inc., Santa Clara, CA, USA), comprised of 143 normal control samples and 42 breast cancer samples. The gene expression profile on probe level was converted into gene symbol level. Subsequent to removing the duplicated symbols, a total of 20,102 gene symbols were obtained for additional analysis.
Pathway data
Information from gene sets representing biological pathways was downloaded from the reactome pathway database (http://www.reactome.org/) (12). Reactome is an online curated resource for human pathway data and provides infrastructure for computation across the biological reaction network (12). Pathways involving a small number of genes are easily understood by researchers. Therefore, for the present study pathways with a gene set of >100 were filtered out. In addition, the present study also eliminated pathways that had a null set with the gene expression profile E-GEOD-10780. Finally, 1,013 pathways were identified, which consisted of 5,182 genes.
Individualized analysis for pathways
To identify altered pathways in an individual breast cancer sample, the iPAS method was employed (10), which includes 4 steps: Data preprocessing (Fig. 1A); gene-level statistics (Fig. 1B); pathway-level statistics (Fig. 1C); and a significant test (Fig. 1D).
Figure 1.
Schematic flow showing the 4 stages of individualized pathway analysis based on the iPAS method using normal reference. iPAS, individualized pathway aberrance score. (A) Data preprocessing schematic flow. (B) Gene-level statistical analysis. (C) Pathway-level statistical analysis. (D) Statistical significance test.
Data pre-processing
Prior to analysis, a standard pre-treatment was conducted to control the quality of the gene expression profiles. For normal genes, background corrections and normalization were carried out using the robust multi-array average (RMA) algorithm and the quantile based algorithm to eliminate the influence of nonspecific hybridization (13,14). The Micro Array Suite 5.0 (MAS 5.0) algorithm was then applied to revise perfect match and mismatch value (15), and median polish method was applied to summarize the expression value (13). All normal control samples were regarded as references (nRef) in the present study. For individual breast cancer cases, the present study performed uniformly standardized normalization following the combination of the single tumor microarray with all nRef samples.
Gene-level statistics
Standardizing the gene expression on the gene-level via mean and standard deviation (SD) from datasets is often used in microarray analysis. In the present study, the individual tumor sample gene expression level was standardized based on the mean and SD of the normal references. This formula was defined as:
Where mean (Nj) symbolized the mean expression value of the genes of the nRef, stdev (Nj) symbolized the SD of the normal, Tij symbolized the expression value of i-th tumor gene and Zij symbolized the standardized expression value of i-th tumor gene, where the number of genes belonging to the gene was i.
Pathway-level statistics based on average Z
The average Z method was selected to evaluate iPAS by utilizing the nRef. A vector Z=(zn) denoted the expression status of a pathway, where zi symbolizes the standardized expression value of the i-th gene and is derived from mean and SD of the nRef. n was the number of genes belonging to the pathway. The iPAS was calculated as following:
Significant measurement
A one sample Wilcoxon-test was conducted for normal and tumor pathway statistics values to estimate the significance of the pathways (16). All collected normal samples for the nRef were sequentially compared with the nRef to yield statistics of the null distribution. A P-value was produced according to comparison between this null distribution and a statistic from a single tumor case, and was adjusted by false discovery rate (FDR). A pathway with P<0.01 was considered as altered pathway compared with nRef.
Hierarchical clustering analysis of altered pathways
To assess the classification performance of altered pathways, a hierarchical clustering analysis was applied across 42 tumor samples and 143 normal control samples using the Gene Cluster 3.0 (Human Genome Center, University of Tokyo, Tokyo, Japan) program. The clustering algorithm was set to complete linkage clustering using an uncentered correlation. Ideally, the samples should be classified into 2 major clusters: Tumor cases and normal controls. The present study tested the method by measuring the percentage of test samples that could be correctly classified. Accuracy is the fraction of correctly classified samples over all samples (17).
TP (true positive) represents the number of positive samples correctly predicted as positive, TN (true negative) represents the number of negative samples correctly predicted as negative, FP (false positive) represents the number of negative samples incorrectly predicted as positive and FN (false negative) represents the number of positive samples incorrectly predicted as negative.
Validation of the altered pathways
The present study applied 2 methods to validate altered pathways obtained from individualized analysis using the nRef, one was comparing with the traditional approach according to DEGs to identify pathways, and the other was by calculating the percentage of each changed pathway in tumor samples.
DEGs based pathway analysis
The linear models for microarray data (Limma) package (18) was utilized to explore DEGs between the patients with breast cancer and the normal controls. Only the genes with FDR adjusted P-values of <0.01 and log fold change of >2 were classed as DEGs. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis for DEGs was conducted based on the Database for Annotation, Visualization and Integrated Discovery (19), which implemented the expression analysis systematic explorer test to selected pathways with the criterion P<0.01 (20).
Pathway changed percent
To validate altered pathways in tumors which were identified by iPAS method combined with nRef, the present study counted the percentage change for each pathway in breast cancer samples. Firstly, by taking the distribution character of each pathway statistic value in normal and tumor samples, the empirical P-value of each pathway in a tumor individual compared with nRef was detected. The amount of P-values <0.01 were then statistically counted in order to obtain the changed percentage for each pathway in all breast cancer cases.
Statistical analysis
In the present study, the one sample Wilcoxon test (using SPSS v.19.0; IBM SPSS, Armonk, NY, USA) was utilized to estimate the significance of the pathways, of which P values also were calculated. P<0.05 was considered to indicate a statistically significant difference. However, to be confident in the validity of these results, P<0.01 was the statistically significant threshold in the current study.
Results
Identification of altered pathways
In the present study, 143 normal control samples in the gene expression profile E-GEOD-10780 were defined as nRef of 42 tumor samples. The present study performed quantile normalization for tumor genes to evaluate their gene-level statistics. A total of 1,013 pathways were identified from the reactome pathway database. The present study extracted gene-level statistic values of all genes enriched in one pathway, and denoted the mean value to pathway-level statistics of this pathway. With a threshold value of P<0.01, a total of 688 altered pathways were explored for breast cancer. The cluster analysis of using Average Z as the iPAS method based on individual breast cancer and normal controls was also shown in Fig. 2. As presented in Fig. 2, TP=31, FP=11, TN=143, while FN=0, thus the accuracy of classification equaled to 94.05%, which indicated that the samples possessed good classification.
Figure 2.
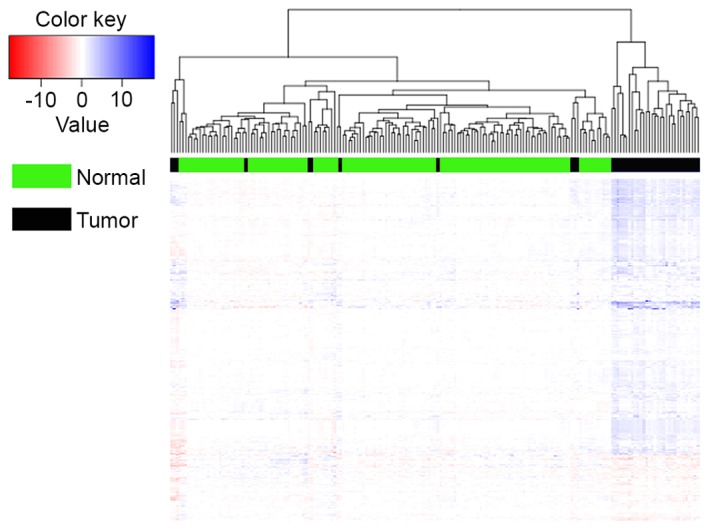
Cluster of using Average Z as the iPAS method based on individual breast cancer and normal controls. iPAS, individualized pathway aberrance score.
In addition, the top 5% of the 688 altered pathways are shown in Table I. Polo-like kinase (PLK) -mediated events, phosphorylation of proteins involved in G1/S transition by active cyclin E/Cdk2 complexes, G2/M DNA replication checkpoint, FGFR2b ligand binding and activation and cyclin A/B1 associated events during G2/M transition were the most significant pathways with a P-value of 4.38E-18.
Table I.
Top 5% of 688 altered pathways with P<0.01 in breast cancer.
| Pathway | P-value |
|---|---|
| Polo-like kinase mediated events | 4.38E-18 |
| Phosphorylation of proteins involved in G1/S transition by active Cyclin E/Cdk2 complexes | 4.38E-18 |
| G2/M DNA replication checkpoint | 4.38E-18 |
| FGFR2b ligand binding and activation | 4.38E-18 |
| Cyclin A/B1 associated events during G2/M transition | 4.38E-18 |
| Deposition of new CENPA-containing nucleosomes at the centromere | 1.27E-17 |
| Nucleosome assembly | 1.27E-17 |
| Kinesins | 1.50E-17 |
| Removal of the flap intermediate from the C-strand | 3.47E-17 |
| G1/S-specific transcription | 4.99E-17 |
| Phosphorylation of emi1 | 4.99E-17 |
| Removal of the flap intermediate | 8.93E-17 |
| Chromosome maintenance | 8.93E-17 |
| G0 and early G1 | 8.93E-17 |
| FGFR1b ligand binding and activation | 1.02E-16 |
| Cyclin B2 mediated events | 1.05E-16 |
| CHL1 interactions | 1.24E-16 |
| Phosphorylation of the APC/C | 1.87E-16 |
| Meiotic recombination | 1.87E-16 |
| RNA polymerase I promoter opening | 2.06E-16 |
| Notch-HLH transcription pathway | 2.33E-16 |
| Type I hemidesmosome assembly | 3.41E-16 |
| E2F mediated regulation of DNA replication | 3.41E-16 |
| Unwinding of DNA | 3.41E-16 |
| Telomere Maintenance | 4.00E-16 |
| Inactivation of APC/C via direct inhibition of the APC/C complex | 4.00E-16 |
| Inhibition of the proteolytic activity of APC/C required for the onset of anaphase by mitotic spindle checkpoint components | 4.00E-16 |
| G2/M checkpoints | 4.00E-16 |
| Mitotic spindle checkpoint | 4.66E-16 |
| DNA strand elongation | 4.66E-16 |
| Processive synthesis on the lagging strand | 4.66E-16 |
| E2F-enabled inhibition of pre-replication complex formation | 5.20E-16 |
| Telomere C-strand (lagging strand) synthesis | 5.80E-16 |
| Activation of the pre-replicative complex | 5.80E-16 |
Gene compositions of altered pathways
Pathways involve several genes, which work together to perform one biological process or to regulate certain biological functions. To additionally identify the functions and properties of altered pathways, the present study investigated their gene compositions at the gene expression level. The PLK-mediated events pathway was comprised of 13 genes (centromere protein F, CENPF; E1A binding protein p300, EP300; forkhead box protein M1, FOXM1; MYB proto-oncogene like 2, MYBL2; polo-like kinase 1, PLK1; lin-37 DREAM MuvB complex component, LIN37; RB binding protein 4, chromatin remodeling factor, RBBP4; WEE 1 G2 checkpoint kinase, WEE1; cyclin B1, CCNB1; protein kinase, membrane associated tyrosine/threonine 1, PKMYT1; cyclin B2, CCNB2; cell division cycle 25A, CDC25A and cell division cycle 25C CDC25C), and Fig. 3 illustrates expression patterns of genes in this pathway across normal and breast cancer samples. The present study identified that the gene-level statistic in tumors was disturbed relative to that in normal samples, and in normal samples the gene-levels for the 13 genes were similar in general. Therefore, it may be inferred that different gene-levels lead to the production of altered pathways in breast cancer compared with nRef.
Figure 3.
Gene level statistics in Polo-like kinase mediated events. Each line represents a sample. Blue, tumor; red, normal.
Comparison with pathways based on DEGs
The present study identified a total of 324 DEGs between breast cancer and normal controls with thresholds of P<0.01 and log fold change of >2. Taking the intersection with 5,182 genes contained in 1,013 pathways, only 155 common genes were detected.
Results of the KEGG pathway enrichment analysis showed that 324 DEGs were enriched in 9 significant pathways under the condition of P<0.01 (Table II). The most significant pathways were focal adhesion (P=4.01E-05), ECM-receptor interaction (P=4.73E-05) and cytokine-cytokine receptor interaction (P=2.90E-04). When performing KEGG enrichment analysis for common genes, notably, the 155 common genes also enriched in the same 9 pathways, however the properties were different, such as P-value, count and enriched genes. The most significant term of common genes was the peroxisome proliferator-activated receptor signaling pathway with P=3.44E-04. In addition, cytokine-cytokine receptor interactions had the largest count of 18, whilst the next was pathways in cancer with a count of 14. Although the DEGs are not entirely included by 5,182 pathway genes, the 9 KEGG pathways were all involved in 688 altered pathways, which indicates that the present method was used to identify altered pathways.
Table II.
Kyoto encyclopedia of genes and genomes pathways with P<0.01 based on DEGs and common genes.
| P-value | Count | |||
|---|---|---|---|---|
| Pathway | DEGs | Common | DEGs | Common |
| Focal adhesion | 4.01E-05 | 5.39E-03 | 17 | 11 |
| Extracellular matrix-receptor interaction | 4.37E-05 | 5.59E-03 | 11 | 7 |
| Cytokine-cytokine receptor interaction | 2.90E-04 | 1.54E-03 | 18 | 14 |
| Peroxisome proliferator-activated receptor signaling pathway | 1.68E-03 | 3.44E-04 | 8 | 8 |
| Pathways in cancer | 3.53E-03 | 9.85E-03 | 18 | 14 |
| Adipocytokine signaling pathway | 6.68E-03 | 9.41E-03 | 7 | 6 |
| Aldosterone-regulated sodium reabsorption | 7.39E-03 | 7.88E-03 | 5 | 5 |
| Chemokine signaling pathway | 8.71E-03 | 9.51E-03 | 11 | 10 |
| Oocyte meiosis | 9.05E-03 | 5.31E-03 | 8 | 8 |
DEGS, differentially expressed genes.
Validation of altered pathways based on changed percent
The present study calculated percentage of changed pathways among 42 breast cancer samples, and listed the 47 pathways with changes in >80% tumor samples (Table III). The 47 pathways were part of 688 altered pathways. Kinesins (KIFs) and PLK mediated events were changed in 39 individuals (92.86%), the next were chromosome maintenance, meiotic recombination, deposition of new centromere protein A-containing nucleosomes at the centromere, nucleosome assembly and G2/M (DNA damage) DNA replication checkpoint changed in 38 individuals (90.48%). If the changed percentage was equal to 50%, a total of 310 terms were obtained, which are also involved in 688 altered pathways. This may contribute to validation of the present results.
Table III.
Altered pathways with a percentage change >80%.
| Altered pathway | Amount | Percent (%) |
|---|---|---|
| Kinesins | 39 | 92.86 |
| Polo-like kinase mediated events | 39 | 92.86 |
| Chromosome maintenance | 38 | 90.48 |
| Meiotic recombination | 38 | 90.48 |
| Deposition of new CENPA-containing nucleosomes at the centromere | 38 | 90.48 |
| Nucleosome assembly | 38 | 90.48 |
| G2/M DNA replication checkpoint | 38 | 90.48 |
| Telomere maintenance | 37 | 88.10 |
| Golgi cisternae pericentriolar stack reorganization | 37 | 88.10 |
| Nuclear factor-kB activation through Fas-associated death domain and receptor interacting protein 1 pathway mediated by caspase-8 and −10 | 37 | 88.10 |
| Phosphorylation of proteins involved in G1/S transition by active Cyclin E/Cdk2 complexes | 37 | 88.10 |
| Meiosis | 36 | 85.71 |
| RNA polymerase I promoter clearance | 36 | 85.71 |
| Amyloids | 36 | 85.71 |
| Packaging of telomere ends | 36 | 85.71 |
| RNA polymerase I promoter opening | 36 | 85.71 |
| Cyclin A/B1 associated events during G2/M transition | 36 | 85.71 |
| Leading strand synthesis | 36 | 85.71 |
| Polymerase switching | 36 | 85.71 |
| Polymerase switching on the C-strand of the telomere | 36 | 85.71 |
| Phosphorylation of emi1 | 36 | 85.71 |
| Meiotic synapsis | 35 | 83.33 |
| RNA polymerase I chain elongation | 35 | 83.33 |
| G2/M checkpoints | 35 | 83.33 |
| Activation of ATR in response to replication stress | 35 | 83.33 |
| DNA strand elongation | 35 | 83.33 |
| Activation of the pre-replicative complex | 35 | 83.33 |
| Extension of telomeres | 35 | 83.33 |
| Telomere C-strand (lagging strand) synthesis | 35 | 83.33 |
| Resolution of AP sites via the multiple-nucleotide patch replacement pathway | 35 | 83.33 |
| Zinc transporters | 35 | 83.33 |
| Repair synthesis for gap-filling by DNA polymerase in TC-NER | 35 | 83.33 |
| E2F-enabled inhibition of pre-replication complex formation | 35 | 83.33 |
| ER quality control compartment | 35 | 83.33 |
| Cyclin B2 mediated events | 35 | 83.33 |
| Synthesis of DNA | 34 | 80.95 |
| RNA polymerase I transcription | 34 | 80.95 |
| Activation of APC/C and APC/CCdc20 mediated degradation of mitotic proteins | 34 | 80.95 |
| DNA damage bypass | 34 | 80.95 |
| Translesion synthesis by Y family DNA polymerases bypasses lesions on DNA template | 34 | 80.95 |
| E2F mediated regulation of DNA replication | 34 | 80.95 |
| G0 and early G1 | 34 | 80.95 |
| Synthesis and interconversion of nucleotide di- and triphosphates | 34 | 80.95 |
| G1/S-specific transcription | 34 | 80.95 |
| Phosphorylation of the APC/C | 34 | 80.95 |
| Removal of the flap intermediate | 34 | 80.95 |
| Chk1/Chk2(Cds1) mediated inactivation of cyclin B/Cdk1 complex | 34 | 80.95 |
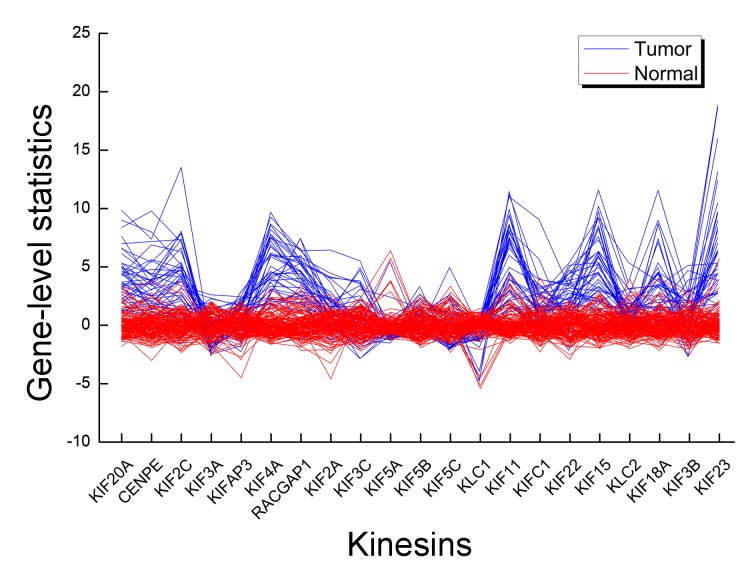
The gene composition of PLK mediated events had been analyzed (Fig. 3), and CCNB1 was the common gene. Its gene-level statistic value across different individual had clear differences. In KIFs, there were 21 genes, KIF20A, centromere protein E (CENPE), KIF2C, KIF3A, KIFAP3, KIF4A, rac GTPase activating protein 1 (RACGAP1), KIF2A, KIF3C, kinesin light chain 1 (KLC1), KIF5A, kinesin light chain 2 (KLC2), KIF5B, KIF5C, KIF11, KIFC1, KIF22, KIF15, KIF18A, KIF3B and KIF23, of which KIF20A and KIF11 were common genes. The standardized gene expression pattern for this pathway differed between tumor and normal (Fig. 4). A number of the genes deviated from the mean of the nRef and expression pattern of a gene varied markedly between tumor and normal samples. For an individual, different genes had their own gene-level statistics.
Figure 4.
Gene level statistics in kinesins. Each line represents a sample. Blue, tumor; red, normal.
Discussion
The present study identified altered pathways in breast cancer using a new method (iPAS method combined with nRef), and validated its feasibility based on the percentage of changed pathways in breast cancer samples and comparison with KEGG pathways. KEGG pathways and pathways with a changed percentage of >50% were parts of the altered pathways, which indicated that the iPAS method to identify altered pathways in breast cancer was feasible. The present results indicated that a total of 688 altered pathways were identified, such as KIF- and PLK-mediated events.
KIFs are a superfamily of microtubule-based motor proteins that exhibit diverse functions in the intracellular transportation of vesicles, organelles and chromosomes, the regulation of microtubule dynamics (21), and of molecular motors engaged in key cellular functions including cell division, mitosis and migration (22,23). Previously, additional mitotic KIFs have been validated as drug targets for cancer drug development particularly for breast cancer, raising the possibility that the range of KIF-based drug targets may expand in the future (24). De et al (25) demonstrated that the over expression of KIF family member C3 (KIFC3), KIFC1, KIF1A, or KIF5A to microtubules opposed the stabilizing effect of docetaxel that prevented cytokinesis and led to apoptosis. Similarly, the over expression of KIFC3, KIF5A, and KIF12 were specific in mediating resistance to docetaxel and not vincristine or doxorubicin. This overexpression of KIFC3, KIF5A and KIF12 correlated with specific taxane resistance in basal-like breast cancer; this ability was eliminated by a mutation of the adenosine triphosphate (ATP)-binding domain of a KIF (26). It had been identified that ANCCA (ATPase family, AAA nuclear coregulator cancer associated) is a key mediator of KIF family deregulation in breast cancer and the crucial role of multiple KIFs in growth and survival of the tumor cells (27). Guerrero-Preston et al (28) suggested that differential promoter methylation of KIF1A in plasma was associated with breast cancer and DNA repair capacity. A negative correlation was identified between KIF2A (KIF family member 2A) expression levels in breast cancer and the survival time of patients with breast cancer (29).
To additionally investigate functions of altered pathways, gene composition based on gene-level statistics were studied. A number of genes expressed deviated from the mean of the nRef, and these fluctuations may cause an alteration between breast cancer and normal controls. Among members of KIFs, KIF20A (KIF family member 20A) and KIF11 (KIS family member 11) were common genes between DEGs and pathway genes. For breast cancer patients, FOXM1 regulated KIF20A expression to modulate mitotic catastrophe caused by interferences in paclitaxel-mediated cell death and senescence (30). KIF11 represented an attractive anticancer target, and the inhibition of KIF11 caused mitotic arrest and apoptosis of multiple cancers, for example, breast cancer (31). Therefore, the KIF members and KIFs pathway had significant effects on breast cancer.
In the PLK mediated events pathway, PLK served a dominant role. PLK family members are known to be functionally involved in mitotic signaling, and in cytoskeletal reorganization in normal and malignant cells (32,33). PLKs are also a family of conserved serine/threonine kinases involved in the regulation of cell cycle progression and in the activation of cyclin-dependent kinase/cyclin complexes during the M-phase of the cell cycle through G2 and mitosis (34). Previous studies reported that PLK1 was a potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer, and was overexpressed in tumors, indicating its involvement in carcinogenesis (35–37). The use of different PLK1 inhibitors has increased knowledge of mitotic regulation and allowed the present study to assess their ability to suppress tumor growth in vivo (38). The PLK2 and PLK3 acted in concert with cyclin-dependent kinase 1-cyclin B1 and aurora kinases to orchestrate a wide range of critical cell cycle events (39). As for other members of PLKs, there was evidence showing that PLK2 and PLK3 acted as tumor suppressors through their functions in the p53 signaling network, which guarded the cell against various stress signals (40,41). It has been identified that there is a significant association between elevated PLK1 and p53 mutation in women with breast cancer (42,43). It has also been verified that PLK3 is a novel independent prognostic marker in breast cancer, which alluded toward a role for PLK overexpression in disease progression (44). Therefore, it could be inferred that PLK mediated events correlate closely with breast cancer.
In conclusion, the iPAS method was suitable for identifying altered pathways (such as KIF- and PLK-mediated events), which may serve an important role in breast cancer progression and are potentially novel predictive and prognostic markers for breast cancer.
References
- 1.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 2.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis C, Siegel R, Bandi P, Jemal A. Breast cancer statistics, 2011. CA Cancer J Clin. 2011;61:408–418. doi: 10.3322/caac.20134. [DOI] [PubMed] [Google Scholar]
- 4.DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013;14:500–515. doi: 10.1016/S1470-2045(13)70076-7. [DOI] [PubMed] [Google Scholar]
- 5.Charif M, Lower EE, Kennedy D, Kumar H, Khan S, Radhakrishnan N, Zhang X. Abstract P3-05-16: The effect of HER-2/neu inhibition on prolonging clinical benefit with fulvestrant treatment for metastatic estrogen receptor positive breast cancer patients treated with trastuzumab. Cancer Res. 2015;75 doi: 10.1158/1538-7445.SABCS14-P3-05-16. [DOI] [Google Scholar]
- 6.Mittendorf EA, Clifton GT, Holmes JP, Clive KS, Patil R, Benavides LC, Gates JD, Sears AK, Stojadinovic A, Ponniah S, Peoples GE. Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: From US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer. 2012;118:2594–2602. doi: 10.1002/cncr.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holder AM, Gonzalez-Angulo AM, Chen H, Akcakanat A, Do KA, Symmans W Fraser, Pusztai L, Hortobagyi GN, Mills GB, Meric-Bernstam F. Increased stearoyl-CoA desaturase 1 expression is associated with shorter survival in breast cancer patients. Cancer Res. 2013;137:319–327. doi: 10.1007/s10549-012-2354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glazko GV, Emmert-Streib F. Unite and conquer: Univariate and multivariate approaches for finding differentially expressed gene sets. Bioinformatics. 2009;25:2348–2354. doi: 10.1093/bioinformatics/btp406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khatri P, Sirota M, Butte AJ. Ten years of pathway analysis: Current approaches and outstanding challenges. PLoS Comput Biol. 2012;8:e1002375. doi: 10.1371/journal.pcbi.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahn T, Lee E, Huh N, Park T. Personalized identification of altered pathways in cancer using accumulated normal tissue data. Bioinformatics. 2014;30:i422–i429. doi: 10.1093/bioinformatics/btu449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen DT, Nasir A, Culhane A, Venkataramu C, Fulp W, Rubio R, Wang T, Agrawal D, McCarthy SM, Gruidl M, et al. Proliferative genes dominate malignancy-risk gene signature in histologically-normal breast tissue. Breast Cancer Res Treat. 2010;119:335–346. doi: 10.1007/s10549-009-0344-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Croft D, O'Kelly G, Wu G, Haw R, Gillespie M, Matthews L, Caudy M, Garapati P, Gopinath G, Jassal B, et al. Reactome: A database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39(Database issue):D691–D697. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- 15.Bolstad B. Affy: Built-in Processing Methods. 2013 [Google Scholar]
- 16.Gehan EA. A Generalized Wilcoxon test for comparing arbitrarily singly-censored samples. Biometrika. 1965;52:203–223. doi: 10.2307/2333721. [DOI] [PubMed] [Google Scholar]
- 17.Mohammadi A, Saraee MH, Salehi M. Identification of disease-causing genes using microarray data mining and Gene Ontology. BMC Med Genomics. 2011;4:12. doi: 10.1186/1755-8794-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 19.da W Huang, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2008;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Simon R. Microarray-based cancer prediction using single genes. BMC Bioinformatics. 2011;12:391. doi: 10.1186/1471-2105-12-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer. 2012;12:527–539. doi: 10.1038/nrc3310. [DOI] [PubMed] [Google Scholar]
- 22.Hirokawa N. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10:682–696. doi: 10.1038/nrm2774. [DOI] [PubMed] [Google Scholar]
- 23.Sharp DJ, Rogers GC, Scholey JM. Microtubule motors in mitosis. Nature. 2000;407:41–47. doi: 10.1038/35024000. [DOI] [PubMed] [Google Scholar]
- 24.Rath O, Kozielski F. Kinesins and cancer. Nat Rev Cancer. 2012;12:527–539. doi: 10.1038/nrc3310. [DOI] [PubMed] [Google Scholar]
- 25.De S, Cipriano R, Jackson MW, Stark GR. Overexpression of kinesins mediates docetaxel resistance in breast cancer cells. Cancer Res. 2009;69:8035–8042. doi: 10.1158/0008-5472.CAN-09-1224. [DOI] [PubMed] [Google Scholar]
- 26.Tan MH, De S, Bebek G, Orloff MS, Wesolowski R, Downs-Kelly E, Budd GT, Stark GR, Eng C. Specific kinesin expression profiles associated with taxane resistance in basal-like breast cancer. Breast Cancer Res Treat. 2012;131:849–858. doi: 10.1007/s10549-011-1500-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou JX, Duan Z, Wang J, Sokolov A, Xu J, Chen CZ, Li JJ, Chen HW. Kinesin family deregulation coordinated by bromodomain protein ANCCA and histone methyltransferase MLL for breast cancer cell growth, survival, and tamoxifen resistance. Mol Cancer Res. 2014;12:539–549. doi: 10.1158/1541-7786.MCR-13-0459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guerrero-Preston R, Hadar T, Ostrow KL, Soudry E, Echenique M, Ili-Gangas C, Pérez G, Perez J, Brebi-Mieville P, Deschamps J, et al. Differential promoter methylation of kinesin family member 1a in plasma is associated with breast cancer and DNA repair capacity. Oncol Rep. 2014;32:505–512. doi: 10.3892/or.2014.3262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Ma S, Ma R, Qu X, Liu W, Lv C, Zhao S, Gong Y. KIF2A silencing inhibits the proliferation and migration of breast cancer cells and correlates with unfavorable prognosis in breast cancer. BMC Cancer. 2014;14:461. doi: 10.1186/1471-2407-14-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khongkow P, Gomes A, Gong C, Man EP, Tsang JW, Zhao F, Monteiro LJ, Coombes RC, Medema RH, Khoo US, Lam EW. Paclitaxel targets FOXM1 to regulate KIF20A in mitotic catastrophe and breast cancer paclitaxel resistance. Oncogene. 2016;35:990–1002. doi: 10.1038/onc.2015.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valensin S, Ghiron C, Lamanna C, Kremer A, Rossi M, Ferruzzi P, Nievo M, Bakker A. KIF11 inhibition for glioblastoma treatment: Reason to hope or a struggle with the brain? BMC Cancer. 2009;9:196. doi: 10.1186/1471-2407-9-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sumara I, Vorlaufer E, Stukenberg PT, Kelm O, Redemann N, Nigg EA, Peters JM. The dissociation of cohesin from chromosomes in prophase is regulated by Polo-like kinase. Mol Cell. 2002;9:515–525. doi: 10.1016/S1097-2765(02)00473-2. [DOI] [PubMed] [Google Scholar]
- 33.Ha G, Kim H, Go H, Lee H, Seimiya H, Chung DH, Lee CW. Tankyrase-1 function at telomeres and during mitosis is regulated by Polo-like kinase-1-mediated phosphorylation. Cell Death Differ. 2012;19:321–332. doi: 10.1038/cdd.2011.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Schubert C, Nigg EA. Polo-like kinases. Current Biol. 2013;23:R225–R227. doi: 10.1016/j.cub.2013.01.066. [DOI] [PubMed] [Google Scholar]
- 35.Spänkuch-Schmitt B, Bereiter-Hahn J, Kaufmann M, Strebhardt K. Effect of RNA silencing of polo-like kinase-1 (PLK1) on apoptosis and spindle formation in human cancer cells. J Natl Cancer Inst. 2002;94:1863–1877. doi: 10.1093/jnci/94.24.1863. [DOI] [PubMed] [Google Scholar]
- 36.Maire V, Némati F, Richardson M, Vincent-Salomon A, Tesson B, Rigaill G, Gravier E, Marty-Prouvost B, De Koning L, Lang G, et al. Polo-like kinase 1: A potential therapeutic option in combination with conventional chemotherapy for the management of patients with triple-negative breast cancer. Cancer Res. 2013;3:813–823. doi: 10.1158/0008-5472.CAN-12-2633. [DOI] [PubMed] [Google Scholar]
- 37.Wierer M, Verde G, Pisano P, Molina H, Font-Mateu J, Di Croce L, Beato M. PLK1 signaling in breast cancer cells cooperates with estrogen receptor-dependent gene transcription. Cell Rep. 2013;27:2021–2032. doi: 10.1016/j.celrep.2013.05.024. [DOI] [PubMed] [Google Scholar]
- 38.Strebhardt K, Ullrich A. Targeting polo-like kinase 1 for cancer therapy. Nat Rev Cancer. 2006;6:321–330. doi: 10.1038/nrc1841. [DOI] [PubMed] [Google Scholar]
- 39.Strebhardt K. Multifaceted polo-like kinases: Drug targets and antitargets for cancer therapy. Nat Rev Drug Discov. 2010;9:643–660. doi: 10.1038/nrd3184. [DOI] [PubMed] [Google Scholar]
- 40.Salvi M, Trashi E, Cozza G, Negro A, Hanson PI, Pinna LA. Tools to discriminate between targets of CK2 vs PLK2/PLK3 acidophilic kinases. Biotechniques. 2012;53 doi: 10.2144/000113866. [DOI] [PubMed] [Google Scholar]
- 41.Salvi M, Trashi E, Cozza G, Franchin C, Arrigoni G, Pinna L. Investigation on PLK2 and PLK3 substrate recognition. Biochim Biophys Acta. 2012;1824:1366–1373. doi: 10.1016/j.bbapap.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 42.King SI, Purdie CA, Bray SE, Quinlan PR, Jordan LB, Thompson AM, Meek DW. Immunohistochemical detection of Polo-like kinase-1 (PLK1) in primary breast cancer is associated with TP53 mutation and poor clinical outcom. Breast Cancer Res. 2012;14:R40. doi: 10.1186/bcr3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iyer RS, Nicol SM, Quinlan PR, Thompson AM, Meek DW, Fuller-Pace FV. The RNA helicase/transcriptional co-regulator, p68 (DDX5), stimulates expression of oncogenic protein kinase, Polo-like kinase-1 (PLK1), and is associated with elevated PLK1 levels in human breast cancers. Cell Cycle. 2014;13:1413–1423. doi: 10.4161/cc.28415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weichert W, Kristiansen G, Winzer KJ, Schmidt M, Gekeler V, Noske A, Müller BM, Niesporek S, Dietel M, Denkert C. Polo-like kinase isoforms in breast cancer: Expression patterns and prognostic implications. Virchows Archiv. 2005;446:442–450. doi: 10.1007/s00428-005-1212-8. [DOI] [PubMed] [Google Scholar]