Abstract
Colon cancer is one of the most common cancers in the world. Multidrug resistance is one of the main reasons for failure of therapy in patients with advanced colon cancer. In previous studies, multiple methods were investigated to reverse the multidrug resistance of colon cancer cells. However, to date, no clinical method has been identified to be satisfactory. Therefore, successful reversal of drug resistance in colon cancer cells still requires new therapeutic strategies or pharmaceuticals. Wild-type p53-induced phosphatase (Wip1), a member of the 2C type serine/threonine protein phosphatase family, is closely associated with the p53 gene, which is the most important tumor-suppressor gene. Wip1 was reported to be associated with the chemosensitivity of breast cancer cells. However, the correlation between the expression of Wip1 gene and the chemosensitivity of colon cancer cells has not been reported yet. In the present study, Wip1-811 small interfering RNA (siRNA) targeting Wip1 was investigated to reverse the multidrug resistance of colon cancer cells. The siRNA duplexes were transfected into RKO colon cancer cells. The messenger RNA (mRNA) expression of Wip1 was measured by reverse transcription-quantitative polymerase chain reaction. The protein level of Wip1 was detected by western blotting. The cell viability was measured by MTS assay. The cell apoptosis and cell cycle were analyzed by flow cytometry. Intracellular adriamycin cumulative concentration was determined using flow cytometry. Wip1-811 siRNA efficiently inhibited the expression of Wip1 at the mRNA and protein levels, and enhanced the sensitivity of RKO colon cancer cells towards chemotherapy, which was accompanied by increased cell apoptosis, following the inhibition of Wip1 gene expression. These results indicate that Wip1 gene silencing could enhance the chemosensitivity of colon cancer cells, which may provide a new potential approach for the reversal of multidrug resistance in colon cancer cells.
Keywords: colon cancer, small interfering RNA, Wip1 gene, chemosensitivity
Introduction
Colon cancer is one of the most common malignant gastrointestinal tumors. The morbidity and mortality of colon cancer in the world as well as in China are gradually increasing (1). The majority of patients with colon cancer are in advanced stage by the time of diagnosis (2); thus, surgery is rarely a sufficient treatment. Consequently, chemotherapy is markedly important in the treatment of colon cancer. However, chemotherapy's effectiveness is limited because colon cancer cells acquire multiple drug resistance (MDR) (3).
To improve the effect of chemotherapy in the treatment of colon cancer, one of the major challenges is reversing MDR in colon cancer cells. In previous studies, calcium antagonists such as verapamil (4) and small interfering RNA (siRNA) targeting multidrug resistance protein 1 (MDR1) messenger RNA (mRNA) were observed to modulate MDR1/P-glycoprotein (P-gp)-dependent MDR by downregulating the expression of MDR1 mRNA and P-gp (5). However, calcium antagonists may cause heart failure and hypotension (6), and the clinical benefit of siRNA targeting MDR1 mRNA has not been reported thus far. Therefore, successful reversal of drug resistance still requires new therapeutic strategies or pharmaceuticals.
Wild-type p53-induced phosphatase (Wip1) is a member of the 2C type serine/threonine protein phosphatase family (7). Wip1 is closely associated with the p53 gene and it can dephosphorylate downstream proteins of the p53 gene, thus causing the degradation of p53 protein and the inhibition of DNA repair and cell apoptosis mediated by p53 (8–10). Thereby, Wip1 promotes tumorigenesis (11). The Wip1 gene has been reported to be overexpressed in a variety of tumors (12,13). Previous studies reported an association between Wip1 and sensitivity towards chemotherapy. For example, self-proliferation of cancer stem cells was inhibited by downregulation of Wip1, which improved the chemosensitivity of breast cancer cells (14). However, the correlation between the expression of the Wip1 gene and the chemosensitivity of colon cancer cells has not been reported yet.
In the present study, RNA interference (RNAi) was used to observe the inhibition of the Wip1 gene induced by a specific siRNA sequence and to investigate the influence of Wip1 gene silencing on the chemosensitivity of colon cancer cells. Therefore, a new potential method may have been identified to reverse the drug resistance of colon cancer cells.
Materials and methods
Cell culture
RKO and COLO 320DM human colon adenocarcinoma cell lines were purchased from Shanghai Cell Bank of Chinese Academy of Sciences (Shanghai, China) and were cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS) (Biological Industries Israel Beit-Haemek, Kibbutz Beit-Haemek, Israel), 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified incubator containing 5% CO2.
Western blotting
Cells were washed twice with PBS and then lysed with 100 µl lysis buffer (91 µl radioimmunoprecipitation assay buffer + 9 µl 10X protease cocktail; Beijing Kangwei Century Biotechnology Co., Ltd., Beijing, China) on ice for 30 min. Cell lysates were centrifuged at 14,000 × g for 20 min at 4°C. The total cellular protein content was determined with BCA™ Protein Assay kit (Beijing Kangwei Century Biotechnology Co., Ltd, Beijing, China). Subsequently, cellular proteins (20 µg) were dissolved in sample loading buffer (Beijing Kangwei Century Biotechnology Co., Ltd.) and subjected to 10% SDS-PAGE. Proteins were electrotransferred to polyvinylidene fluoride membranes (200 mA, 90 min). The membranes were rinsed with PBS and blocked with 5% nonfat milk in PBS for 90 min at room temperature. The membranes were then incubated with primary polyclonal anti-Wip1 (1:1,000; GeneTex, Inc., Irvine, CA, USA) or monoclonal anti-GAPDH antibodies (cat. no., MB001H; 1:1,000; Bioworld Technology, Inc., St. Louis Park, MN, USA) in 5% nonfat milk overnight at 4°C. Following primary antibody incubation, the membranes were rinsed in TBS containing Tween-20 (TBS-T) wash buffer four times (10 min each). The membranes were then incubated with a secondary antibody (cat. no., E030120-02; horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G; 1:5,000; EarthOx Life Sciences, Millbrae, CA, USA) for 1 h at room temperature and rinsed in TBS-T wash buffer four times (5 min each). The protein-antibody complexes were visualized by chemiluminescence (ECL Plus Western Blotting Substrate; Thermo Fisher Scientific, Inc.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using RNAiso Plus (Takara Biotechnology Co., Ltd., Dalian, China) according to the manufacturer's protocol. Single stranded complementary DNA (cDNA) was synthesized from 1 µg RNA using cDNA Synthesis kit (Takara Biotechnology Co., Ltd.). The newly synthesized cDNA was amplified by PCR using an iCycler iQ™ Real-Time PCR Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The reaction mixture contained 2 µl of cDNA template, 0.4 µl of 10 µM Wip1 sense primer (5′-CAGGGAAACTTTACCAATGAA-3′), 0.4 µl of 10 µM Wip1 antisense primer (5′-ACGAACCAGGGCAGGTATAT-3′), 10 µl of SYBR Premix Ex Taq™ (Takara Bio Inc., Otsu, Japan) and 7.2 µl of distilled H2O. The final reaction volume was 20 µl. Both sense (5′-CCTGGATACCGCAGCTAGGA-3′) and antisense (5′-GCGGCGCAATACGAATGCCCC-3′) 18S ribosomal RNA (rRNA) primers were used as internal controls. The cycling conditions were as follows: 95°C for 30 sec to denature the cDNA and primers, followed by 40 cycles at 95°C for 5 sec and 60°C for 20 sec. Dissociation curve analysis (15) was performed according to the following conditions: 95°C for 0 sec, 60°C for 15 sec and 95°C for 0 sec. The amplification products of Wip1 and 18S rRNA were 180 and 112 bp in size, respectively. The relative expression of Wip1 mRNA was calculated by comparing it with the mRNA expression of the negative control, which was set as 1.
siRNA synthesis and transfection
The siRNA sequences targeting Wip1 (GenBank accession no. NM008493) were designed by Suzhou Jima Gene Co. Ltd. (Suzhou, China). Wip1 siRNA duplexes (Wip1-811 siRNA), positive control (GAPDH) siRNA and negative control siRNA were synthesized by Suzhou Jima Gene Co. Ltd. For Wip1-811 siRNA, the sense strand was 5′-GGGUGGUUCUUGGAAUUCATT-3′ and the antisense strand was 5′-UGAAUUCCAAGAACCACCCTT-3′. Cells treated with Lipofectamine™ 2000 (Thermo Fisher Scientific, Inc.) and Opti-MEM® I Reduced Serum Medium (Thermo Fisher Scientific, Inc.) were set as the liposome control. The siRNA duplexes were transfected using Lipofectamine™ 2000 according to the protocol recommended by the manufacturer. Serial concentrations of Wip1 siRNA, including 5, 10, 25, 50 and 100 nmol/l, were used to screen the optimal concentration of Wip1 siRNA. A series of transfection times, including 24, 48, 72 and 96 h, were used to screen the optimal transfection time.
MTS assay
Cell viability assays were performed by MTS assay. Cells were seeded at 1×104 cells/well in 96-well microtiter plates. After 24 h of incubation, Wip1-811 siRNA transfection was performed. A series of diluted concentrations of anti-tumor drugs, including 5-fluorouracil (5-FU; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), oxaliplatin (Sigma-Aldrich; Merck KGaA) and adriamycin (Sigma-Aldrich; Merck KGaA), were added to the cells 24 h after transfection. Then, the cells were incubated for 3 days at 37°C in 5% CO2. Subsequently, 20 µl of MTS solution (Promega Corporation, Madison, WI, USA) was added to the cells and incubated for 3 h at 37°C, followed by agitation for 5 min. Optical density (OD) values were read on a Synergy HT Multi-Detection Microplate Reader (Bio-Tek Instruments, Inc., Winooski, VT, USA) at a wavelength of 570 nm. Cell viability was calculated by comparison with the OD value of the control, and the half-maximal inhibitory concentration (IC50) of the anti-tumor drug in each group was further calculated.
Analysis of cell apoptosis and cell cycle
RKO colon cancer cells were plated at 5×105 cells/well in 12-well plates in RPMI-1640 medium with 10% FBS. After 24 h of incubation, Wip1-811 siRNA transfection was performed. 5-FU or oxalipatin were added 24 h after transfection to a final concentration of 5 µmol/l. Then, cells were incubated at 37°C and 5% CO2 for 48 h. Subsequently, the cells were harvested and washed twice with cold PBS, and then divided into two tubes. One tube of cells was used for analysis of cell apoptosis, in which 1×106 cells/ml of cell suspension was obtained by adding 5 µl of Annexin V-FITC (BD Biosciences, San Jose, CA, USA) and 200 µl of 1X binding buffer (BD Biosciences). Propidium iodide (10 µl) (Thermo Fisher Scientific, Inc.) was next added and allowed to react in the dark for 30 min. Next, the cell suspension was subjected to analysis of cell apoptosis. The other tube of cells was prepared for analysis of the cell cycle. Briefly, the cells were fixed with 2 ml of 70% (v/v) ethanol at 4°C, and then resuspended by adding 200 µl of PBS. The next day, the fixed cells were ready for analysis of the cell cycle. Flow cytometry analysis was performed with a FACSVerse™ flow cytometer (BD Biosciences). For each sample, 10,000 cells were analyzed.
Intracellular adriamycin accumulation assay
The fluorescence intensity of intracellular adriamycin was determined using flow cytometry according to a standard method (16). Cells were plated at 2×105 cells/well in 6-well plates 24 h prior to siRNA transient transfection, and then incubated for 48 h. Next, adriamycin (final concentration, 10 µmol/l) was added to the cells and incubated for 2 h. The cells were then harvested, washed twice with cold PBS and placed in ice-water to block the reaction until analysis. After 30 min, the fluorescence intensity of the cells was determined by FACSVerse™ flow cytometry at an excitation wavelength of 488 nm and a detection wavelength of 575 nm. COLO 320DM colon cancer cells transfected with MDR1 siRNA (#4123; Thermo Fisher Scientific, Inc.) were used as the positive control and compared with COLO 320DM colon cancer cells (5).
Statistical analysis
All measurement data were present as mean ± standard deviation (SD). The differences among multiple mean values were evaluated using one-way analysis of variance (ANOVA) and post-hoc Tukey's test. The differences between two mean values were estimated using independent-samples t-test. All of the statistical analyses were processed with the statistical analysis software SPSS, version 19.0 (IBM Corp, Armonk, NY, USA).
Results
Optimal inhibitory concentration of Wip1 siRNA on the mRNA and protein expression of Wip1 in RKO colon cancer cells
In order to investigate the inhibitory effect of siRNA on the mRNA and protein expression of Wip1, RKO colon cancer cells were seeded in a 24-well plate. Serial concentrations of Wip1-811 siRNA (range, 5–100 nmol/l) were transfected into RKO colon cancer cells. Cells cultured in medium without liposomes or siRNA treatment were used as controls. The liposome control was treated only with Lipofectamine® 2000. Negative controls were treated with a control siRNA containing no homology to any human gene. Cells of each group were harvested 24 or 48 h after transfection. RT-qPCR detected the expression of Wip1 mRNA. Western blotting examined the expression of Wip1 protein. Fig. 1A shows the influence of different concentrations of Wip1-811 siRNA on the relative mRNA expression of Wip1 in RKO colon cancer cells 24 h after siRNA transfection. No significant differences were observed in Wip1 mRNA levels between the control, liposome control and negative control groups 24 h after transfection. However, there was a significant difference between the Wip1 siRNA groups and the negative control group (P<0.05; Fig. 1A). This indicated that different concentrations of Wip1 siRNA decreased Wip1 mRNA expression 24 h after transfection. The expression of Wip1 mRNA was the lowest when the concentration of Wip1-811 siRNA was 50 nmol/l. The results from RT-qPCR at 48 h post-transfection were similar to those at 24 h post-transfection (Fig. 1B). No significant differences were observed in Wip1 protein expression between the control, liposome control and negative control groups 24 h after transfection (Fig. 2A). However, there was a significant difference between the Wip1 siRNA groups, except for 5 nmol/l siRNA, and the negative control group (P<0.05). This indicated that a Wip1 siRNA range of 10–100 nmol/l inhibits Wip1 protein expression 24 h after transfection. Wip1 protein expression was the lowest when the concentration of Wip1-811 siRNA was 50 nmol/l (Fig. 2A). The results from western blot at 48 h post-transfection were similar to those at 24 h post-transfection, except for 5 nmol/l siRNA (Fig. 2B). Therefore, the optimal concentration of Wip1-811 siRNA appeared to be 50 nmol/l based on the results from 24 and 48 h post-transfection.
Figure 1.
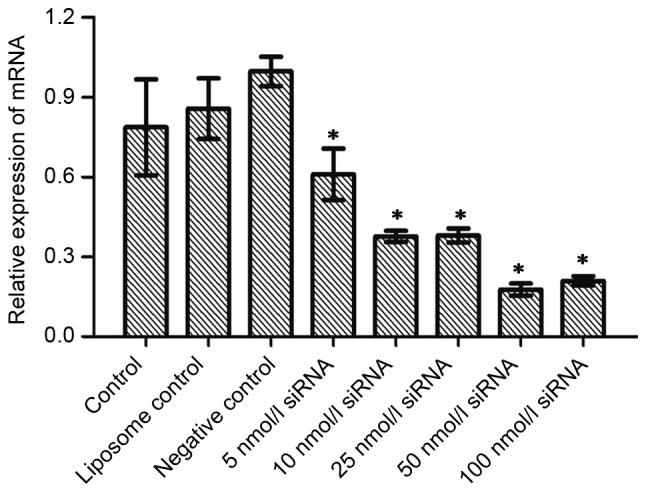
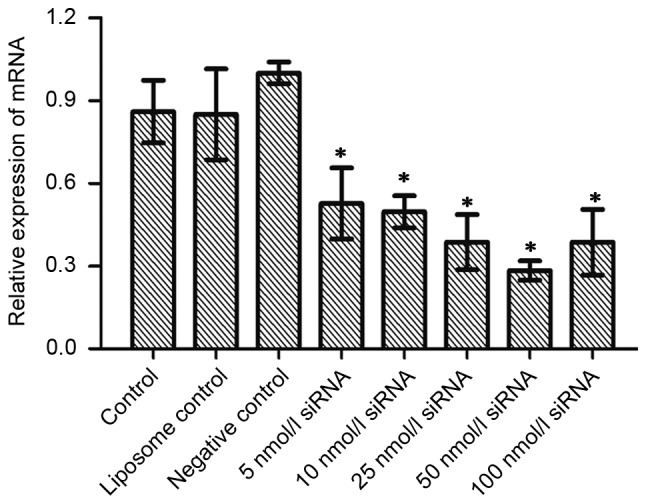
Influence of different concentrations of Wip1-811 siRNA on the relative mRNA expression of Wip1 in RKO colon cancer cells (A) 24 and (B) 48 h after transfection. *P<0.05 vs. negative control. Wip1, wild-type p53-induced phosphatase; siRNA, small interfering RNA; mRNA, messenger RNA.
Figure 2.
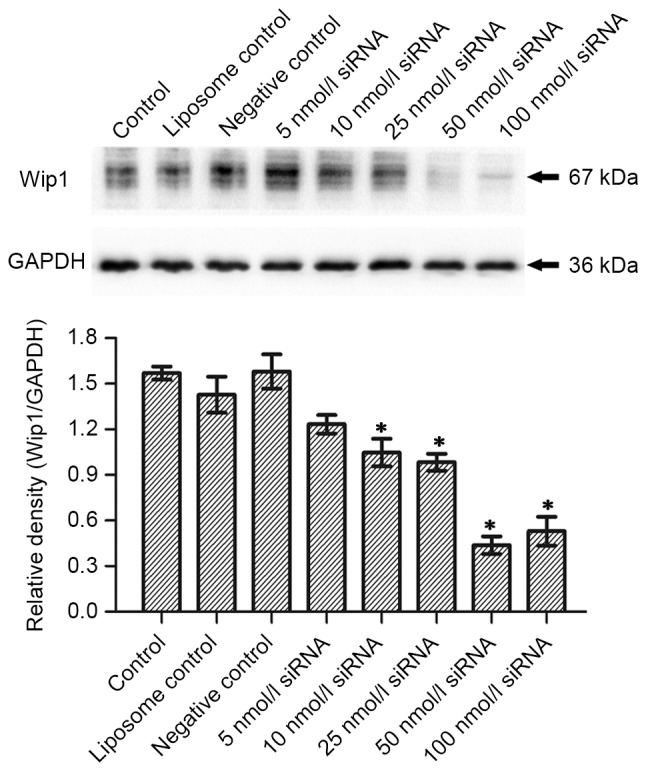
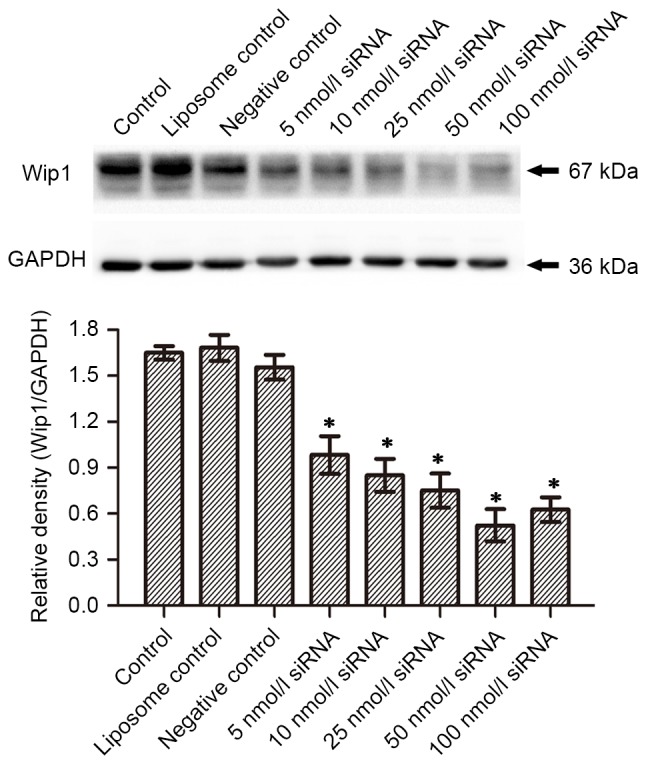
Influence of different concentrations of Wip1-811 siRNA on the protein expression of Wip1 in RKO colon cancer cells (A) 24 and (B) 48 h after transfection. *P<0.05 vs. negative control. Wip1, wild-type p53-induced phosphatase; siRNA, small interfering RNA.
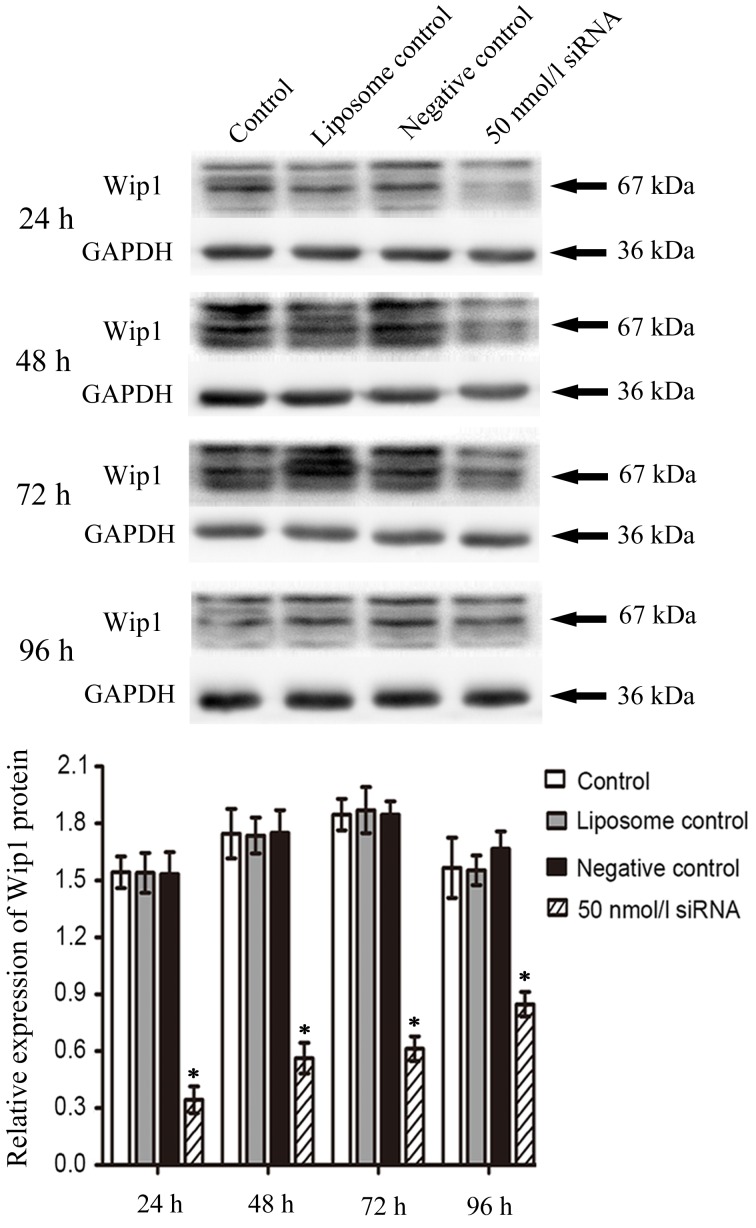
Duration of the inhibitory effect of Wip1 siRNA on the mRNA and protein expression of Wip1 in RKO colon cancer cells
In order to investigate the duration of the inhibition of Wip1 siRNA on the mRNA and protein expression of Wip1 following transient transfection, 50 nmol/l of Wip1-811 siRNA was transfected into RKO colon cancer cells. Cells were harvested at 24–96 h post-transfection. As shown by RT-qPCR in Fig. 3, the relative expression of Wip1 mRNA in RKO colon cancer cells in the Wip1 siRNA groups was significantly lower than that in negative control groups at any time point. In addition, the longer the time post-transfection, the lower the inhibitory effect of Wip1-811 siRNA. The western blot analysis was consistent with the results from RT-qPCR (Fig. 4). According to the above results, the optimal time of transfection of Wip1-811 siRNA was determined to be between 24 and 48 h.
Figure 3.
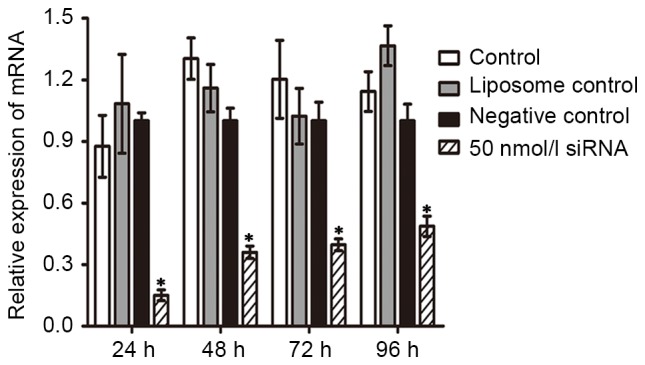
Influence of Wip1-811 siRNA on the relative mRNA expression of Wip1 in RKO colon cancer cells after transfection for 24–96 h. *P<0.05 vs. negative control at the same time point. Wip1, wild-type p53-induced phosphatase; siRNA, small interfering RNA; mRNA, messenger RNA.
Figure 4.
Influence of Wip1-811 siRNA on the protein expression of Wip1 in RKO colon cancer cells after transfection for 24–96 h. *P<0.05 vs. negative control at the same time point. Wip1, wild-type p53-induced phosphatase; siRNA, small interfering RNA.
Wip1 gene silencing enhances the chemosensitivity of RKO colon cancer cells
Wip1-811 siRNA (50 nmol/l) was transfected into RKO colon cancer cells, and 24 h after transfection, serial concentrations of 5-FU, oxaliplatin and adriamycin were added to treat RKO colon cancer cells for 48 h. According to the results of MTS assay, the cell viability of RKO colon cancer cells without chemotherapy treatment in the Wip1-811 siRNA group exhibited no significant difference compared with that of the control, liposome control and negative control groups. The cell viability of RKO colon cancer cells in these control groups and in the Wip1-811 siRNA group decreased along with increasing concentrations of the antitumor drugs (data not shown). The IC50 of the Wip1-811 siRNA group following treatment with 5-FU (25.32±2.59 µmol/l) was significantly decreased when compared with that of the negative control group (56.88±6.08 µmol/l). Similarly, the IC50 of the Wip1-811 siRNA group following treatment with oxaliplatin (18.74±2.21 µmol/l) was significantly lower than that of the negative control group (43.60±3.72 µmol/l). In addition, the IC50 of the Wip1-811 siRNA group following treatment with adriamycin (0.88±0.08 µmol/l) was significantly lower than that of the negative control group (2.13±0.20 µmol/l) (Table I).
Table I.
IC50 of 5-FU, oxaliplatin and adriamycin in different groups.
| IC50 (µmol/l, mean ± standard deviation) | |||
|---|---|---|---|
| Group | 5-FU | Oxaliplatin | Adriamycin |
| Control | 66.38±9.26 | 45.08±4.74 | 2.22±0.21 |
| Liposome control | 59.95±6.58 | 41.80±3.74 | 2.01±0.20 |
| Negative control | 56.88±6.08 | 43.60±3.72 | 2.13±0.20 |
| Wip1-811siRNA (50 nmol/l) | 25.32±2.59a | 18.74±2.21b | 0.88±0.08c |
P<0.05, 50 nmol/l siRNA vs. negative control.
P<0.05, 50 nmol/l siRNA vs. negative control.
P<0.05, 50 nmol/l siRNA vs. negative control. IC50, half-maximal inhibitory concentration 5-FU, 5-fluorouracil; siRNA, small interfering RNA.
The results presented in Table I demonstrate that Wip1-811 siRNA alone was not able to kill RKO colon cancer cells. However, a larger number of RKO colon cancer cells were killed when treated with Wip1-811 siRNA combined with 5-FU, oxaliplatin and adriamycin compared with those in the negative control group.
Downregulation of Wip1 gene expression increases cell apoptosis induced by chemotherapeutic drugs
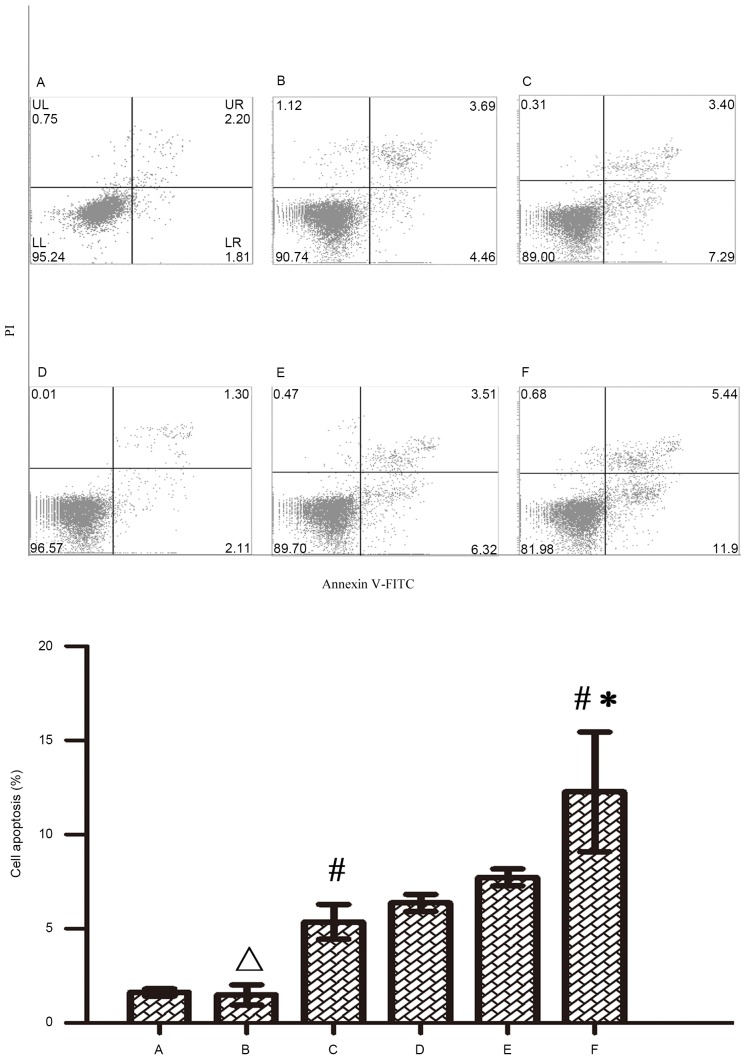
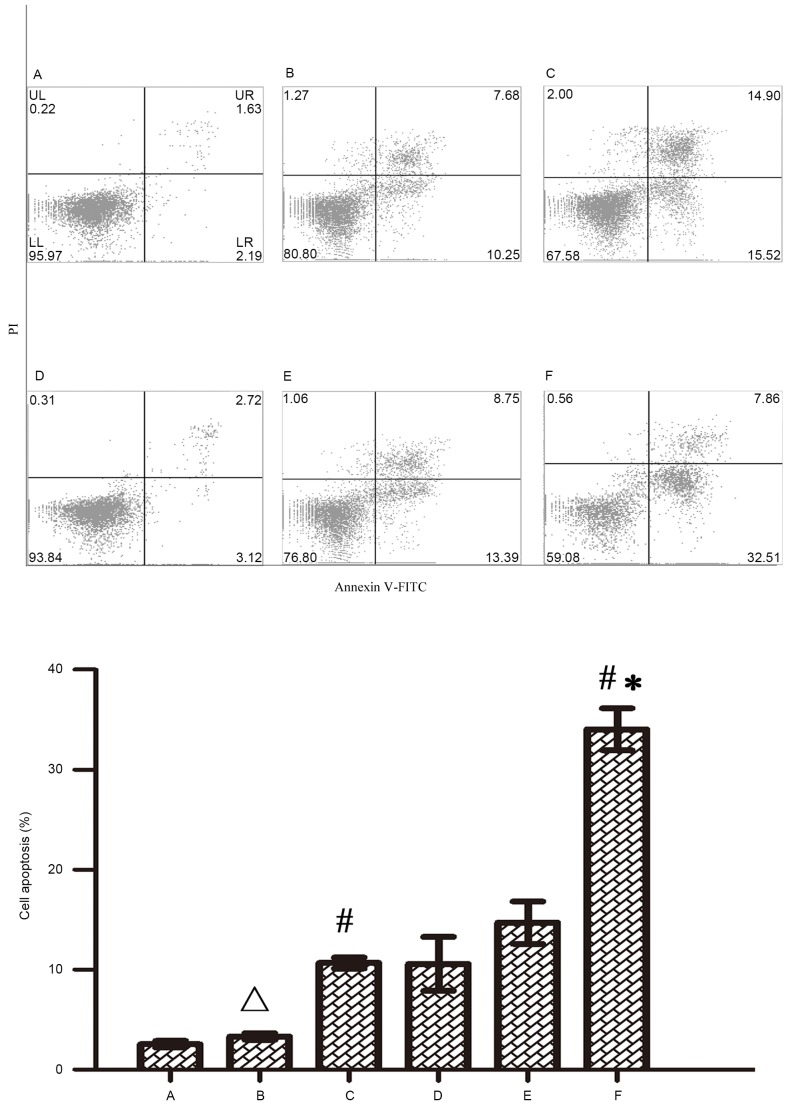
In order to investigate the mechanism of Wip1 gene silencing on the enhanced chemosensitivity of RKO colon cancer cells, based on the results of MTS assay, 5 µmol/l of 5-FU and oxaliplatin were applied to treat RKO colon cancer cells for 48 h after transfection, and cell apoptosis was then detected by flow cytometry. Figs. 5 and 6 indicate that both 5-FU and oxaliplatin could induce cell apoptosis in RKO colon cancer cells. Wip1-811 siRNA alone could not induce cell apoptosis in RKO colon cancer cells without treatment of chemotherapeutic drug. However, Wip1-811 siRNA could increase the cell apoptosis of RKO colon cancer cells when in combination with antitumor drugs, compared with that of the negative control. The results indicate that the downregulation of Wip1 gene expression could increase the cell apoptosis of RKO colon cancer cells induced by antitumor drugs.
Figure 5.
Influence of Wip1-811 siRNA on cell apoptosis in RKO colon cancer cells treated with 5-FU. A, Control; B, Control+5-FU; C, Negative control+5-FU; D, Wip1-811 siRNA; E, Liposome+5-FU; F, Wip1-811 siRNA+5-FU. ∆P>0.05 vs. control; #P<0.05 vs. Wip1-811 siRNA group; *P<0.05 vs. negative control. Wip1, wild-type p53-induced phosphatase; siRNA, small interfering RNA; 5-FU, 5-fluorouracil; Annexin V-FITC, Annexin V-fluorescein isothiocyanate; PI, Propidium iodide; UL, upper left; UR, upper right; LL, lower left; LR, lower right.
Figure 6.
Influence of Wip1-811 siRNA on cell apoptosis in RKO colon cancer cells treated with oxaliplatin. A, Control; B, Control+oxaliplatin; C, Negative control+oxaliplatin; D, Wip1-811 siRNA; E, Liposome+5oxaliplatin; F, Wip1-811 siRNA+oxaliplatin. ∆P>0.05 vs. control; #P<0.05 vs. Wip1-811 siRNA group; *P<0.05 vs. negative control. Wip1, wild-type p53-induced phosphatase; siRNA, small interfering RNA; Annexin V-FITC, Annexin V-fluorescein isothiocyanate; PI, Propidium iodide; UL, upper left; UR, upper right; LL, lower left; LR, lower right.
Influence of Wip1 gene silencing on the cell cycle of RKO colon cancer cells
As shown in Table II, there was no significant difference in the cell cycle between the siRNA group and the control group (P>0.05). This result indicates that Wip1-811 siRNA by itself had no influence on the cell cycle of RKO colon cancer cells in the absence of antitumor drugs. 5-FU could induce RKO colon cancer cell arrest in G1 phase. Therefore, 5-FU could increase the percentage of cells in G1 phase and decrease the percentage of cells in S phase (P<0.05). Oxaliplatin could induce RKO colon cancer cell arrest in G2/M phase (P<0.05). However, the percentage of cells in each cell cycle phase was not changed following Wip1 siRNA transfection in combination with 5-FU or oxaliplatin treatment compared with that following 5-FU or oxaliplatin treatment alone. This result indicates that Wip1 gene silencing has no significant influence on the cell cycle of RKO colon cancer cells induced by 5-FU or oxaliplatin.
Table II.
Influence of Wip1-811 siRNA on the cell cycle of RKO colon cancer cells treated with antitumor drugs.
| Treatment | siRNA | G0/G1 phase (%, mean ± standard deviation) | S phase (%, mean ± standard deviation) | G2/M phase (%, mean ± standard deviation) |
|---|---|---|---|---|
| 5-Fluorouracil (µmol/l) | ||||
| 0 | Control | 50.5±7.3 | 35.0±2.5 | 14.6±7.0 |
| Wip-811 siRNA | 49.9±2.4a | 33.5±4.1 | 16.6±6.2 | |
| 5 | Control | 60.9±3.8b | 28.9±1.5 | 10.3±5.2 |
| Wip-811 siRNA | 64.8±4.3c | 20.7±4.4 | 14.4±5.5 | |
| Oxaliplatin (µmol/l) | ||||
| 0 | Control | 59.0±6.4 | 28.8±5.8 | 12.2±0.6 |
| Wip-811 siRNA | 54.5±4.6 | 33.8±5.3 | 11.7±1.7a | |
| 5 | Control | 60.4±3.4 | 11.9±1.5 | 27.7±1.9b |
| Wip-811 siRNA | 58.5±3.1 | 12.3±2.7 | 28.2±6.7c | |
P>0.05, non-drug plus siRNA group vs. control.
P<0.05, chemotherapy drug group vs. non-drug group.
P>0.05, chemotherapy drug plus siRNA group vs. control. Wip1, wild-type p53-induced phosphatase; siRNA, small interfering RNA.
Influence of Wip1 gene silencing on intracellular adriamycin accumulation in RKO colon cancer cells
To investigate if Wip1-811 siRNA could increase intracellular antitumor drug accumulation, an intracellular adriamycin accumulation assay was performed using COLO 320DM and RKO colon cancer cells. Table III shows that, when treated with adriamycin, the fluorescence intensity of COLO 320DM colon cancer cells was 196.45 AU. However, the fluorescence intensity increased to 410.63 AU when cells were treated with adriamycin combined with the positive control #4123 MDR1 siRNA (P<0.05). As shown in Table III, when RKO colon cancer cells were treated with adriamycin, adriamycin fluorescence could be detected in all groups, including the control, liposome control and negative control groups, as well as in the Wip1-811 siRNA group. However, there was no significant difference in the fluorescence intensity of adriamycin among these groups (P>0.05). This indicates that Wip1-811 siRNA had no effect on intracellular adriamycin accumulation in RKO colon cancer cells.
Table III.
Fluorescence intensity of adriamycin in RKO colon cancer cells following transfection with Wip1-811 siRNA.
| Group | Fluorescence intensity of adriamycin (AU, mean ± standard deviation) |
|---|---|
| Control | 395.23±29.85 |
| Liposome control | 425.21±18.91 |
| Negative control | 431.19±6.28a |
| Wip1-811 siRNA | 441.61±23.49b |
| COLO 320DM colon cancer cells | 196.45±20.08 |
| Positive control | 410.63±30.57c |
P>0.05, Wip1-811 siRNA vs. negative control.
P>0.05, negative control vs. control.
P<0.05, positive control vs. COLO 320DM colon cancer cells. Wip1, wild-type p53-induced phosphatase; siRNA, small interfering RNA.
Discussion
The p53 gene is one of the most important tumor-suppressor genes, and its mutation and functional inactivation exist in more than half of human cancers (17). The intact function of p53 is crucial to prevent cancer development. Wip1, which is encoded by protein phosphatase magnesium-dependent 1 delta, is a serine/threonine protein phosphatase belonging to the type 2C protein phosphatase family (7). Wip1 is activated by various stresses, and is involved in several cellular processes such as tumorigenesis and aging (18). Wip1 is closely associated with p53 (8). Wip1, which is overexpressed in numerous cancers, including breast cancer (19), medulloblastoma (20), pancreatic neuroendocrine tumor (21), liver cancer (22) and stomach cancer (23), is recognized as a novel oncogene inhibiting several p53-dependent tumor-suppressor signaling pathways, including the Ataxia telangiectasia mutated-checkpoint kinase 2-p53, p38 mitogen-activated protein kinase-p53 and nuclear factor-κB signaling pathways (19). Discher et al (14) reported that Wip1 was associated with the chemosensitivity of cancer, since downregulation of Wip1 gene expression could inhibit the self-proliferation of breast cancer stem cells and enhance the chemosensitivity of MCF-7 breast cancer cells to doxorubicin. However, the influence of Wip1 gene on the chemosensitivity of colon cancer cells is still unclear.
In the present study, Wip1-811 siRNA targeting Wip1 mRNA was transfected into RKO colon cancer cells to investigate the influence of Wip1 gene silencing on the chemosensitivity of colon cancer cells. It was observed that Wip1-811 siRNA could effectively suppress Wip1 gene expression in RKO colon cancer cells. MTS assay revealed that Wip1-811 siRNA alone had no influence on the cell proliferation of RKO colon cancer cells. However, Wip1-811 siRNA combined with 5-FU, oxaliplatin or adriamycin could kill more RKO colon cancer cells than treatment with 5-FU, oxaliplatin or adriamycin alone. By comparing the IC50 of 5-FU, oxaliplatin or adriamycin in each group, it was observed that the IC50 values of these drugs in the Wip1 siRNA group were significantly lower than those in the three control groups. This indicates that Wip1 gene silencing could enhance the chemosensitivity of RKO colon cancer cells.
Cell apoptosis and cell cycle analyses by flow cytometry revealed that Wip1-811 siRNA alone had no influence on cell proliferation, cell apoptosis or cell cycle. However, when combined with antitumor drugs, the cell viability of RKO colon cancer cells in the Wip1 siRNA group was significantly lower than that in the control groups. In addition, the cell apoptosis of RKO colon cancer cells in the Wip1 siRNA group was significantly increased compared with that in the control groups. There was no significant difference on the cell cycle of RKO colon cancer cells in the Wip1 siRNA group and the other groups. This indicated that Wip1 siRNA enhanced the chemosensitivity of RKO colon cancer cells via augmentation of cell apoptosis but not alteration of the cell cycle. Nopel-Dünnebacke et al (24) reported that the downregulation of Wip1 gene expression could increase the p53 expression level and directly induce cell apoptosis. High expression levels of p53 could improve the outcome of chemotherapy for patients with colon cancer who accepted regimens based on 5-FU and oxaliplatin. Bisteau et al (25) reported that the inhibition of Wip1 gene expression could increase the accumulation of wild-type p53 protein and induce the cyclin D1 expression, which was accompanied by an increased in the percentage of cells in S phase. Therefore, the inhibition of Wip1 gene expression enhanced the chemosensitivity of liver cancer cells. However, the present study demonstrated that the downregulation of Wip1 gene expression alone could not increase cell apoptosis or change the cell cycle of RKO colon cancer cells. Thus, the alternative mechanism of enhanced chemosensitivity in RKO colon cancer cells by Wip1 gene silencing still requires to be further investigated. Brazina et al (26) proposed that Wip1 was one of the negative feedback key factors of death-associated protein 6 (Daxx) phosphorylation. High expression of Wip1 could inhibit Daxx phosphorylation induced by DNA damage and prevent cell apoptosis from occurring, thus increasing the resistance of cells to stimulation (26). Wip1 gene silencing may inhibit the self-proliferation of cancer stem cells and therefore participate in the regulation of the chemosensitivity of cancer cells (27).
The classical reversal of multidrug resistance is targeting drug efflux pumps to increase intracellular antitumor drug accumulation in cancer cells. However, in the present study, intracellular adriamycin accumulation assay by flow cytometry revealed that there was no significant difference between the Wip1-811 siRNA group and the three control groups. Therefore, it can be inferred that Wip1 gene silencing enhanced the chemosensitivity of RKO colon cancer cells not via drug efflux pumps.
In the present study, Wip1 siRNA was transiently transfected into RKO colon cancer cells to successfully inhibit the gene expression of Wip1. The downregulation of Wip1 gene expression could enhance the chemosensitivity of RKO colon cancer cells, which provided a new potential approach for the reversal of multidrug resistance in colon cancer. However, siRNA transient transfection has certain disadvantages such unsatisfactory inhibition of the target gene and short time effects (5). Furthermore, the results of the present study were obtained in vitro. In the future, a stable expression vector for Wip1-811 siRNA must be constructed. Furthermore, long-term, stable RNAi by Wip1-811 siRNA in vivo must also be investigated.
In conclusion, Wip1-811 siRNA could induce Wip1 gene silencing. Wip1-811 siRNA could enhance the chemosensitivity of RKO colon cancer cells via augmentation of cell apoptosis but not alteration of the cell cycle or intracellular antitumor drug accumulation in RKO colon cancer cells.
Acknowledgements
The present study was supported by grant no. [2013]163 from the Key Laboratory of Malignant Tumor Molecular Mechanism and Translational Medicine of Guangzhou Bureau of Science and Information Technology, and grant no. KLB09001 from the Key Laboratory of Malignant Tumor Gene Regulation and Target Therapy of Guangdong Higher Education Institutes.
References
- 1.Wiseman M. The second world cancer research Fund/American institute for cancer research expert report: Food, nutrition, physical activity, and the prevention of cancer: A global perspective; Proc Nutr Soc; 2008; pp. 253–256. [DOI] [PubMed] [Google Scholar]
- 2.Douillard JY, Cunningham D, Roth AD, Navarro M, James RD, Karasek P, Jandik P, Iveson T, Carmichael J, Alakl M, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: A multiple multicentre randomised trial. Lancet. 2000;355:1041–1047. doi: 10.1016/S0140-6736(00)02034-1. [DOI] [PubMed] [Google Scholar]
- 3.Redmond SM, Joncourt F, Buser K, Ziemiecki A, Altermatt HJ, Fey M, Margison G, Cerny T. Assessment of P-glycoprotein, glutathione-based detoxifying enzymes and O6-alkylguanine-DNA alkyltransferase as potential indicators of constitutive drug resistance in human colorectal tumors. Cancer Res. 1991;51:2092–2097. [PubMed] [Google Scholar]
- 4.Van de Vrie W, Gheuens EE, Durante NM, De Bruijn EA, Marquet RL, Van Oosterom AT, Eggermont AM. In vitro and in vivo chemosensitizing effect of cyclosporine A on an intrinsic multidrug-resistant rat colon tumour. J Cancer Res Clin Oncol. 1993;119:609–614. doi: 10.1007/BF01372724. [DOI] [PubMed] [Google Scholar]
- 5.Xia Z, Zhu Z, Zhang L, Royal C, Liu Z, Chen Q, Adam BL. Specific reversal of MDR1/P-gp-dependent multidrug resistance by RNA interference in colon cancer cells. Oncol Rep. 2008;20:1433–1439. [PubMed] [Google Scholar]
- 6.Damiani D, Michieli M, Michelutti A, Melli C, Cerno M, Baccarani M. D-verapamil downmodulates P170-associated resistance to doxorubicin, daunorubicin and idarubicin. Anticancer Drugs. 1993;4:173–180. doi: 10.1097/00001813-199304000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Fiscella M, Zhang H, Fan S, Sakaguchi K, Shen S, Mercer WE, Woude GF Vande, O'Connor PM, Appella E. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner; Proc Natl Acad Sci USA; 1997; pp. 6048–6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Y. Serine/threonine phosphatases: Mechanism through structure. Cell. 2009;139:468–484. doi: 10.1016/j.cell.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Donehower LA. Phosphatases reverse p53-mediated cell cycle checkpoints; Proc Natl Acad Sci USA; 2014; pp. 7172–7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu X, Nannenga B, Donehower LA. PPM1D dephosphorylates Chk1 and p53 and abrogates cell cycle checkpoints. Genes Dev. 2005;19:1162–1174. doi: 10.1101/gad.1291305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demidov ON, Timofeev O, Lwin HN, Kek C, Appella E, Bulavin DV. Wip1 phosphatase regulates p53-dependent apoptosis of stem cell and tumorigenesis in the mouse intestine. Cell Stem Cell. 2007;1:180–190. doi: 10.1016/j.stem.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 12.Li ZT, Zhang L, Gao XZ, Jiang XH, Sun LQ. Expression and significance of the Wip1 proto-oncogene in colorectal cancer. Asian Pac J Cancer Prev. 2013;14:1975–1979. doi: 10.7314/APJCP.2013.14.3.1975. [DOI] [PubMed] [Google Scholar]
- 13.Zhao M, Zhang H, Zhu G, Liang J, Chen N, Yang Y, Liang X, Cai H, Liu W. Association between overexpression of Wip1 and prognosis of patients with non-small cell lung cancer. Oncol Lett. 2016;11:2365–2370. doi: 10.3892/ol.2016.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Discher DE, Janmey P, Wang YL. Tissue cells feel and repond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- 15.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 16.Li J, Xu LZ, He KL, Guo WJ, Zheng YH, Xia P, Chen Y. Reversal effects of nomegestrol acetate on multidrug resistance in adriamycin-resistant MCF7 breast cancer cell line. Breast Cancer Res. 2001;3:253–263. doi: 10.1186/bcr303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang F, Syrjänen S, Kurvinen K, Syrjänen K. The p53 tumor suppressor gene as a common cellular target in human carcinogenesis. Am J Gastroenterol. 1993;88:174–186. [PubMed] [Google Scholar]
- 18.Lu G, Wang Y. Functional diversity of mammalian type 2C protein phosphatase isoforms: New tales from an old family. Clin Exp Pharmacol Physiol. 2008;35:107–112. doi: 10.1111/j.1440-1681.2007.04843.x. [DOI] [PubMed] [Google Scholar]
- 19.Yu E, Ahn YS, Jang SJ, Kim MJ, Yoon HS, Gong G, Choi J. Overexpression of the Wip1 gene abrogates the p38 MAPK/p53/Wip1 pathway and silences p16 expression in human breast cancers. Breast Cancer Res Treat. 2007;101:269–278. doi: 10.1007/s10549-006-9304-y. [DOI] [PubMed] [Google Scholar]
- 20.Baxter EW, Milner J. p53 regulates LIF expression in human medulloblastoma cells. J Neurooncol. 2010;97:373–382. doi: 10.1007/s11060-009-0043-x. [DOI] [PubMed] [Google Scholar]
- 21.Hu W, Feng Z, Modica I, Klimstra DS, Song L, Allen PJ, Brennan MF, Levine AJ, Tang LH. Gene Amplifications in well-differentiated pancreatic neuroendocrine tumors inactivate the P53 pathway. Genes Cancer. 2010;1:360–368. doi: 10.1177/1947601910371979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu YH, Bulavin DV. Wip1-dependent signaling pathways in health and diseases. Prog Mol Biol Transl Sci. 2012;106:307–325. doi: 10.1016/B978-0-12-396456-4.00001-8. [DOI] [PubMed] [Google Scholar]
- 23.Fuku T, Semba S, Yutori H, Yokozaki H. Increased wild-type p53-induced phosphatase 1 (Wip1 or PPM1D) expression correlated with downregulation of checkpoint kinase 2 in human gastric carcinoma. Pathol Int. 2007;57:566–571. doi: 10.1111/j.1440-1827.2007.02140.x. [DOI] [PubMed] [Google Scholar]
- 24.Nöpel-Dünnebacke S, Schulmann K, Reinacher-Schick A, Porschen R, Schmiegel W, Tannapfel A, Graeven U. Prognostic value of microsatellite instability and p53 expression in metastatic colorectal cancer treated with oxaliplatin and fluoropyrimidine-based chemotherapy. Z Gastroenterol. 2014;52:1394–1401. doi: 10.1055/s-0034-1366781. [DOI] [PubMed] [Google Scholar]
- 25.Bisteau X, Caldez MJ, Kaldis P. The complex relationship between liver cancer and the cell cycle: A story of multiple regulations. Cancers (Basel) 2014;6:79–111. doi: 10.3390/cancers6010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brazina J, Svadlenka J, Macurek L, Andera L, Hodny Z, Bartek J, Hanzlikova H. DNA damage-induced regulatoryinterplay between DAXX, p53, ATM kinase and Wip1 phosphatase. Cell Cycle. 2015;14:375–387. doi: 10.4161/15384101.2014.988019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang X, Wan G, Mlotshwa S, Vance V, Berger FG, Chen H, Lu X. Oncogenic Wip1 phosphatase is inhibited by miR-16 in the DNA damage signaling pathway. Cancer Res. 2010;70:7176–7186. doi: 10.1158/0008-5472.CAN-10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]