Abstract
Aim
The Safe Passage Study, conducted by the Prenatal Alcohol in SIDS and Stillbirth Network, is investigating contributions of prenatal alcohol exposure to fetal and infant demise. This current report presents physiological data from full term infants with no prenatal exposure to alcohol or maternal smoking.
Methods
Data are from 666 infants from the Northern Plains (North and South Dakota) and South Africa. A standardized protocol assessed cardiorespiratory function during baseline and head-up tilts shortly after birth and at 1 month of age.
Results
Analyses revealed significant increases in heart rate and decreases in BP from the newborn to 1 month time period as well as diminished heart rate responses to head-up tilt in 1 month old infants.
Conclusion
The Safe Passage Study was successful in characterizing physiology in a large number of infants at sites known to have elevated risks for SIDS. Results demonstrate that even with low prenatal adverse exposures, there are significant changes in cardiorespiratory function as infants enter the window of increased risk for SIDS.
Keywords: Autonomic Development, Blood Pressure, Heart Rate, Respiration, SIDS
INTRODUCTION
Sudden Infant Death Syndrome (SIDS) remains the most common cause of death in infants from 1 month to 1 year of age (1). Despite intensive research, the causes for SIDS remain unknown. However, there is mounting evidence of anatomical and biochemical alterations in SIDS victims in brainstem regions that are involved in autonomic nervous regulation, particularly as related to serotonergic circuits (2). Another consistent finding related to SIDS is the strong age dependency with nearly all deaths occurring before 6 months of age (1). Many studies have investigated infant cardiorespiratory measures during sleep and at ages associated with increased risk for SIDS (3–11). However, there is as yet no consensus as to what physiological variables and test conditions are most likely to reveal state dependent vulnerabilities that might account for and predict SIDS.
The Prenatal Alcohol in SIDS and Stillbirth (PASS) Network was formed to investigate prenatal alcohol exposure and risk for (SIDS), stillbirth and fetal alcohol spectrum disorders (FASD). The PASS network’s Safe Passage Study recruited nearly 12,000 subjects from sites in North and South Dakota and in Cape Town, South Africa. The Safe Passage Study assessed participants from pregnancy through one year of age to test the primary hypotheses that prenatal alcohol exposure increases risk for SIDS, stillbirth and fetal alcohol spectrum disorders(12).
Secondary hypotheses of the Safe Passage Study were to investigate effects of prenatal exposures on central and autonomic nervous system function in the fetus and infant prior to development of adverse outcomes. The potential impact of this study is to better define mechanisms that underlie vulnerability to stillbirth, SIDS and FASD. Information gained is expected to provide early markers of abnormal nervous system development and thus, identify fetuses and infants at greatest need for increased surveillance and early interventions.
The Safe Passage Study implemented protocols that were uniformly conducted at all test sites. These included assessments of infant heart rate (HR), breathing frequency (f), heart rate variability (HRV), and blood pressure (BP) within the first four days of life and again at 1 month postnatal age. Assessments were made while infants were asleep during a baseline period and following three 45° head-up tilts. Here we report cardiorespiratory findings for infants unexposed prenatally to maternal smoking, alcohol or drugs of abuse. The aims of this paper are to; 1) present details of the physiological assessment protocols used in the PASS Network Safe Passage Study, 2) establish for full term infants with no prenatal exposure to alcohol, smoking or drugs of abuse, normative values and ranges for physiological parameters, 3) replicate and extend prior working showing increases in HR, decreases in HRV, and diminished HR responses to head-up tilting as infants enter the period of increased risk for SIDS, and 4) present new sleep state and age-related findings for this subset of unexposed infants. The methods and normative data here provide the baseline for subsequent analyses focused on the effects of a wide range of adverse prenatal exposures in the entire Safe Passage cohort.
METHODS
Subjects
Institutional Review Board approvals for the Safe Passage Study were obtained from sponsoring organizations at the participating clinical sites in the Northern Plains (North and South Dakota) and South Africa, as well as for the Data Coordinating Center and the Physiologic Assessment Center. Infants were singletons born 37–41 weeks postmenstrual age (PMA) with birth weights >=2500g. No infants required resucitation at birth or admission to the neonatal intensive care unit. Prenatal alcohol exposure information was obtained using a modification of the Timeline Follow-back (TLFB) interview(13). Mothers of these infants reported no alcohol consumption, no smoking, and no drugs of abuse during pregnancy. No evidence of diabetes, hypertension or preeclampsia during pregnancy was found in the medical records of these mothers. As of January 2016, 666 infants met these criteria. Enrollment of these subjects occurred at prenatal clinics affiliated with each clinical site between 6 weeks gestation up to, but not including, the time of delivery (12). The racial distribution of the 666 infants was 69.2% mixed ancestry from South African, and 18.6% white, 11.0% American Indian and 1.5% unspecified from the North and South Dakota sites. Females comprised 53.4% of the 666 infants. A majority of the infants (83.3%) were delivered vaginally with an average GA and BW at delivery of 39.7 weeks and 3376g respectively. All infants had 5 minute Apgar scores of 7 or greater. At the time of the one month assessments 54% of the infants were fed with breast milk, 12% formula and 34% a combination of breast milk and formula.
Data Acquisition
Physiological assessments were completed between 9am and 4pm. ECG and respiration were acquired using custom hardware and software systems. After feeding (~30 min), ECG leads were placed on the infants (left abdominal, left and right scapula). A respiratory inductance belt (Ambulatory Monitoring Inc.) was placed around the infant’s chest. ECG and respiration signals were acquired at 500, and 20 samples per second respectively. A BP cuff (width = 3 – 5 cm) was placed around the infant’s leg below the left knee. The distance from the middle of the cuff to the level of the heart was measured to allow for computation of hydrostatic pressure while infants were in the 45° head-up position (0.8 mmHg/cm). BP was measured using a clinical monitor (CASMED 740 Vital Signs Monitor). Infants were placed prone in a custom bassinet that enabled rapid (~5 sec) tilting to the 45° head-up position. A video camera filmed the infant’s face during the protocol.
Assessment Protocol
Newborn infants were tested 12–96h after delivery. In the North and South Dakota sites, tests occurred before discharge from the hospital. In Cape Town infants were discharged <24h after delivery. Therefore, at this site, infants returned to testing facilities at either Tygerberg or Karl Bremer Hospital 48–96h after birth for newborn assessments. Infants were assessed again at 1 month (28±7 days) corrected age. Three factors contributed to the decision to collect data at 1 month rather than at multiple time points or at an older age within the window of maximum risk for SIDS. First, multiple assessment time points after the neonatal period were not feasible due to budgetary limitations. Second, as babies age, they are less likely to sleep during acquisition at the follow-up visit. Thus, the 1 month time point was thought to produce less data loss than at older ages. Third, a major goal of the SPS study was to obtain as much data as possible, physiological and other wise, prior to demise from SIDS. Postponing the age of assessment until 2, 3, or 4 months increased the risk of not obtaining prospective physiological data.
A standardized protocol was used at both ages in which ECG, respiration and BP were recorded during a 10 minute baseline period and in response to three rapid (~3–5 seconds) 450 head-up tilts while the infant was in the prone position. The prone position was chosen as it is a long recognized SIDS risk sleeping position(14) that may accentuate the physiological challenge associated with tilting. A clinometer was attached to the basinet to measure the baby’s tilt position. Figure 1 depicts the protocol time course. In this figure, blocks of different sizes represent analysis epochs of various sizes, 15 to 90 seconds (see caption for Figure 1). The figure depicts a 30 second block just prior to each tilt; this was the baseline period for each tilt. After reaching the head up position, a 15 sec epoch was assessed for the maximum minus minimum HR to reflect the immediate biphasic response to head-up tilting (10, 15). Following this period there were 3 30 second periods, the average HR during the last two of these was taken as the sustained response to tilt, which, in newborns, is higher than during the baseline period(16). Finally, prior to returning to the flat position, a BP was taken.
Figure 1.
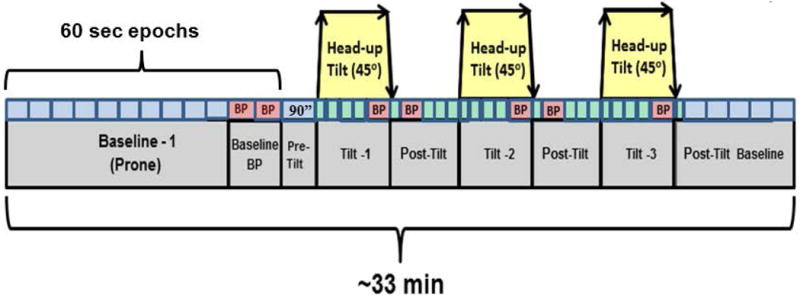
Schematic of cardiorespiratory assessments. Small blocks above the segments of the protocol (e.g. Baseline-1, Tilt-1) are epochs used for the current analyses. Epoch sizes (small blocks) varied in durations. Baseline 1 epochs, epochs during which blood pressure was measured, and all but the first epoch comprising the post tilt period are 60 seconds in duration. The epoch preceding the first head up tilt was 90 seconds. The epochs immediately after head-up or tilts back to flat were 15 seconds in duration. All other epochs were 30 seconds in duration.
Data Upload
Physiological signal, behavioral, and video files were uploaded to the data center in Boston. These files were then sent to a server at the Physiology Assessment Center at the Columbia University Medical Center in New York City.
Quality Assurance
Oversight of data acquisition was accomplished through protocols implemented by the Clinical Sites and by site visits (2–3 visits per year) during which equipment was inspected and test sessions were observed. These visits were supplemented by remote viewing of assessments and monthly calls with the site research staff. Although data were collected for research purposes, the protocol allowed research staff to refer infants based on findings of clinical significance: unexplained cyanosis, pallor or tone change, respiratory distress and bradycardia.
Prior to processing, signals were displayed to ascertain study duration, data range, occurrence of artifact and overall quality. This initial visual screening was also used to verify that the acquisition systems were performing as expected and adherence to the study protocol.
Data Processing
Processing of physiological signals involved several steps. First, electrical line noise was filtered. Next, the ECG was subjected to automated peak detection algorithms and respiratory tracings were marked using a published algorithm(17). Raw ECG and respiratory waveforms along with their superimposed peak marks were displayed and marks were manually corrected when necessary. Quality of the ECG was generally excellent and took a few minutes per file to mark and visually check. Marking of breaths was more time consuming due to movement artifact, requiring up to 20 minutes for each file. Research assistants were trained on these marking procedures until they achieved 80% concordance on training records. Signals from the clinometer were also marked to denote start and stop times for the head-up tilt sequences.
Sleep State Coding
Cardiorespiratory variables differ as a function of sleep state(5, 18, 19). Accordingly, prior to analyses of physiological data, an algorithm for coding active or quiet sleep states (AS and QS) based on breathing rate variability was implemented. Details of the state coding method and its validation were recently published(20). Breathing variability was chosen for this coding because variability in breath to breath intervals is the best single physiological measure of these states during the first 6 months of life(21). This coding, particularly the distinction between AS or awake, was supplemented by research assistant key pad entries of time-locked behavioral codes indicating when infants were seen to be awake, crying or fussy.
Derived Variables (Baseline)
Mean and median values for physiological variables were computed for each of 10 one-minute epochs prior to two baseline BP measurements and initiation of the sequence of three head-up tilts (see Figure 1). For each minute, mean and median R-wave to R-wave intervals (RRi) were computed, and from the median RRi, mean heart rate (HR) was computed. Also computed were standard deviations of RRis (SD-RRi), the square root of the mean of the squared successive differences in RRis (rMSSD) and, from spectral analyses, high frequency (0.5 to 1.5 Hz, i.e. f range for infants) variability in RRi (HF-RRi). HF-RRi and rMSSD were taken as indirect indices of parasympathetic modulation of HR(22, 23). RRi spectra were calculated for each minute using an interval method for computing Fourier transforms(24). Prior to computing these spectra, the mean of the RRi series was subtracted from each value in the series and the residual series was filtered using a Hanning window(25). Estimates of spectral power were adjusted to account for power attenuation produced by the Hanning filter(25). Also computed were mean and median breath-to-breath intervals, and from these, breathing rates as well as standard deviations of breath-to-breath intervals (SD-BBi). For each heart and respiratory measure, median values across epochs within each sleep state were the final baseline estimates. At the end of the baseline period two BP measures were taken over a course of about 2 minutes. All summary variables were computed separately for AS and QS epochs.
Derived Variables (Head-up Tilt)
For each of the cardiorespiratory variables described above, changes in response to head-up tilting were computed. For HR and breathing rate change scores, median values during the 30 second period immediately before each tilt were subtracted from median values during the last minute in the head-up position (~45 seconds after reaching the head-up position). After returning to the flat position, 165 seconds elapsed before the next head-up tilt, a time determined to be sufficient for all variables to return, on average, to baseline. Medians over the three tilts are reported. For HR, acute responses to tilts were also computed as maximum HR – minimum HR during the first 15 seconds in the head-up position. The median maximum-minimum HR over the 3 tilts is reported. The pre-tilt values for systolic and diastolic BP were obtained ~90 seconds before tilts and the head-up BP values were taken just before returning to the horizontal position. Median changes in BP over the three tilts are reported. The three tilts occurred according to predefined protocol times (Figure 1). Sleep state for each tilt was defined as the state the infant was in during the 30 second baseline preceding each tilt.
Data Cleaning
Multiple steps excluded data contaminated by artifact. Such values occur during periods of noisy ECG which are difficult to mark and are generally associated with excessive infant movements. R-wave marking during such segments can produce RR-intervals which are not within a normal physiological range. For RRi analyses, values less than 0.3 seconds (200 bpm) or greater than 0.75 seconds (80 bpm) were excluded from further computations. Beat-to-beat changes in RRi greater than 60 msec (i.e. ~20 bpm when HR=140) bpm) were excluded. Breath-to-breath intervals less than 0.5 seconds (120 breaths/min) or greater than 2.0 seconds (30 breaths/min) were excluded. Systolic and diastolic BPs were excluded when the pulse rate derived from the BP monitor differed by more than 10 bpm from the closest preceding ECG-derived HR. Following these procedures, an evaluation for statistical outliers was performed (see Results).
Statistical Analyses
Not all subjects provided data in both sleeps states and/or at both ages in each state. Accordingly, to determine effects of age and/or sleep states, mixed models regression analyses (MMRA) were performed with age (or state) as categorical fixed effects and random intercepts for individual participants to account for dependence of repeated measures. For each MMRA, the total number of individual subjects and the number of subjects for which there were repeated measures, i.e. babies with data in both sleep states or at both ages, are reported.
RESULTS
Data Capture
666 infants met criteria for inclusion; 463 from South Africa, 203 from the North and South Dakota sites. Physiological assessments were attempted for 456 infants at the newborn period (12–96 hours of age) and 482 infants at 1 month (28 ± 7 days); 510 subjects had assessments at either the newborn or 1 month period, and 227 at both ages.
In total, 955 files were processed. Baseline data for 68 infants were excluded because babies were on the mothers’ laps or were being fed during the baseline period. Of 887 baseline studies, 32 (3.6%) had no usable data (3.3% at the newborn and 3.9% at the 1 month time points).
Outlier Data
For each variable, statistical outliers were identified (>1.5 × interquartile range above the 75th percentile or <1.5 × interquartile range below the 25th percentile). For purposes of establishing normative values for the Safe Passage Study these outliers were removed from the data set. However, in future analyses, using cut off values derived in this current report, outliers will be quantified as potential markers of outcomes or exposures.
Table 1 presents summaries for newborn HR, SD-RRi, rMSSD, HF-RRi, f, SD-BBi, and systolic and diastolic BP during the 10 minute baseline period prior to initiation of head-up tilts. Results are presented for active sleep (AS) and quiet sleep (QS). Shown are the original number of subjects prior to removal of outliers: low and high cutoff values for defining statistical outliers, number of subjects after removing outliers, percentage of outlier data and, means, SDs, and 5th and 95th percentiles for the cleaned data. Table 2 shows results from studies at 1 month of age. Tables 3 and 4 show summaries for changes in response to head-up tilts at the newborn and 1 month time points.
Table 1.
Normative Values (mean, SD, 5th and 95th percentiles) for Newborn Baseline by Sleep State. Outlier cutoffs were set at 1.5 × IQR above the 75th or below the 25th percentile. The 5th and 95th percentiles were determined after removing outliers.
| Variable | original N |
low cutoff |
high cutoff |
final N | (outliers) | Mean ± SD | 5th | percentile 95th |
|---|---|---|---|---|---|---|---|---|
| HR (bpm, AS) | 359 | 104 | 150 | 343 | 4.46% | 126±10 | 109 | 144 |
| HR (bpm, QS) | 99 | 101 | 144 | 92 | 7.07% | 122±10 | 106 | 139 |
| SD-RRi (sec, AS) | 359 | 0.005 | 0.049 | 339 | 5.57% | 0.025±0.01 | 0.011 | 0.042 |
| SD-RRi (sec, QS) | 99 | 0.000 | 0.040 | 96 | 3.03% | 0.019±0.01 | 0.008 | 0.034 |
| log10rMSSD-RRi (sec, AS) | 359 | −2.47 | −1.40 | 346 | 3.62% | −1.94±0.23 | −2.32 | −1.55 |
| log10rMSSD-RRi (sec, QS) | 99 | −2.49 | −1.40 | 97 | 2.02% | −1.96±0.25 | −2.34 | −1.50 |
| log10 HF-RRi (sec2, AS) | 357 | −7.84 | −5.93 | 352 | 1.40% | −6.88±0.42 | −7.52 | −6.17 |
| log10 HF-RRi (sec2, QS) | 99 | −7.86 | −5.95 | 98 | 1.01% | −6.90±0.44 | −7.67 | −6.20 |
| f (breaths/min, AS) | 360 | 32.7 | 79.9 | 344 | 4.44% | 54.7±9.8 | 40.0 | 71.4 |
| f (breaths/min, QS) | 94 | 29.1 | 61.2 | 89 | 5.32% | 43.4±7.2 | 33.4 | 58.9 |
| SD-BBi (sec, AS) | 360 | 0.175 | 0.430 | 338 | 6.11% | 0.304±0.056 | 0.215 | 0.404 |
| SD-BBi (sec, QS) | 94 | 0.080 | 0.300 | 87 | 7.45% | 0.183±0.049 | 0.105 | 0.281 |
| Systolic BP (mmHg, AS) | 178 | 61 | 106 | 169 | 5.06% | 82±9 | 68 | 102 |
| Systolic BP (mmHg, QS) | 40 | 56 | 106 | 38 | 5.00% | 80±10 | 67 | 97 |
| Diastolic BP (mmHg, AS) | 178 | 33 | 69 | 167 | 6.18% | 50±8 | 37 | 65 |
| Diastolic BP (mmHg, QS) | 40 | 29 | 65 | 39 | 2.50% | 47±8 | 36 | 62 |
Table 2.
Normative Values (mean, SD, 5th and 95th percentiles) for 1 Month Baseline by Sleep State. Outlier cutoffs were set at 1.5 × IQR above the 75th or below the 25th percentile. The 5th and 95th percentiles were determined after removing outliers.
| Variable | original N |
low cutoff |
high cutoff |
final N | (outliers) | Mean ± SD | 5th | percentile 95th |
|---|---|---|---|---|---|---|---|---|
| HR (bpm, AS) | 336 | 126 | 170 | 314 | 6.55% | 147±10 | 132 | 164 |
| HR (bpm, QS) | 102 | 126 | 158 | 95 | 6.86% | 141±8 | 129 | 155 |
| SD-RRi (sec, AS) | 336 | 0.006 | 0.036 | 324 | 3.57% | 0.021±0.01 | 0.010 | 0.032 |
| SD-RRi (sec, QS) | 102 | 0.005 | 0.026 | 95 | 6.86% | 0.014±0.01 | 0.006 | 0.023 |
| log10rMSSD-RRi (sec, AS) | 336 | −2.46 | −1.71 | 323 | 3.87% | −2.08±0.16 | −2.33 | −1.80 |
| log10rMSSD-RRi (sec, QS) | 102 | −2.46 | −1.67 | 96 | 5.88% | −2.08±0.16 | −2.35 | −1.83 |
| log10 HF-RRi (sec2, AS) | 336 | −7.72 | −6.39 | 318 | 5.36% | −7.06±0.28 | −7.51 | −6.59 |
| log10 HF-RRi (sec2, QS) | 101 | −7.76 | −6.29 | 93 | 7.92% | −7.07±0.32 | −7.61 | −6.54 |
| f (breaths/min, AS) | 334 | 37.8 | 76.6 | 315 | 5.69% | 56.1±8.6 | 42.2 | 71.5 |
| f (breaths/min, QS) | 100 | 21.6 | 72.9 | 96 | 4.00% | 45.8±9.1 | 34.2 | 63.9 |
| SD-BBi (sec, AS) | 334 | 0.172 | 0.376 | 293 | 12.28% | 0.273±0.043 | 0.202 | 0.349 |
| SD-BBi (sec, QS) | 100 | 0.071 | 0.282 | 93 | 7.00% | 0.164±0.045 | 0.085 | 0.242 |
| Systolic BP (mmHg, AS) | 146 | 63 | 108 | 136 | 6.85% | 83 ±10 | 69 | 102 |
| Systolic BP (mmHg, QS) | 61 | 64 | 91 | 55 | 9.84% | 76±6 | 68 | 87 |
| Diastolic BP (mmHg, AS) | 146 | 29 | 62 | 143 | 2.05% | 45±7 | 36 | 58 |
| Diastolic BP (mmHg, QS) | 61 | 30 | 51 | 55 | 9.84% | 39±4 | 32 | 47 |
Table 3.
Normative Values (mean, SD, 5th and 95th percentiles) Newborn Responses to Tilt by Sleep State. Outlier thresholds (1.5 × IQR above the 75th or below the 25th percentile). AS and QS data were obtained in Active and Quiet Sleep epochs as defined by high or low variation in respiratory rate. Δ indicates the median change over 3 possible 45° head up tilts from the 30 second period immediately before the tilt to a 60 second period 50 seconds after achieving the head-up position (i.e. sustained change). Δ acute HR is the difference between the maximum and minimum HRs recorded during the first 15 seconds after achieving the head up position. For BP measures, the baseline period immediately preceded the baseline for HR and RSP measures and the head-up tilt measures were taken immediately after the sustained HR and RSP values.
| Variable | original N |
low cutoff |
high cutoff |
final N | (outliers) | Mean ± SD | 5th | percentile 95th |
|---|---|---|---|---|---|---|---|---|
| Δ sustained HR (bpm, AS) | 340 | −8.1 | +12.7 | 297 | 12.65% | 2.0±4.4 | −5.6 | 10.2 |
| Δ sustained HR (bpm, QS) | 129 | −6.5 | +12.3 | 118 | 8.53% | 2.6±4.2 | −4.8 | 9.4 |
| Δ rMSSD-RRi (sec, AS) | 340 | −0.005 | +0.005 | 273 | 19.71% | 0.000±0.002 | −0.004 | +0.003 |
| Δ rMSSD-RRi (sec, QS) | 129 | −0.010 | +0.006 | 117 | 9.30% | −0.001±0.003 | −0.008 | +0.004 |
| Δ acute HR (bpm, AS) | 338 | 5.5 | 40.4 | 318 | 5.92% | 22.0±7.4 | 11.1 | 35.9 |
| Δ acute HR (bpm, QS) | 128 | 3.0 | 42.6 | 125 | 2.34% | 22.3±7.7 | 10.8 | 37.0 |
| Δ f (breaths/min, AS) | 317 | −19.1 | +10.6 | 292 | 7.89% | −3.8±6.3 | −15.0 | +6.0 |
| Δ f (breaths/min, QS) | 117 | −11.2 | +6.2 | 113 | 3.42% | −2.5±5.5 | −13.3 | +7.0 |
| Δ Systolic BP (mmHg, AS) | 179 | −23.2 | +12.5 | 166 | 7.3% | −5.3±6.4 | −16.1 | +5.3 |
| Δ Systolic BP (mmHg, QS) | 68 | −18.1 | +10.4 | 66 | 2.9% | −4.3±4.8 | −11.6 | +2.7 |
| Δ Diastolic BP (mmHg, AS) | 180 | −20.9 | +10.1 | 168 | 6.7% | −5.0±5.3 | −13.5 | +4.7 |
| Δ Diastolic BP (mmHg, QS) | 70 | −17.1 | +7.3 | 68 | 2.9% | −4.6±5.1 | −12.5 | +5.2 |
Table 4.
Normative Values (mean, SD, 5th and 95th percentiles) 1 Month Responses to Tilt by Sleep State. Outlier thresholds (1.5 × IQR above the 75th or below the 25th percentile). AS and QS data were obtained in Active and Quiet Sleep epochs as defined by high or low variation in respiratory rate. Δ indicates the median change over 3 possible 45° head up tilts from the 30 second period immediately before the tilt to a 60 second period 50 seconds after achieving the head-up position (i.e. sustained change). Δ acute HR is the difference between the maximum and minimum HRs recorded during the first 15 seconds after achieving the head up position. For BP measures, the baseline period immediately preceded the baseline for HR and RSP measures and the head-up tilt measures were taken immediately after the sustained HR and RSP values.
| Variable | original N |
low cutoff |
high cutoff |
final N | (outliers) | Mean ± SD | 5th | percentile 95th |
|---|---|---|---|---|---|---|---|---|
| Δ sustained HR (bpm, AS) | 300 | −8.1 | +11.9 | 260 | 13.33% | 1.2±4.3 | −5.5 | +9.5 |
| Δ sustained HR (bpm, QS) | 155 | −6.4 | +6.1 | 145 | 6.45% | 0.4±2.9 | −4.2 | +5.6 |
| Δ rMSSD-RRi (sec, AS) | 300 | −0.004 | +0.004 | 279 | 7.00% | 0.000±0.002 | −0.003 | +0.003 |
| Δ rMSSD-RRi (sec, QS) | 155 | −0.004 | +0.005 | 148 | 4.52% | 0.000±0.002 | −0.004 | +0.004 |
| Δ acute HR (bpm, AS) | 315 | 7.1 | 44.0 | 291 | 7.62% | 23.8±7.5 | 12.0 | 36.4 |
| Δ acute HR (bpm, QS) | 155 | 4.1 | 42.2 | 146 | 5.81% | 21.7±8.6 | 8.8 | 37.4 |
| Δ f (breaths/min, AS) | 283 | −15.4 | +8.8 | 278 | 1.77% | −3.2±6.2 | −14.6 | +7.1 |
| Δ f (breaths/min, QS) | 138 | −13.6 | +7.4 | 132 | 4.35% | −2.6±5.5 | −12.2 | +6.6 |
| Δ Systolic BP (mmHg, AS) | 181 | −20.7 | +11.1 | 164 | 9.4% | −5.4±6.5 | −15.8 | +6.3 |
| Δ Systolic BP (mmHg, QS) | 109 | −18.2 | +10.5 | 96 | 11.9% | −3.9±5.6 | −11.4 | +5.8 |
| Δ Diastolic BP (mmHg, AS) | 182 | −19.9 | +7.2 | 162 | 11.0% | −6.2±5.7 | −16.8 | +3.8 |
| Δ Diastolic BP (mmHg, QS) | 109 | −16.9 | +4.0 | 96 | 11.9% | −5.9±3.8 | −11.4 | +0.2 |
Effects of Age and Sleep State; Baseline Data
Figure 2 shows means (±SE) for HR, breathing rate, and BP during the 10 minute baseline prior to initiation of head–up tilts for newborn and 1 month assessments. Means were computed after removal of outliers. For HR (Figure 2A) in QS, there were 187 measurements (174 infants, 13 at both ages). The MMRA indicated a significant effect of age (p<0.001) with QS HR estimated to be 19.1 bpm higher at 1 month. For HR in AS there were 657 measurements (474 infants, 183 at both ages), and HR in AS was 21.0 bpm higher at 1 month (p<0.001). In newborns, HR was ~3.5 bpm higher in AS than in QS (p<0.001; 435 observations (386 subjects, 49 with both sleep states). At 1 month, HR was ~3.4 bpm higher in AS than in QS (p<0.001; 409 observations, 351 subjects, 58 with both states). There were no significant differences in HR between sites by either sleep state or age.
Figure 2.
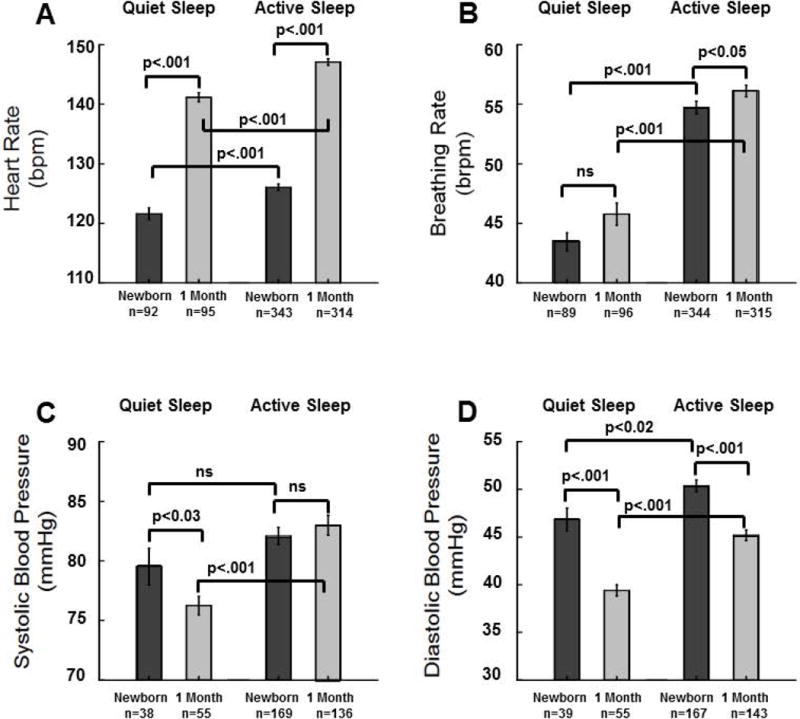
Means±SE for Active Sleep (AS) and Quiet Sleep (QS) heart rate (HR) (A), breathing rate (B), systolic BP (C), and diastolic BP (D) during the 10 minute baseline prior to head–up tilts for newborns and at 1 month. Values were computed after removal of statistical outliers. Results from mixed models regression indicated the following significant effects: A) in both QS and AS HR is higher at 1 month (p<0.001), in newborns and at 1 month HR is higher in AS than in QS (p<0.001); B) there is no significant effect of age for breathing rate in QS, in AS breathing rate is higher at 1 month than in newborns (p<0.03), at both ages breathing rate is higher in AS than in QS (p<0.001); C) in QS systolic BP is lower at 1 month (p<0.03); D) diastolic BP is lower at 1 month in both sleep states (p<0.001).
For breathing rate (Figure 2B) in QS, there were 185 measurements (172 infants, 13 at both ages). There was no significant effect of age. For breathing rate in AS, there were 659 measurements (475 infants, 184 at both ages). Breathing rate in AS was ~1.5 breaths/min higher at 1 month (p<0.03). During the newborn period BR was ~9.2 breaths/min higher in AS than in QS (p<0.001; 433 observations, 385 subjects, 48 with both states). At 1 month, breathing rate was ~8.4 breaths/min higher in AS than in QS (p<0.001; 411 observations = 411, 354 subjects, 57 with both states).
Two baseline BP measurements were made but the first often aroused infants; thus, only the second was used for analyses. For QS systolic BP (Figure 2C) there were 93 measurements (89 infants, 4 at both ages). There was a significant effect of age (p<0.03) with QS systolic BP estimated to be 3.5 mmHg lower at 1 month. For systolic BP in AS there was no significant difference between ages (305 measurements, 267 infants, 37 at both ages). For QS diastolic BP (Figure 2D) there were 94 measurements (90 infants, 4 at both ages). There was a significant effect of age (p<0.001) with QS diastolic BP ~8.0 mmHg lower at 1 month. For AS diastolic BP there were 310 measurements (272 infants, 38 at both ages). Diastolic BP in AS was ~5.2 mmHg lower at 1 month (p<0.001).
Because there was only one BP measurement at baseline a given subject could not have results from both sleep states. Therefore, t-tests were used to assess state differences in BP. In newborns there was no significant difference in systolic BP between QS and AS. At 1 month, systolic BP was ~6.7 mmHg higher in AS than in QS (p<0.001). Diastolic BP in newborns was ~3.5 mmHg higher in AS than in QS (p<0.02). At 1 month, diastolic BP was ~5.8 mmHg higher in AS than in QS (p<0.001).
For QS global HR variability (SD-RRi, Figure 3A) there were 191 measurements (179 infants, 12 at both ages). There was a significant effect of age (p<0.001) with QS SD-RRi ~0.005 sec lower at 1 month than in newborns. For AS SD-RRi there were 663 measurements (477 infants, 186 at both ages) and SD-RRi was ~0.005 sec lower at 1 month (p<0.001). For newborns, SD-RRi was ~0.006 sec higher in AS than in QS (p<0.001; 435 observations, 385 subjects, 50 with both states). At 1 month, SD-RRi was ~0.006 sec higher in AS than in QS (p<0.001; 419 observations, 359 subjects, 60 with both sleep states).
Figure 3.
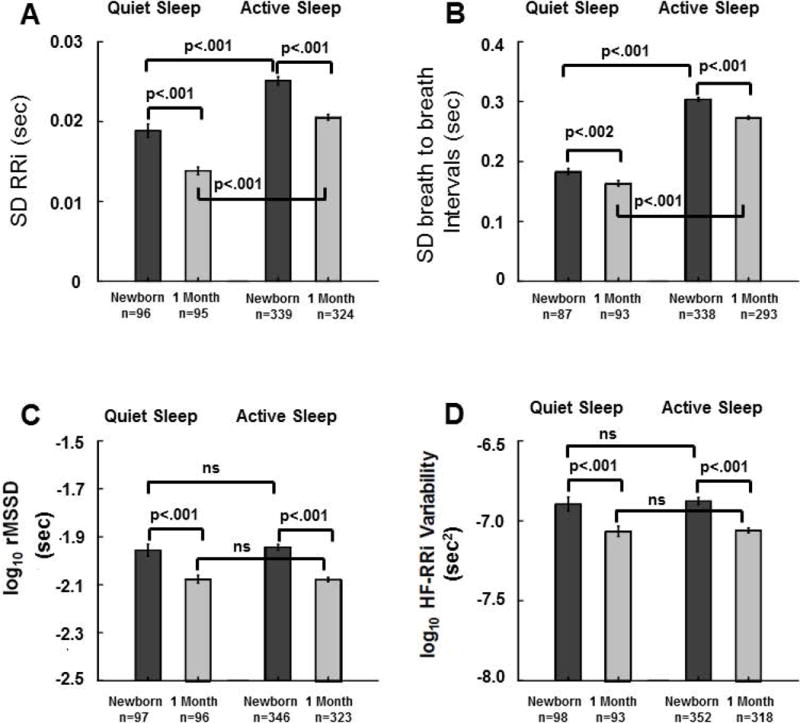
Means±SE for Active Sleep (AS) and Quiet Sleep (QS) standard deviation of r-wave to r-wave intervals (SD-RRi) (A), standard deviation of breath to breath intervals (SD-BBi) (B), root mean square of successive differences in RRi, a time domain measure beat to beat variability in heart rate (rMSSD of RRi (log10)) (C), and a spectral measure of high frequency variability in RRi (HF-RRi variability (log10)) (D) during the 10 minute baseline prior to head–up tilts for newborns and at 1 month. Values were computed after removal of statistical outliers. Results from mixed models regression indicated the following significant effects: A) in both QS and AS SD-RRi is lower at 1 month (p<0.001), in newborns and at 1month SD-RRi is higher in AS than in QS (p<0.001); B) for breathing rate variability there was a significant decrease with age in QS and AS (p<0.002, p<0.001), and SD-BBi was significantly higher in AS than QS at both ages (p<0.001); C) the time domain measure of beat-to-beat HRV (rMSSD) was significantly lower at 1 month for both sleep states (p<0.001), and there were no significant effects of sleep state at either age; D) the spectral measure of high frequency variability in HR (HF-RRi) was significantly lower at 1 month in both QS (p<0.002) and AS (p<0.001), andthere were no significant effects of sleep state on HF-RRi at either age.
For QS breathing rate variability (SD-BBi, Figure 3B) there were 180 measurements (168 infants, 12 at both ages). There was a significant effect of age (p<0.002) with QS SD-BBi ~0.020 sec lower at 1 month. In AS there were 631 measurements (461 infants, 170 at both ages) and SD-BBi in AS was ~0.030 sec lower at 1 month (p<0.001). In newborns, SD-BBi was ~0.119 sec higher in AS than in QS (p<0.001; 425 observations, 381 subjects, 44 with both states). At 1 month, SD-BBi was ~0.109 sec higher in AS than in QS (p<0.001; 486 observations, 335 subjects, 51 with both states).
For the time domain measure of beat-to-beat HRV (rMSSD, Figure 3C) in QS there were 193 measurements (180 infants, 13 at both ages). There was a significant effect of age (p<0.001) with QS rMSSD ~0.121 (log10 msec) lower at 1 month. In AS there were 669 measurements (478 infants, 191 at both ages) and rMSSD in AS was ~0.139 (log10 msec) lower at 1 month (p<0.001). There was no significant effect of sleep state on rMSSD during the newborn period (443 observations, 392, subjects, 51 with both states) or at 1 month (419 observations, 361 subjects = 361, 58 with both states).
For the spectral measure of high frequency variability in HR (HF-RRi, Figure 3D) in QS there were 191 measurements (178 infants, 13 at both ages). There was a significant effect of age (p<0.002) with QS HF-RRi ~0.173 (log10 msec2) lower at 1 month. In AS there were 670 measurements (479 infants, 191 at both ages) and HF-RRi in AS was ~0.194 (log10 msec2) lower at 1 month (p<0.001). There was no significant effect of sleep state on HF-RRi either in newborns (450 observations, 398 subjects, 52 with both states) or at 1 month (411 observations, 354 subjects, 57 with both sleep states).
Effects of Age and Sleep State; Head-up Tilt Data (Figures 4 and 5)
Figure 4.
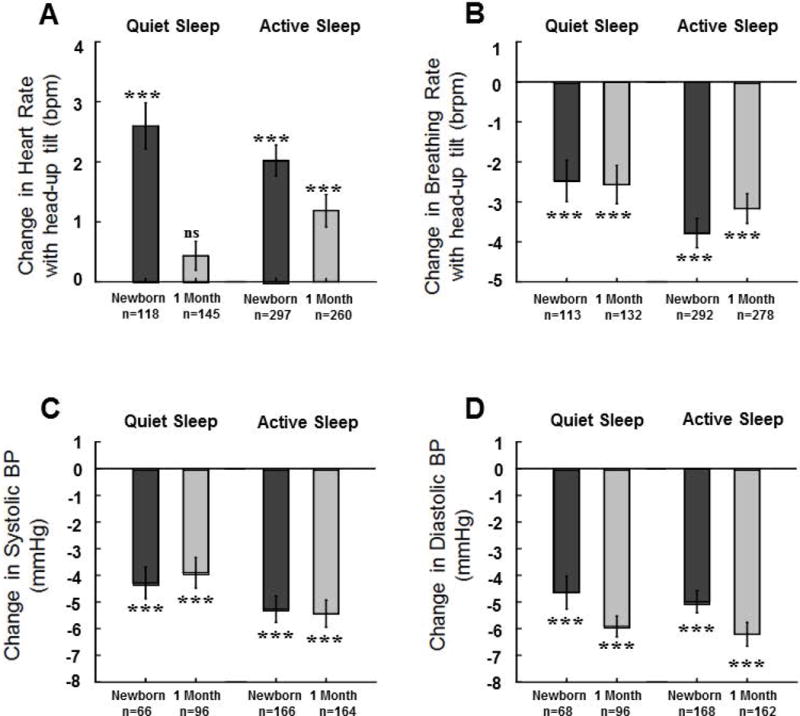
Means±SE changes from baseline following 45° head-up tilts for Active Sleep (AS) and Quiet Sleep (QS) heart rate (HR) (A), breathing rate (B), systolic BP (C), and diastolic BP (D) for newborns and at 1 month. Values were computed after removal of statistical outliers. *** indicates changes following tilts were significant at the p<0.001 level, ns indicates the change was not significant. Results from mixed models regression indicated the following additional significant effects: A) changes in HR with tilt were greater in newborns than at 1 month for QS (p<0.001) and AS (p<0.02); B) there were no significant effects of age on changes in breathing rate, the decrease in breathing rate with head-up tilting in newborns was greater in AS than in QS (p<0.03), at 1 month there was no significant effect of sleep state; C) there were no significant effects of age for changes in systolic BP in either sleep state; D) changes in diastolic BP in AS were greater at 1 month than in newborns (p<0.04).
Figure 5.
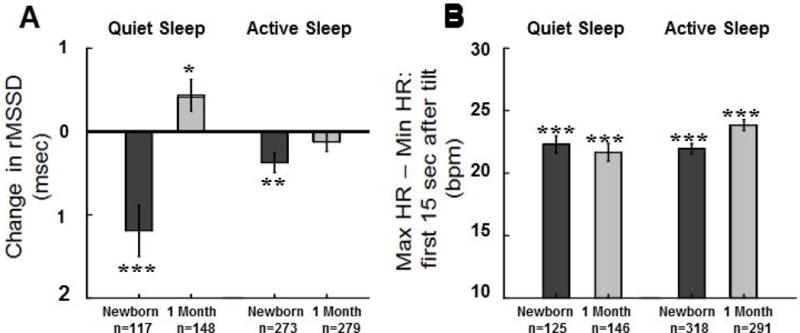
Means±SE changes from baseline following 45° head-up tilts during AS and QS in the root mean square of successive differences in RRi (rMSSD) A), and maximum – minimum heart rate B) for newborns and at 1 month. Values were computed after removal of statistical outliers. *, **, and *** indicates changes were significant at p<0.05, p<0.01, and p<0.001 levels respectively, ns indicates the change was not significant. Results from mixed models regression indicated the following significant effects: A) there was a significant difference in the change in rMSSD between newborn and 1 month of age (p<0.001), in newborns the decrease in rMSSD was greater in QS than in AS (p<0.002), at 1 month, there was a significant difference in the change in rMSSD between AS and QS (p<0.003); B) maximum – minimum HR following tilt showed no significant effect of age during QS, but in AS this HR response was greater at 1 month than in newborns (p<0.002), this measure of HR response to head-up tilting did not differ by state in the newborn period but was greater in AS at 1 month of age (p<0.002).
There were significant increases in HR with head-up tilt in both sleep states in the newborn period and at 1 month (within-subjects t-tests of change scores, p<0.001). However, at 1 month, in QS, there were no significant HR changes with head-up tilt (Figure 4A). In QS, there were 263 measurements (226 infants, 37 at both ages). There was a significant effect of age (p<0.001) with change in HR ~2.2 bpm greater in newborns. For change in HR following tilt in AS there were 557 measurements (420 infants, 137 at both ages) and change in HR in AS was ~0.8 bpm greater in newborns (p<0.02). There was no significant effect of state in change in HR either for newborns (415 observations, 341 subjects, 74 with both states) or at 1 month (405 observations, 314 subjects, 91 with both sleep states).
There were significant decreases in breathing rate (Figure 4B) with head-up tilt in both sleep states at both ages (within subjects t-tests of change scores, p<0.001). For QS breathing rate following head-up tilt there were 245 measurements (211, 34 at both ages). There was no significant effect of age. In AS there were 570 measurements (432 infants, 138 at both ages) with no significant effect of age. In newborns, the decrease in breathing rate was ~1.4 breaths per minutegreater in AS than in QS (p<0.03; 405 observations 342 subjects, 63 with both states). At 1 month, there was no significant effect of sleep state in breathing rate (410 observations, 318 subjects, 92 with both states).
As shown in Figures 4C and 4D, there were significant decreases in both systolic and diastolic BP with head-up tilts in both sleep states at both ages (within-subjects t-tests of change scores, p<0.001). For systolic BP (Figure 4C) following tilt in QS there were 162 measurements (143 infants, 19 at both ages) with no significant effect of age. In AS there were 330 measurements (284 infants, 46 at both ages) with no significant effect of age.
For QS diastolic BP following tilt (Figure 4D) there were 164 measurements (147 infants, 17 at both ages) with a nearly significant effect of age (p<0.07); decreases in BP at 1month were slightly greater (~1.3 mmHg) than in newborns. In AS there were 330 measurements (285 infants, 45 at both ages). The decreases in BP at 1 month were significantly greater (~1.3 mmHg, p<0.04) than in newborns.
In newborns and at 1 month there were 492 measurements of change in systolic BP from 329 infants, 163 in both sleep states. The decreases in systolic BP were ~1.3 mmHg greater in AS at both ages (p<0.05). There were no significant effects of sleep state at either age with regard to changes in diastolic BP following tilt.
In newborns, the rMSSD measure of beat-to-beat variability showed significant decreases following head-up tilts in both QS and AS (Figure 5A, within subjects t-tests of change scores, p<0.001, p<0.01 respectively). At 1 month, rMSSD increased significantly in QS (within-subjects t-tests of change scores, p<0.05) and showed no significant change in AS. For rMSSD following tilt in QS there were 265 measurements (227 infants, 38 at both ages). There was a significant difference in the change in rMSSD between newborn and 1 month of age of ~1.6 msec (p<0.001). In AS there were 552 measurements (415 infants, 137 at both ages) and there was no significant effect of age. In newborns the decrease in rMSSD was (~0.8 msec) greater in QS than in AS (p<0.002; 390 observations, 329 subjects = 329, 61with both sleep states). At 1 month, there was a significant (~0.6 msec) difference in the change in rMSSD between AS and QS (p<0.003, 427 observations, 325 subjects, 102 with both sleep states). As noted above, rMSSD increased significantly in QS at 1 month with no change in AS.
A pattern of acute increases in HR followed by decreases is typical of normal infants(10, 15) and, in adults, is mediated by vagal withdrawal and activation(26). These acute, biphasic responses to tilt have been measured by others as changes occurring over a certain number of heart beats following the tilt (15). However, given that HR changes with age, we opted to assess these changes within a given time frame (i.e. 15secs). These acute responses to tilt are, by definition always positive, i.e. maximum – minimum HF (Figure 5B). This measure of dynamic range of HR response to tilt showed no significant effect of age during QS (271 observations=271, 230 subjects, 41 at both ages= 41). In AS this HR response was ~1.9 bpm greater at 1 month versus newborn (p<0.002, 609 observations, 445 subjects, 164 at both ages). This measure of HR tilt response did not differ by state in the newborn period (443 observations, 367 subjects = 367, 73 with both sleep states), but was ~2.4 bpm greater in AS at 1 month of age (p<0.002, 437 observations, 334 subjects, 103 with both sleep states).
Unfortunately, it was not possible to study infants in both the prone and supine sleep positions in comparison their normal sleep position. However, at the 1 month visit, mothers were asked in what position their infants were placed (prone, side, supine) on the previous night. Of the 477 who answered this question, 117 said “prone” (24.5%), 200 (41.9%) said “side”, and 160 (33.5%) said “supine”. We tested whether usual sleep position affected the 28 physiological variables (8 during baseline × 2 states + 6 following tilt × 2 sleep states) measured at 1 month. Only 2 variables were found to be significantly related to the prior night’s sleep position. First, baseline diastolic blood pressure during active sleep was lowest in infants who, the night before, slept in the supine position (prone = 44.8, side= 47.0, supine = 43.7; group effect, p<0.05). That is, infants who changed sleep position from low risk to high risk had the lowest BPs. Second, the decrease in systolic blood pressure following head-up tilting during quiet sleep while prone was greatest in babies who had slept in the prone position the night before (prone = −6.1, side= −3.8, supine = −0.3; group effect, p<0.05).
DISCUSSION
A major goal of the Safe Passage Study was to obtain physiological data on as many of the ~12,000 enrolled subjects as possible during early infant time periods. This current report focusses on measurement of infant HR, respiration, and BP during a baseline period and in response to a sequence of three 45° head-up tilts in a subset of unexposed infants. There were many obstacles to conducting a standardized protocol across two continents over a period of nearly a decade. However, ~77% (510/666) of the subset of unexposed infants were assessed at either the newborn or 1 month time points and some data were obtained in ~97% of these infants. The physiological results obtained are in agreement with a number of prior studies and also provide new findings with regard to effects of age and sleep state on newborn and 1 month cardiorespiratory physiology.
Many studies have made measurements of infant physiology, and prior to one year of age most of these were conducted during sleep. Three prior studies report data similar to the current study. One of these assessed newborns and infants at 2 months of age, although these results were without regard to sleep state(16). In this study mean HR and breathing rate at the newborn period were 123 bpm and 46 breaths per minute respectively, and 133 bpm and 37 breaths per minute at 2 months. While the newborn data from this prior study are similar to the current results (Table 1), heart and breathing rates of the 1 month old infants in the current study are considerably higher than the rates recorded at 2 months in the prior work, suggesting the high rates recorded at 1 month in the current study may be transient. Another study reported baseline HR and HRV by sleep state for newborn infants from a Northern Plains (South Dakota) American Indian reservation (27). Values for mean HR in QS and AS in this study (124 and 128 bpm respectively), SD-RRi (22.4 and 28.4 msec QS and AS respectively), and rMSSD (10.3 and 9.5 msec QS and AS respectively) are similar to those in the current report (Table 1). Values reported here for newborn and 1 month HR during sleep and those for breathing rate during QS are within a few beats/min and breaths/min of normative values derived from a systematic review of many published studies(28); however, values for breathing rates in AS in the Safe Passage Study are about 10 breaths/min faster that those found in this review. Perhaps sleep position (prone) and/or time after feed (~30 minutes) in the Safe Passage Study might be related to this discrepancy, but it is not clear why this would be more apparent in AS.
Another report on infant physiology provided normative values for newborn infant HR and RRi variability when recorded over a 24 hour period without regard to sleep state(29). The 5th and 95th percentiles for HR were 114 bpm and 143 bpm, and for rMSSD, these thresholds were 1.15 and 1.52 (log10 msec; i.e. 14 and 33 msec) respectively. These HR means are similar to those in the current study (see Table 1; 5th and 95th for HR in AS 109 and 149 bpm). However, the 5th and 95th for log10rMSSD in AS, −2.32 and −1.55 (5 and 28 msec) respectively, in the current study are lower than in the Mehta et al. report. In addition, the 5th and 95th percentiles for SD of RR-intervals in the prior report were 30 and 75 msec respectively which are considerably greater than the 11 and 42 msec values reported here. However, the 24 hour recordings of Mehta and colleagues included periods of awake, transitional sleep and crying, all of which would contribute to increased variability in HR. In addition to the most comparable studies above, there are other reports of infant cardiorespiratory physiology, both during sleep and following head-up tilts(3–11). Although none of these has recorded data at exactly the same ages or for all of the same variables as in the Safe Passage Study, the values for comparable measures are quite similar to those reported here. Taken together, the Safe Passage Study norms are well within values established in the literature.
Results from the current analyses demonstrate that full term infants not exposed prenatally to alcohol, maternal smoking or drugs of abuse, show significant changes in cardiorespiratory control as infants enter the period of increased risk for SIDS, some of which are sleep state dependent. This is of relevance since SIDS typically occurs in a sleep period. Of particular note are the dramatic 15 to 20 bpm increases in HR, in both sleep states, from the newborn to 1 month assessments. Although we have previously noted these substantial increases in HR as infants enter the period of increased risk for SIDS(16), what has not been reported is that over this same period BP, particularly diastolic pressure, decreases. Thus, at 1 month of age, infants may have more difficulty responding to conditions that decrease BP and/or increase HR and cardiac output. This is notable in light of the proposed circulatory failure mechanism for SIDS(30, 31). Consistent with a diminished capacity to respond to physiological challenges at this age, the current data replicates previous results from our group showing smaller HR responses to head-up tilt in the 1 to 2 month old age range(16). In QS at 1 month, the absence of significant increases in HR in response to head-up tilting was paralleled by increases in both time and frequency domain indices of parasympathetic activity (rMSSD, HF-RRi variability). Since vagal withdrawal is the expected response to this challenge, which is seen in newborns, this finding suggests that at 1 month of age babies are responding to the challenge of a decrease in BP with an unusual physiological response mode, i.e. vagal activation. Future analyses will elucidate effects of prenatal alcohol and smoking on these physiological response biomarkers and their potential association with SIDS with the ultimate goals of helping women, families, physicians, and scientists find ways to improve pregnancy outcomes and infant health.
Limitations
Although mixed models regression analyses, using a combination of repeated and cross-sectional measures, revealed robust changes in physiological parameters, it was not always possible to obtain repeated physiological measures during the newborn and 1 month old time points and thus, characterize individual differences in developmental patterns. This limitation was especially true for data in QS for which only 13 infants had data in this state at both ages.
In order to insure a high degree of generalizability of results, the Safe Passage Study was intentionally designed to include a wide range of geographic, ethnic, racial, SES and prenatal exposure backgrounds. This design also dictates that the normative results presented here, including outlier cutoffs, may be broader than those that would be obtained from more narrowly defined cohorts. However, because of this heterogeneity, the ranges reported here are likely to encompass the ranges of more restricted samples. In addition, robust effects of age and state reported here were found even given the heterogeneity of the Safe Passage sample and are thus likely to be representative of more homogeneous cohorts.
The Safe Passage Study employed rigorous and detailed methodologies for documenting exposures. As in virtually all prenatal exposure studies, assessment of exposure relied on maternal self-report. It is worthy of note, however, that, in limited samples, meconium biomarkers of alcohol exposure provided good agreement with the self-report measures(32). Yet, in the current study, we must assume that there is some degree of exposure under-reporting.
KEY NOTES.
The Safe Passage Study from the Prenatal Alcohol in SIDS and Stillbirth Network successfully applied a standard physiological protocol in a large population of infants from the US and South Africa.
Healthy infants with low prenatal adverse exposures show significant developmental changes in cardiorespiratory function from birth to one month of age including diminished heart rate responses to head up tilt and low resting diastolic blood pressure.
At 1 month of age, infants may have more difficulty responding to conditions that decrease blood and/or increase heart rate.
Acknowledgments
The authors gratefully acknowledge the cooperation of the study participants, PASS investigators, the PASS Steering Committee Chairman Gary D.V. Hankins, MD, and members of the NICHD advisory safety monitoring board: Elizabeth Thom, PhD (Chair); Reverend Phillip Cato, PhD; James W. Collins, Jr, MD, MPH; Terry Dwyer, MD, MPH; George Macones, MD; Philip A. May, PhD; Jeff Murray, MD; Richard M. Pauli, MD, PhD; Raymond W. Redline, MD; and Michael Varner, MD.
The PASS Research Network is supported by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Institute on Deafness and Other Communication Disorders through the Cooperative Agreement Mechanism (U01HD055154, U01 HD045935, U01 HD055155, U01HD045991, and U01 AA016501).
FUNDING: This research was supported by grants U01HD055154, U01HD045935, U01HD055155, U01HD045991 and U01AA016501 issued by the National Institute on Alcohol Abuse and Alcoholism, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the National Institute on Deafness and Other Communication Disorders. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service or the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Development, the National Institute on Alcohol Abuse and Alcoholism, or the National Institute on Deafness and Other Communication Disorders.
ABBRREVIATIONS
- PASS
Prenatal Alcohol in SIDS and Stillbirth
- SIDS
sudden infant death syndrome
- FASD
fetal alcohol spectrum disorders
- HR
heart rate
- f
breathing frequency
- HRV
heart rate variability
- BP
blood pressure
- EEG
electroencephalographic
- g
grams
- h
hours
- PMA
postmenstrual age
- TLFB
Time Line Follow Back
- ECG
electrocardiogram
- AS
active sleep
- QS
quiet sleep
- RRi
R-wave to R-wave interval
- SD
standard deviation
- HF
high frequency
- rMSSD
square root of the mean of the squared successive differences in RRis
- Hz
Hertz
- bpm
beats per minute
- msec
milliseconds
The following institutions and researchers, in addition to those listed as authors on this paper, comprise the PASS Network.
DCAC: Co-Director: Lisa M. Sullivan, PhD; Biostatistics: Tara Tripp, MA; Project Management/Regulatory Affairs: Julie M. Petersen, BA, Rebecca A. Young, MPH; Data Management/Information Technology: Travis Baker, BS, Derek Petersen, BS, Gregory Toland MS.
DBPC: Director: Hannah C. Kinney, MD, Assistant Director: Robin L. Haynes, PhD; Co- investigators: David S. Paterson, PhD, Kevin G. Broadbelt, PhD, Kyriacos Markianos, PhD, Ingrid A.Holm, MD, Theonia Boyd, MD, Drucilla Roberts,MD, Richard G. Goldstein, MD, Hanno Stein, PhD; Technicians: Claire Maggiotto, BS, Catherine Hassett, BS.
CCS NP: Co-investigators: Donald Habbe, MD, H. Eugene Hoyme, MD, William Massello III, MD, Bradley Randall, MD, Mary Ann Sens, MD, PhD, Catherine Stoos, MD, Peter Van Eerden, MD; Project Management: Whitney Adler,BA, Elizabeth Berg, RN, Jessica Gromer, RN, Bethany Norton, MA, Liz Swenson, RN, Deb Tobacco, MA.
CCS SA: Project Management: Erna Carstens, RN, Jean Coldray, Nat Dipl, Mandy Potter,RN, Lucy Brink, MSc, Rosemary Meyer, BTech, Carlie du Plessis, RN, Elaine Geldenhuys, Nat Dipl
PAC: Project Management: Carmen Condon, BA, Johnston T. Grier, MS, Daianna Rodriguez, BA; Data Analysis: Emilia F. Vignola, BA, Margaret C. Shair, BA, Tracy Thai, MA and Joseph J. Violaris, MD.
NIH: Project Scientists: Marian Willinger, PhD (NICHD), Dale Herald, PhD (NIAAA), Howard J. Hoffman, PhD (NIDCD), Chuan-Ming Li, MD, PhD (NIDCD); Program Officers: Bill Dunty, PhD (NIAAA), Tonse Raju, MD, DCH (NICHD), Gordon B. Hughes, MD (NIDCD)
Further, the following individuals made significant contributions to the research and warrant recognition: DCAC: Idania Ramirez, MPH, Jamie Collins, MA, Laura Spurchise, MPH; DBPC: Richard A. Belliveau, BA, Kristin McMillan, BA, Megan Minter, MS.
The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service (IHS) or the National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Development (NICHD), the National Institute on Alcohol Abuse and Alcoholism (NIAAA), or the National Institute on Deafness and Other Communication Disorders (NIDCD).
Footnotes
CONFLICT OF INTEREST: The authors have no conflicts of interest to declare.
References
- 1.Carlin RF, Moon RY. Risk Factors, Protective Factors, and Current Recommendations to Reduce Sudden Infant Death Syndrome: A Review. JAMA Pediatr. 2017;171:175–80. doi: 10.1001/jamapediatrics.2016.3345. [DOI] [PubMed] [Google Scholar]
- 2.Kinney HC, Richerson GB, Dymecki SM, Darnall RA, Nattie EE. The brainstem and serotonin in the sudden infant death syndrome. Annu Rev Pathol. 2009;4:517–50. doi: 10.1146/annurev.pathol.4.110807.092322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Franco P, Lipshut W, Valente F, Adams S, Groswasser J, Kahn A. Cardiac autonomic characteristics in infants sleeping with their head covered by bedclothes. J Sleep Res. 2003;12:125–32. doi: 10.1046/j.1365-2869.2003.00340.x. [DOI] [PubMed] [Google Scholar]
- 4.Horne RS, Parslow PM, Harding R. Respiratory control and arousal in sleeping infants. Paediatr Respir Rev. 2004;5:190–8. doi: 10.1016/j.prrv.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Horne RS, Witcombe NB, Yiallourou SR, Scaillet S, Thiriez G, Franco P. Cardiovascular control during sleep in infants: Implications for Sudden Infant Death Syndrome. Sleep Med. 2010;11:615–21. doi: 10.1016/j.sleep.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Kahn A, Groswasser J, Rebuffat E, Sottiaux M, Blum D, Foerster M, et al. Sleep and cardiorespiratory characteristics of infant victims of sudden death: a prospective case-control study. Sleep. 1992;15:287–92. doi: 10.1093/sleep/15.4.287. [DOI] [PubMed] [Google Scholar]
- 7.Schechtman VL, Raetz SL, Harper RK, Garfinkel A, Wilson AJ, Southall DP, et al. Dynamic analysis of cardiac R-R intervals in normal infants and in infants who subsequently succumbed to the sudden infant death syndrome. Pediatr Res. 1992;31:606–12. doi: 10.1203/00006450-199206000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Schechtman VL, Harper RK, Harper RM. Development of heart rate dynamics during sleep-waking states in normal infants. Pediatr Res. 1993;34:618–23. doi: 10.1203/00006450-199311000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Galland BC, Reeves G, Taylor BJ, Bolton DP. Sleep position, autonomic function, and arousal. Arch Dis Child Fetal Neonatal Ed. 1998;78:F189–94. doi: 10.1136/fn.78.3.f189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Edner A, Katz-Salamon M, Lagercrantz H, Milerad J. Heart rate response profiles during head upright tilt test in infants with apparent life threatening events. Arch Dis Child. 1997;76:27–30. doi: 10.1136/adc.76.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen G, Vella S, Jeffery H, Lagercrantz H, Katz-Salamon M. Cardiovascular stress hyperreactivity in babies of smokers and in babies born preterm. Circulation. 2008;118:1848–53. doi: 10.1161/CIRCULATIONAHA.108.783902. [DOI] [PubMed] [Google Scholar]
- 12.Dukes KA, Burd L, Elliott AJ, Fifer WP, Folkerth RD, Hankins GD, et al. The safe passage study: design, methods, recruitment, and follow-up approach. Paediatr Perinat Epidemiol. 2014;28:455–65. doi: 10.1111/ppe.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobell MB, Sobell LC, Klajner F, Pavan D, Basian E. The reliability of a timeline method for assessing normal drinker college students’ recent drinking history: utility for alcohol research. Addict Behav. 1986;11:149–61. doi: 10.1016/0306-4603(86)90040-7. [DOI] [PubMed] [Google Scholar]
- 14.Willinger M, Hoffman HJ, Hartford RB. Infant sleep position and risk for sudden infant death syndrome: report of meeting held January 13 and 14, 1994, National Institutes of Health, Bethesda, MD. Pediatrics. 1994;93:814–9. [PubMed] [Google Scholar]
- 15.Yiallourou SR, Walker AM, Horne RS. Prone sleeping impairs circulatory control during sleep in healthy term infants: implications for SIDS. Sleep. 2008;31:1139–46. [PMC free article] [PubMed] [Google Scholar]
- 16.Fifer WP, Greene M, Hurtado A, Myers MM. Cardiorespiratory responses to bidirectional tilts in infants. Early Hum Dev. 1999;55:265–79. doi: 10.1016/s0378-3782(99)00026-2. [DOI] [PubMed] [Google Scholar]
- 17.Korten JB, Haddad GG. Respiratory waveform pattern recognition using digital techniques. Computers in biology and medicine. 1989;19:207–17. doi: 10.1016/0010-4825(89)90009-7. [DOI] [PubMed] [Google Scholar]
- 18.Harper RM, Hoppenbrouwers T, Sterman MB, McGinty DJ, Hodgman J. Polygraphic studies of normal infants during the first six months of life. I. Heart rate and variability as a function of state. Pediatr Res. 1976;10:945–8. doi: 10.1203/00006450-197611000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Horne RS. Cardio-respiratory control during sleep in infancy. Paediatr Respir Rev. 2014;15:163–9. doi: 10.1016/j.prrv.2013.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Isler JR, Thai T, Myers MM, Fifer WP. An automated method for coding sleep states in human infants based on respiratory rate variability. Dev Psychobiol. 2016;58:1108–15. doi: 10.1002/dev.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harper RM, Schechtman VL, Kluge KA. Machine classification of infant sleep state using cardiorespiratory measures. Electroencephalography and clinical neurophysiology. 1987;67:379–87. doi: 10.1016/0013-4694(87)90126-x. [DOI] [PubMed] [Google Scholar]
- 22.Hedman AE, Hartikainen JE, Tahvanainen KU, Hakumaki MO. The high frequency component of heart rate variability reflects cardiac parasympathetic modulation rather than parasympathetic ‘tone’. Acta physiologica Scandinavica. 1995;155:267–73. doi: 10.1111/j.1748-1716.1995.tb09973.x. [DOI] [PubMed] [Google Scholar]
- 23.Spiers JP, Silke B, McDermott U, Shanks RG, Harron DW. Time and frequency domain assessment of heart rate variability: a theoretical and clinical appreciation. Clin Auton Res. 1993;3:145–58. doi: 10.1007/BF01819000. [DOI] [PubMed] [Google Scholar]
- 24.DeBoer RW, Karemaker JM, Strackee J. Comparing spectra of a series of point events particularly for heart rate variability data. IEEE Trans Biomed Eng. 1984;31:384–7. doi: 10.1109/TBME.1984.325351. [DOI] [PubMed] [Google Scholar]
- 25.Harris FJ. On the use of windows for harmonic analysis with the discrete Fourier transform. Proc IEEE. 1978;66:51–83. [Google Scholar]
- 26.Ewing DJ, Hume L, Campbell IW, Murray A, Neilson JM, Clarke BF. Autonomic mechanisms in the initial heart rate response to standing. Journal of applied physiology: respiratory, environmental and exercise physiology. 1980;49:809–14. doi: 10.1152/jappl.1980.49.5.809. [DOI] [PubMed] [Google Scholar]
- 27.Fifer WP, Fingers ST, Youngman M, Gomez-Gribben E, Myers MM. Effects of alcohol and smoking during pregnancy on infant autonomic control. Dev Psychobiol. 2009;51:234–42. doi: 10.1002/dev.20366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fleming S, Thompson M, Stevens R, Heneghan C, Pluddemann A, Maconochie I, et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review of observational studies. Lancet. 2011;377:1011–8. doi: 10.1016/S0140-6736(10)62226-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mehta SK, Super DM, Connuck D, Salvator A, Singer L, Fradley LG, et al. Heart rate variability in healthy newborn infants. Am J Cardiol. 2002;89:50–3. doi: 10.1016/s0002-9149(01)02162-2. [DOI] [PubMed] [Google Scholar]
- 30.Harper RM, Bandler R. Finding the failure mechanism in Sudden Infant Death Syndrome. Nat Med. 1998;4:157–8. doi: 10.1038/nm0298-157. [DOI] [PubMed] [Google Scholar]
- 31.Matthews T. Sudden infant death syndrome–a defect in circulatory control? Child: care, health and development. 2002;28(Suppl 1):41–3. doi: 10.1046/j.1365-2214.2002.00012.x. [DOI] [PubMed] [Google Scholar]
- 32.Himes SK, Dukes KA, Tripp T, Petersen JM, Raffo C, Burd L, et al. Clinical sensitivity and specificity of meconium fatty acid ethyl ester, ethyl glucuronide, and ethyl sulfate for detecting maternal drinking during pregnancy. Clin Chem. 2015;61:523–32. doi: 10.1373/clinchem.2014.233718. [DOI] [PMC free article] [PubMed] [Google Scholar]


