Abstract
Patients who have liver cirrhosis and liver cancer also have reduced farnesoid X receptor (FXR). The current study analyzes the effect of diet through microbiota that affect hepatic inflammation in FXR knockout (KO) mice. Wild-type and FXR KO mice were on a control (CD) or Western diet (WD) for 10 months. In addition, both CD- and WD-fed FXR KO male mice, which had hepatic lymphocyte and neutrophil infiltration, were treated by vancomycin, polymyxin B, and Abx (ampicillin, neomycin, metronidazole, and vancomycin). Mice were subjected to morphological analysis as well as gut microbiota and bile acid profiling. Male WD-fed FXR KO mice had the most severe steatohepatitis. FXR KO also had reduced Firmicutes and increased Proteobacteria, which could be reversed by Abx. In addition, Abx eliminated hepatic neutrophils and lymphocytes in CD-fed, but not WD-fed, FXR KO mice. Proteobacteria and Bacteroidetes persisted in WD-fed FXR KO mice even after Abx treatment. Only polymyxin B could reduce hepatic lymphocytes in WD-fed FXR KO mice. The reduced hepatic inflammation by antibiotics was accompanied by decreased free and conjugated secondary bile acids as well as changes in gut microbiota. Our data revealed that Lactococcus, Lactobacillus, and Coprococcus protect the liver from inflammation.
The liver is constantly exposed to gut-derived components as 70% of hepatic blood supply comes from enterohepatic circulation. Gut-derived signaling dictates liver health. Bacteria-generated lipopolysaccharide (LPS), which stimulates IL-6–mediated Janus activating kinase 2–STAT3 activation, is essential for liver regeneration.1 However, excessive LPS causes hepatic inflammation and dampens liver regeneration capability.2, 3 Moreover, the transition of steatosis to steatohepatitis plays a crucial role in hepatocyte proliferation and liver carcinogenesis. Thus, it is important to understand the effect of the gut in contributing to liver inflammation. Increased hepatic inflammatory signaling can be due to excessive lipid and sugar intake that generates reactive oxygen species, stressing the metabolic system.4 It can also be due to inappropriate activation and homing of neutrophils.5 Moreover, hepatic inflammation is associated with intestinal bacterial overgrowth, increased gut permeability, and reduced immunological defenses. Therefore, gut-derived signaling has a significant impact on the liver health.3, 6
Bile acids (BAs) are no longer considered solely for lipid absorption; they are signaling molecules with diverse effects on regulating host immunity and inflammation.6, 7, 8, 9, 10, 11, 12, 13, 14 Clinical data revealed that the receptor for BAs [ie, farnesoid X receptor (FXR)] is reduced in liver and colon cancers.15, 16 Similarly, mice that lack FXR develop spontaneous liver cancer and have increased susceptibility to colitis, cholestasis, and colon cancer.17 However, tissue-specific FXR knockout (KO) mice, including liver and intestines, do not spontaneously develop liver cancer.18, 19 These findings clearly indicate the interactive effect of hepatic and gut FXR in contributing to liver carcinogenesis. Moreover, elevated BAs found in FXR KO mice contribute to liver carcinogenesis because cholestyramine can prevent it.17, 20, 21 Because of the significance of BAs in inflammation and carcinogenesis, the current study uncovers whether the specific BAs that contribute to or protect from hepatic inflammation in response to a Western diet (WD) intake in FXR KO mice.
BAs that flow through the gut-liver axis have a pivotal role in maintaining liver and intestinal health. Free and conjugated primary and secondary BAs are generated by hepatic as well as microbial enzymes. Bile salt hydrolase in Bifidobacterium and Lactobacillus deconjugates BAs, and bacterial 7α-dehydroxylase converts primary into secondary BAs.3 Thus, eubiosis is essential for BA homeostasis. In contrast, WD-induced dysbiosis leads to dysregulated BA synthesis and increases the risk of liver and colon cancer. Therefore, there is an intimate relationship between gut microbiota and BAs, and it would be important to identify the microbiota and their associated BAs, which contribute to hepatic inflammation.
In the current study, we challenged wild-type (WT) and FXR KO mice of both sexes with a control diet (CD) and WD, followed by antibiotic treatments, to uncover gut bacteria as well as BAs that have a protective or detrimental effect on hepatic inflammation. Our data showed that WD and FXR inactivation increased hepatic inflammatory signaling with lymphocyte and neutrophil infiltration. Diet, sex, and different antibiotic treatments altered the gut microbiota and had different effects on hepatic inflammation. The specific bacteria and BAs that may contribute to or protect the liver from inflammation are identified.
Materials and Methods
Mice
Specific pathogen-free C57BL/6 mice and FXR KO mice22 of both sexes were housed in steel microisolator cages at 22°C with a 12-hour light/dark cycle and were given a CD (5.2% fat, 12% sucrose, and 0.01% cholesterol, w/w) or WD (21.2% fat, 34% sucrose, and 0.2% cholesterol, w/w) from Harlan Teklad (Madison, WI), after weaning (3 weeks, 6 to 10 mice per group) and were euthanized at the age of 10 months. Experiments were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals23 under protocols approved by the Institutional Animal Care and Use Committee of the University of California, Davis (Sacramento, CA).
Antibiotic Treatment
The treatment regimens included vancomycin (Vcm; 500 mg/L, Gram-positive coverage), polymyxin B (PolyB; 100 mg/L, Gram-negative coverage), and Abx (broad-spectrum coverage) that included ampicillin (1 g/L), neomycin (1 g/L), metronidazole (1 g/L), and vancomycin (500 mg/L) in drinking water for 12 weeks. All three treatments have been shown to disrupt the gastrointestinal microbiota.24, 25
Biochemical Analysis
Serum alanine aminotransferase (ALT; Pointe Scientific, Canton, MI) and lipopolysaccharide (Thermo Fisher Scientific, Rockford, IL) levels were quantified according to the manufacturers' instructions.
Quantification of Bile Acids
Sample preparation was performed based on published methods.11 Hepatic BAs were detected on a Prominence Ultra Fast Liquid Chromatograph system (Shimadzu, Kyoto, Japan) coupled to an API 4000 QTRAP mass spectrometer (Sciex, Redwood City, CA) operated in the negative ionization mode. Chromatography was performed on a Kinetex C18 column (50 × 2.1 mm, 2.6 μm particle size) maintained at 40°C preceded by a high-pressure column prefilter. The mobile phase consisted of a gradient of methanol delivered at a flow rate of 0.4 mL/minute. Mass spectrometer parameters were described in our previous publication.11
Gene Expression Profiling
RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) and reverse transcribed into cDNA. Real-time quantitative RT-PCR was performed on an ABI 7900HT Fast real-time PCR system using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA). The mRNA levels were normalized to the level of Gapdh mRNA.
Flow Cytometry
Freshly isolated spleen cells (106) were first incubated with Fc block and then labeled with anti-mouse antibodies, including phycoerythrin-Cy5–conjugated anti-CD62L, allophycocyanin (APC)-Cy7–conjugated anti-CD25, phycoerythrin-Cy5–conjugated anti-CD44, fluorescein isothiocyanate–conjugated anti-CD4, and APC-Cy7–conjugated CD25, APC anti-F4/80, and APC Cy7 anti-CD11b (BD Pharmingen, San Diego, CA), and Alexa Fluor 700–conjugated anti-CD8 and Pacific Blue–conjugated anti-CD44 (BioLegend, San Diego, CA) and a staining buffer consisting of 1% fetal bovine serum, 1 mmol/L EDTA, and 0.02% azide in Dulbecco's phosphate-buffered saline (Mediatech, Herndon, VA). List mode data files were collected using a Fortessa cell analyzer with FACSDiva software version 8.0 (BD Biosciences, San Jose, CA). All data sets were analyzed using FlowJo software version 10 (Tree Star Inc., Ashland, OR).
16S rRNA Gene Sequencing of Gut Microbial Communities
Cecal DNA was isolated using the ZR Fecal DNA miniprep kit (Zymo Research, Irvine, CA). Illumina sequencing of barcoded 16S rRNA gene amplicons of genomic DNA was performed based on published methods.11, 26, 27, 28 Variable region 4 of the 16S rRNA gene was amplified and sequenced. Sequence reads were analyzed using QIIME software version 1.9.1.29
Bioinformatics and Statistical Analysis
Functional profiles of microbial communities were predicted using phylogenetic investigation of communities by reconstruction of unobserved states, and linear discriminant analysis effect size was performed to identify pathways that were statistically different.30 Spearman correlations were performed with the R program (The R Foundation; http://www.r-project.org). Unpaired t-test, Mann-Whitney U test, and one-way analysis of variance were performed by using GraphPad Prism software version 6.0 (GraphPad Software, Inc., La Jolla, CA). Data are expressed as means ± SD. Differences between groups in microbiota family and genus level were calculated by Kruskal-Wallis tests. P values were adjusted for multiple comparisons using false discovery rate. P < 0.05 was considered statistically significant.
Results
The Effect of Diet and FXR Inactivation on Hepatic Phenotypes
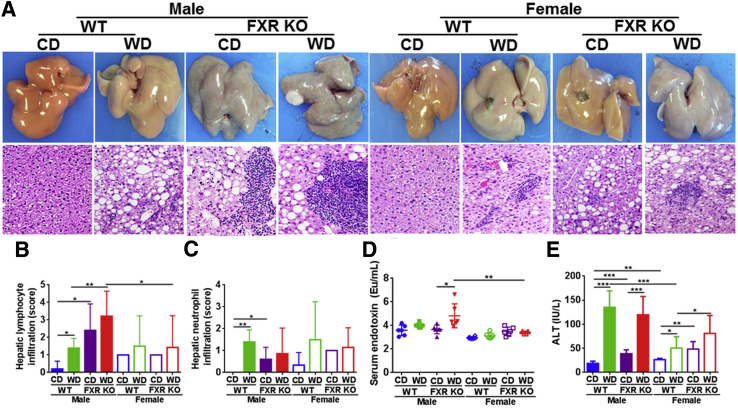
When mice were 10 months old, WD intake and FXR inactivation both induced steatosis, which was more severe in males than females (Figure 1A). However, the most striking finding was that only FXR KO mice had massive lymphocyte and neutrophil infiltration, which was more severe in males than females (Figure 1, A–C). In addition, only WD-fed male FXR KO mice had fatty adenoma, which was grossly visible (Figure 1A). Serum LPS levels were much higher in WD-fed male FXR KO mice than the female counterparts (Figure 1D). In addition, serum ALT level was increased by both WD intake and FXR inactivation (Figure 1E). Together, our data support that the induced serum endotoxin, ALT, and hepatic inflammatory cell infiltration contributed to the development of steatohepatitis in FXR KO mice in a male-dominant manner.
Figure 1.
Histology and inflammation score in control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR knockout (KO) mice of both sexes. A: Representative liver morphology and hematoxylin and eosin–stained liver sections. Hepatic lymphocyte (B) and neutrophil (C) infiltration score was graded on a scale of 0 (absent), 1 (rare), 2 (mild), 3 (moderate), and 4 (severe). Serum endotoxin (D) and alanine (E) level. Data are expressed as means ± SD (B–E)n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 [based on a single variable of comparison (ie, diet, genotype, or sex)]. Original magnification, ×40 (A). ALT, serum alanine aminotransferase.
The Effect of Antibiotics on Hepatic Inflammation
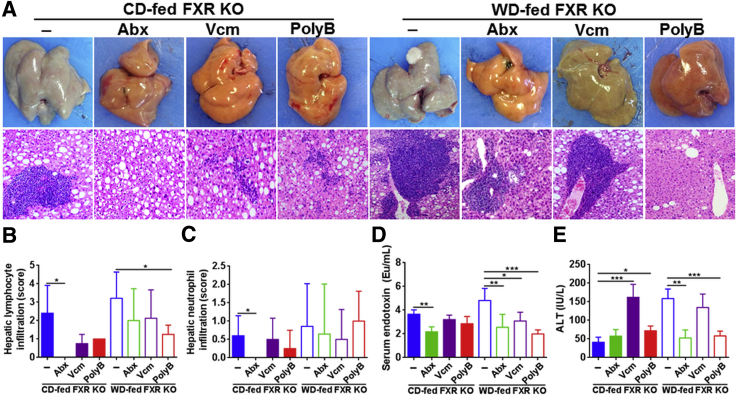
To determine the role of gut microbes in hepatic inflammation, antibiotics were used to treat male FXR KO mice. Broad-spectrum coverage Abx (a cocktail of ampicillin, neomycin, metronidazole, and vancomycin) completely prohibited neutrophils and lymphocytes in CD-fed FXR KO mice (Figure 2, A–C), which was accompanied by a reduced serum LPS level (Figure 2D). Vcm and PolyB seem to reduce hepatic lymphocytes, but the effect was not statistically significant (P = 0.07 and P = 0.11, respectively) (Figure 2B). In addition, neutrophil infiltration remained after the Vcm and PolyB treatment (Figure 2C). In contrast to CD-fed mice, Abx was not able to eliminate neutrophils and lymphocytes in WD-fed FXR KO mice. Only PolyB significantly reduced lymphocyte infiltration in WD-fed FXR KO mice, which was consistent with the reduction of serum LPS and ALT (Figure 2, D and E). However, elimination of Gram-positive bacteria by Vcm increased serum ALT in CD-fed FXR KO mice (Figure 2E).
Figure 2.
Histology and inflammation score in control diet (CD)– and Western diet (WD)–fed FXR KO mice treated with antibiotics Abx, Vcm, and PolyB. A: Representative liver morphology and hematoxylin and eosin–stained liver sections. Hepatic lymphocyte (B) and neutrophil (C) infiltration score was graded on a scale of 0 (absent), 1 (rare), 2 (mild), 3 (moderate), and 4 (severe). Serum endotoxin (D) and ALT (E) level. Data are expressed as means ± SD (B–E). n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. Original magnification, ×40 (A). ALT, serum alanine aminotransferase; PolyB, polymyxin B; Vcm, vancomycin.
The Effect of Diet and FXR Status on Hepatic Inflammatory Signaling
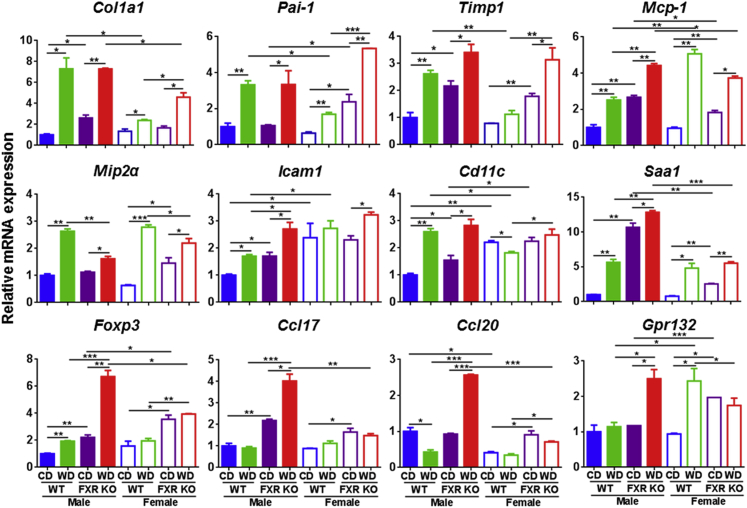
WD intake and FXR inactivation increased the mRNA levels of hepatic profibrogenic markers collagen type 1α1, plasminogen activator inhibitor-1, and tissue inhibitor of metalloproteinases (Figure 3). Similarly, monocyte chemotactic protein 1 (Mcp-1), macrophage inflammatory protein 2α, intercellular adhesion molecule 1, and leukocyte integrin α (Cd11c) mRNA levels were induced by WD or FXR KO (Figure 3). The enhanced effect due to WD plus FXR KO in inducing inflammatory signaling was revealed by the mRNA level of serum amyloid a1, forkhead box P3, chemokine (C-C motif) ligands 17 and 20, and lysophosphatidylcholine receptor [G-protein–coupled receptor (Gpr) 132], which had the highest expression levels in WD-fed FXR KO male mice, consistent with the observed liver pathology (Figure 3).
Figure 3.
Hepatic gene expression in control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR knockout (KO) mice of both sexes. Data are expressed as means ± SD. n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 [based on a single variable of comparison (ie, diet, genotype, or sex)].
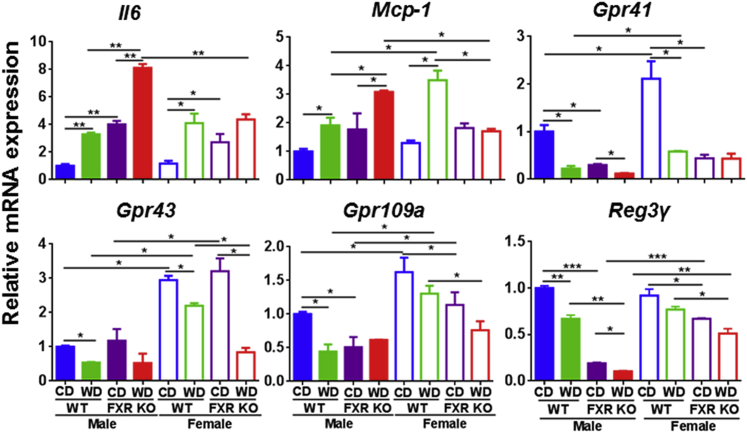
Because Abx reduced hepatic neutrophils and lymphocytes, gut microbiota likely contributed to hepatic inflammation. Thus, we studied the inflammatory signaling in the ileum, where the enterohepatic circulation takes place. Consistently, ileal mRNA levels of Il-6 and Mcp-1 were increased by WD intake and FXR deficiency (Figure 4). Gram-positive bacteria that generated short-chain fatty acids (SCFAs) increase the expression of intestinal tight junction genes to prevent leaky gut and have an anti-inflammatory effect.31 WD intake and/or FXR inactivation reduced the mRNA levels of SCFA receptors that include Gpr 41, 43, and 109a (Figure 4). These findings suggested WD intake as well as FXR inactivation might have a negative impact on SCFA receptor-regulated signaling. In addition, regenerating islet-derived protein 3γ, an antimicrobial peptide, was also reduced by WD intake and FXR inactivation in the ileum (Figure 4).
Figure 4.
Ileal gene expression in control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR knockout (KO) mice of both sexes. Data are expressed as means ± SD. n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 [based on a single variable of comparison (ie, diet, genotype, or sex)].
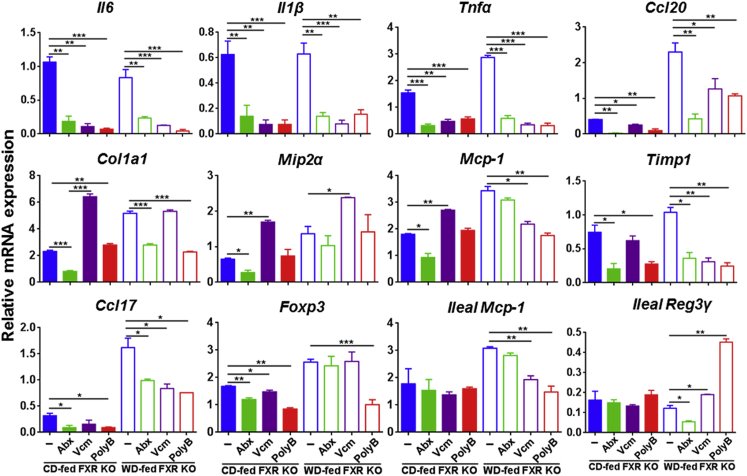
All three antibiotics reduced the expression of hepatic inflammatory genes, such as Il6, Il1b, Tnfa, and Ccl20, in both CD- and WD-fed FXR KO mice (Figure 5). However, Abx had the best effect in reducing the expressions of hepatic collagen type 1α1, macrophage inflammatory protein 2α, and Mcp-1, but Vcm increased them in CD-fed FXR KO mice (Figure 5). In addition, Abx and PolyB reduced tissue inhibitor of metalloproteinases mRNA in both CD- and WD-fed FXR KO mice. Moreover, many of the inflammatory genes, such as Cxcl2, Ccl2, Foxp3, Ccl17, and Ccl20, had higher expression levels in WD- than CD-fed FXR KO after Abx treatment (Figure 5). Furthermore, PolyB-reduced lymphocytes in WD-fed FXR KO mice were accompanied by reduced levels of many hepatic inflammatory genes as well as ileal Mcp-1 and increased ileal regenerating islet-derived protein 3γ mRNA level (Figure 5).
Figure 5.
Hepatic and ileal gene expression in Abx-, vancomycin (Vcm)-, and polymyxin B (PolyB)–treated control diet (CD)– and Western diet (WD)–fed FXR knockout (KO) male mice. Data were relative to CD-fed WT male mice. Data are expressed as means ± SD. n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
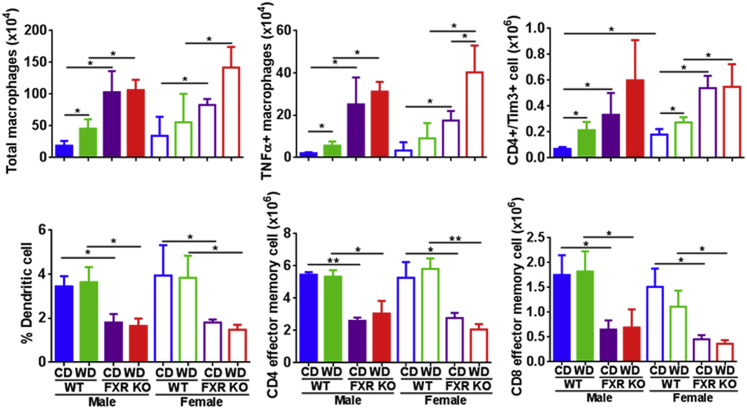
The spleen plays an important role in modulating immune response by removing antibody-coated bacteria and blood cells. Flow cytometry data revealed that WD or FXR inactivation substantially increased total macrophages, tumor necrosis factor-α+ macrophages, and CD4+/Tim3+ (T-cell immunoglobulin domain and mucin domain-3) in both sexes (Figure 6). FXR inactivation also reduced the percentage of dendritic cells (DCs) and the number of CD4 and CD8 effector memory T cells, indicating compromised immunity (Figure 6).
Figure 6.
Flow-cytometric phenotyping of spleen cells in control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR knockout (KO) mice of both sexes. Data are expressed as means ± SD. n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01 [based on a single variable of comparison (ie, diet, genotype, or sex)]. TNF-α, tumor necrosis factor-α.
Bile Acid Profiles and Their Relationships with Hepatic Inflammatory Signaling
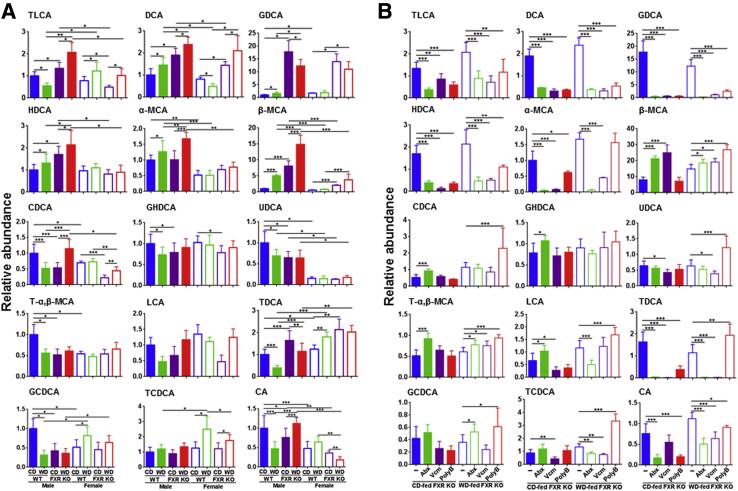
Based on the significance of FXR in regulating hepatic inflammation, hepatic BAs were quantified (Figure 7A). WD-fed FXR KO male mice, which had the greatest hepatic inflammatory signaling, had elevated hepatic taurine-conjugated lithocholic acid (TLCA), deoxycholic acid (DCA), glycodeoxycholic acid (GDCA), hyodeoxycholic acid, and α-muricholic acid and β-muricholic acid (α-MCA and β-MCA, respectively) (Figure 7A). β-MCA had the biggest fold induction in response to WD intake and FXR inactivation in male mice. Moreover, the increase of hepatic GDCA, which is a known IL-6 inducer and tumor promoter, was FXR KO specific.32 In addition, FXR inactivation reduced hepatic chenodeoxycholic acid (CDCA), glycohyodeoxycholic acid, ursodeoxycholic acid, and taurine-α, β-MCA in one or both sexes (Figure 7A).
Figure 7.
Hepatic bile acid profile. A: Relative concentration of hepatic bile acid profile in control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR KO mice of both sexes. B: Relative concentration of hepatic bile acid profile in Abx-, Vcm-, and PolyB-treated CD- and WD-fed FXR KO mice (data were relative to CD-fed WT male mice). Data are expressed as means ± SD. n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. CA, cholic acid; GCDCA, glycochenodeoxycholic acid; GHDCA, glycohyodeoxycholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; T-α, taurine-α; TCDCA, taurine-conjugated chenodeoxycholic acid; TDCA, taurodeoxycholic acid; UDCA, ursodeoxycholic acid.
All three antibiotic treatments reduced TLCA, DCA, GDCA, and hyodeoxycholic acid, indicating gut microbiota association with the production of those secondary BAs (Figure 7B). Abx, which totally eliminated neutrophils and lymphocytes in CD-fed mice, increased CDCA, glycohyodeoxycholic acid, and taurine-α, β-MCA, suggesting their anti-inflammatory role (Figure 7B). In WD-fed groups, PolyB-reduced hepatic lymphocytes were accompanied by induction of CDCA, ursodeoxycholic acid, and taurine-conjugated chenodeoxycholic acid, and those changes were not found in Abx- and Vcm-treated groups (Figure 7B). Increased CDCA and its conjugated forms, such as glycochenodeoxycholic acid and taurine-conjugated chenodeoxycholic acid, as well as reduced TLCA, DCA, GDCA, hyodeoxycholic acid, and cholic acid, were commonly found in Abx-treated CD-fed or PolyB-treated WD-fed FXR KO mice, of which both showed reduced hepatic inflammation.
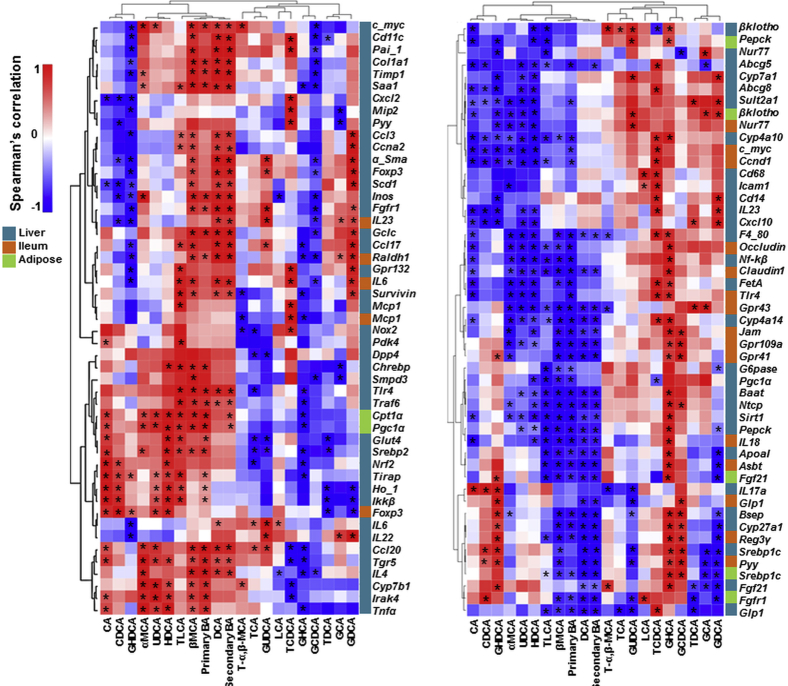
To understand the pathobiological effects of those specific BA profiles, we have studied the expression of >150 genes in liver, ileum, and adipose tissues that have roles in inflammation and metabolism. Spearman correlation analysis revealed that the hepatic concentrations of TLCA, β-MCA, DCA, and free primary and secondary BAs, which clustered together, were positively associated with the expression of inflammation genes, such as hepatic Cd11c, Col1a1, Timp1, Saa1, Foxp3, Ccl17, Tlr4, Ccl20, Il4, and ileal Il6 (Supplemental Figure S1). In addition, their concentrations were negatively associated with hepatic metabolism genes, including hepatic Cyp4a14, Sirt1, Pepck, Bsep, Cyp27a1, Srebp1c, and Glp1, ileal Claudin 1, Jam, Il18, Reg3γ, and Pyy, and adipose Fgf21 and Srebp1c (Supplemental Figure S1). Moreover, hepatic GDCA concentrations were positively associated with hepatic Ccna2, α-sma, Foxp3, Ccl17, Gpr132, and Il22, and ileal Il23, Il6, and Mcp-1, but negatively associated with hepatic mRNA levels of hepatic G6pase, Pepck, and Fgf21, and ileal Reg3γ, Glp1, and Pyy, and adipose Fgf21 and Fgfr1 genes (Supplemental Figure S1).
Gut Microbiota Profiles and Their Relationships with Hepatic Inflammatory Signaling
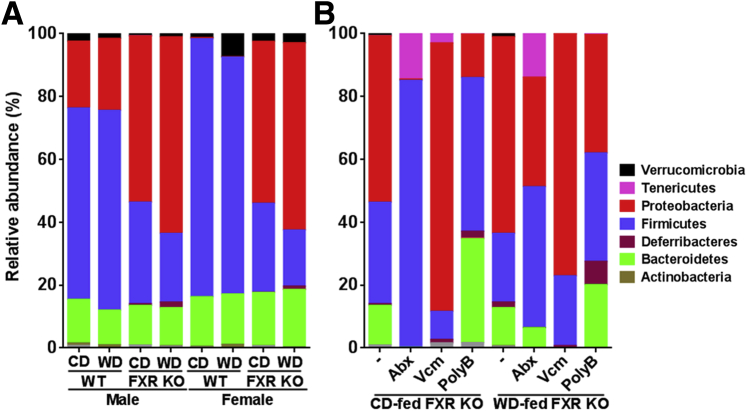
16S rRNA gene sequencing of genomic DNA from cecal samples revealed that FXR inactivation substantially reduced Firmicutes and increased Proteobacteria relative abundance in both sexes (Figure 8A and Supplemental Table S1). The WD-fed FXR KO mice had the highest relative abundance of Proteobacteria and Deferribacteres among the eight studied groups (Figure 8A). However, Abx treatment of CD-fed FXR KO mice, which completely eliminated hepatic neutrophils and lymphocytes, had opposite effects (Figure 8B). The relative abundance of Firmicutes and Tenericutes increased in both CD- and WD-fed FXR KO mice treated with Abx (Figure 8B and Supplemental Table S2). In addition, Proteobacteria and Bacteroidetes persisted after Abx treatment, which were accompanied by the persistent inflammation found in WD-fed FXR KO mice. Vcm increased the relative abundance of Proteobacteria in both CD- and WD-fed mice. In contrast, PolyB, which reduced hepatic lymphocytes in WD-fed mice, substantially decreased Proteobacteria and increased Firmicutes relative abundance in WD-fed FXR KO mice (Figure 8B). The sex difference in hepatic inflammation was apparent. Accordingly, FXR inactivation reduced the relative abundance of Verrucomicrobia phylum in males, but increased it in CD-fed WT females. In addition, Deferribacteres were more abundant compared with other microbes in male FXR KO than the female counterparts (Figure 8A and Supplemental Table S1).
Figure 8.
Relative abundance of cecal microbiota at phylum level of control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR knockout (KO) mice of both sexes (A) and antibiotic-treated CD- and WD-fed FXR KO male mice (B). Data are given as means. PolyB, polymyxin B; Vcm, vancomycin.
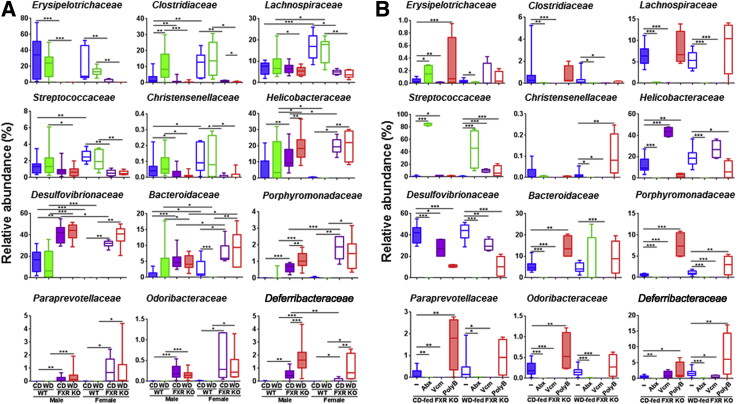
Under Firmicutes, the relative abundance of Erysipelotrichaceae and Clostridiaceae was substantially reduced because of FXR inactivation (Figure 9A). In addition, the reduction of Lachnospiraceae, Streptococcaceae, and Christensenellaceae in FXR KO was more apparent in females than males (Figure 9A). Furthermore, in CD-fed mice, Abx substantially increased relative abundance of Streptococcaceae, which might account for reduced inflammation (Figure 9B). Moreover, PolyB-reduced inflammation was accompanied by increased relative abundance of Christensenellaceae in WD-fed FXR KO mice (Figure 9B).
Figure 9.
Relative abundance of cecal microbiota at family level of control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR knockout (KO) mice of both sexes (A) and antibiotic-treated CD- and WD-fed FXR KO male mice (B). Box plots display the median, 25th percentile, and 75th percentile; whiskers display minimum and maximum values. n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. PolyB, polymyxin B; Vcm, vancomycin.
Under the Proteobacteria phylum, the relative abundance of Helicobacteraceae and Desulfovibrionaceae substantially increased because of FXR inactivation (Figure 9A). Consistently, Abx and PolyB reduced them in both CD- and WD-fed FXR KO mice (Figure 9B).
Under Bacteroidetes, the relative abundance of Bacteroidaceae, Porphyromonadaceae, Paraprevotellaceae, and Odoribacteriaceae families was increased in FXR KO mice (Figure 9A), and Abx was able to eliminate them in CD-fed FXR KO mice (Figure 9B). The bacteria that account for antibiotic-reduced hepatic inflammation might not be the same bacteria that caused hepatic inflammation in FXR KO mice. For example, the relative abundance of Deferribacteraceae (Deferribacteres phylum) was increased by FXR KO, and was further increased by WD, but PolyB, which reduced hepatic lymphocytes, also increased the relative abundance of Deferribacteraceae in WD-fed FXR KO mice (Figure 9B).
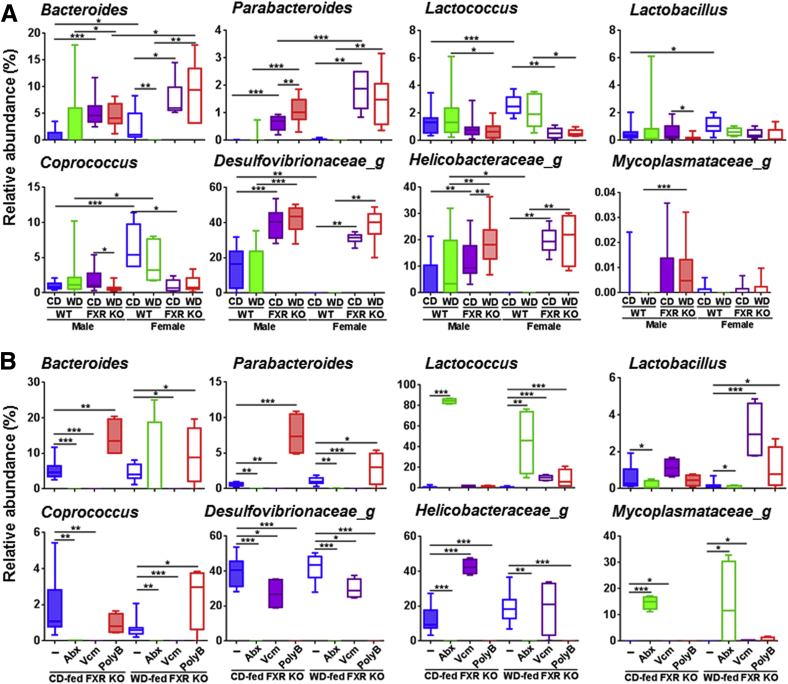
At the genus level, the lack of FXR increased the relative abundance of genera Bacteroides and Parabacteroides (Bacteroidetes phylum), which were also reduced by Abx in CD-fed FXR KO mice, suggesting their role in hepatic inflammation (Figure 10, A and B). In addition, Abx dramatically increased Lactococcus genus (Firmicutes phylum). Moreover, PolyB also increased the relative abundance of Lactococcus in WD-fed FXR KO mice. Furthermore, PolyB substantially increased the relative abundance of genera Lactobacillus and Coprococcus (Firmicutes phylum) in WD-fed FXR KO, suggesting the protective effects of those bacteria (Figure 10B). Furthermore, the relative abundance of genera Desulfovibrionaceae_g and Helicobacteraceae_g (Proteobacteria phylum) was significantly increased because of FXR inactivation, and both Abx and PolyB substantially reduced them (Figure 10, A and B). Under Tenericutes phylum, genus Mycoplasmataceae_g, which increased because of FXR deficiency in males, was further increased by Abx in both CD- and WD-fed FXR KO mice (Figure 10, A and B).
Figure 10.
Relative abundance of cecal microbiota at genus level of control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR knockout (KO) mice of both sexes (A) and antibiotic-treated CD- and WD-fed FXR KO male mice (B). Box plots display the median, 25th percentile, and 75th percentile; whiskers display minimum and maximum values. n ≥ 6 per group. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. PolyB, polymyxin B; Vcm, vancomycin.
Functional Analysis of Gut Microbiota
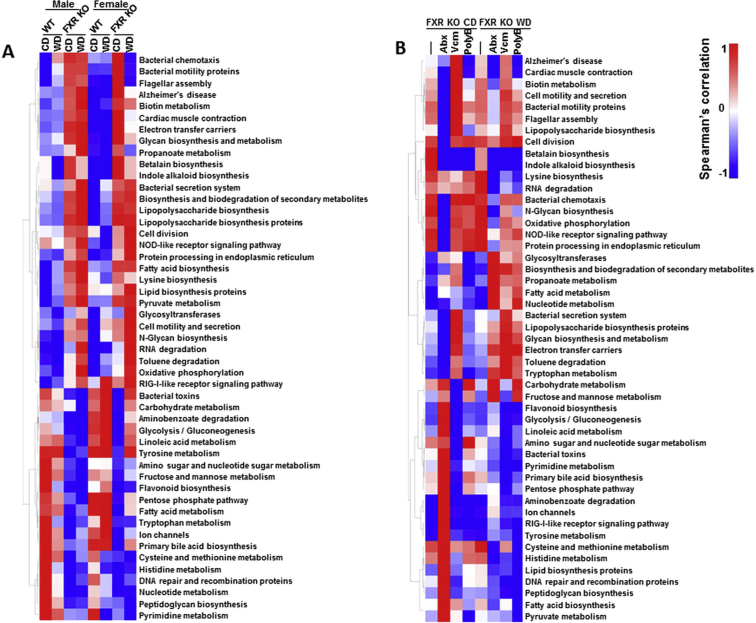
FXR deficiency had a higher impact than diet or sex in shifting microbiota function based on the phylogenetic investigation of communities by reconstruction of unobserved states analysis (Supplemental Table S3). In addition, there were 49 pathways with a significant difference between WT and FXR KO mice (Supplemental Figure S2A). WD-fed FXR KO male mice, which had the most hepatic lymphocyte infiltration, had enriched pathways in Alzheimer disease, cardiac muscle contraction, biotin metabolism, and betalain biosynthesis. These pathways were reduced by Abx in CD-fed mice and PolyB in WD-fed mice (Supplemental Figure S2). In addition, Abx reduced bacterial motility proteins, LPS biosynthesis, and bacterial chemotaxis in CD-fed FXR KO mice. Moreover, PolyB reduced inflammation in WD-fed FXR KO mice, which was accompanied by increased tryptophan, carbohydrate, and fructose metabolism as well as reduced fatty acid biosynthesis pathways (Supplemental Figure S2B).
Discussion
Our novel data revealed there is an apparent sex difference in hepatic inflammation. The amount of hepatic lymphocytes, serum endotoxin levels, and ALT levels were higher in males than females in several study groups. The expression levels of proinflammatory genes, such as hepatic Saa1, Foxp3, Ccl17, and Ccl20, as well as ileal Il6 were higher in male than female WD-fed FXR KO mice. All those cytokines are implicated in hepatic inflammation, as shown in different models. For example, serum amyloid a1 has a known effect of inducing the migration, adhesion, and tissue infiltration of monocytes and neutrophils in humans.33, 34, 35 In addition, Foxp3 is induced because of hepatitis C virus infection.36 Ccl17 is closely associated with intestinal inflammation and hepatitis C virus infection.37, 38, 39, 40 Moreover, Ccl20 is highly induced in alcoholic patients and LPS-induced liver injury.41 In addition, our data revealed that the substantial induction of those hepatic cytokine genes was accompanied by elevated hepatic Col1a1 in a male-predominant manner, suggesting males are more susceptible to the development of steatohepatitis or fibrosis. Furthermore, WD-fed male FXR KO mice had reduced ileal Reg3γ, which has a protective effect against the infection of Gram-positive bacteria.42 Consistently, increased Reg3γ found in PolyB-treated WD-fed FXR KO might account for reduced hepatic inflammation. Moreover, although Abx reduced almost all of the proinflammatory signaling in CD-fed FXR KO mice, WD-fed FXR KO mice still had elevated hepatic Ccl17 and reduced ileal Reg3γ as well as hepatic lymphocytes after Abx treatment. These findings clearly indicate the protective role of regenerating islet-derived protein 3γ in inflammation.
A sex difference in microbiota was also noted. Lachnospiraceae, Clostridiaceae, Streptococcaceae, and Christensenellaceae are likely to have a protective effect against hepatic inflammation because their relative abundance is increased in the female mice. This scenario is in part supported by the finding that Lachnospiraceae-generated SCFAs have anti-inflammatory and histone deacetylation inhibitory properties.43, 44, 45 Consistently, the expression level of ileal SCFA receptors (Gpr41, Gpr43, and Gpr109A) was higher in female than male WT mice. Among those three receptors, GPR109A is butyrate specific, and its mRNA reduction folds were greater in males than females because of WD intake or FXR activation. Moreover, ileal mRNA levels of Gpr43, which can be bound by propionate and butyrate, were higher in females than in males as well, with the exception of WD-fed FXR KO groups. Christensenellaceae also generate SCFAs and are enriched in lean individuals.46, 47 Moreover, Clostridiaceae and Streptococcaceae also have many known health benefits.48, 49 Together, the sex difference in those bacteria may in part explain the sex disparity of liver disease, including cancer.
FXR deactivation increased hepatic lymphocytes and neutrophils, indicating the presence of both chronic and acute inflammation. Neutrophils are usually the first responders to the acute phase of inflammation, especially in the case of bacterial infection.50 Through chemotaxis controlled by cytokines, neutrophils migrate through the blood vessels, reaching the interstitial tissue and then the liver. In the spleen, FXR KO mice had increased total macrophages as well as tumor necrosis factor-α+ macrophage and CD4+/Tim3+ cells, but reduced DCs. It has been shown that elevated Tim3 causes T-cell disruption and exhaustion, leading to inflammation and compromised immunity.51, 52 In addition, the significance of FXR in regulating inflammation through DCs has been revealed in the dextran sulfate sodium–induced colitis model and other intestinal models.53, 54 Furthermore, activation of FXR by obeticholic acid can retain DCs and have an anti-inflammatory effect.53 The consistent reduction of DCs in the liver and intestinal inflammation clearly indicates their protective effect, which is regulated by FXR.
The beneficial and detrimental effects of BAs depend on their hydrophobicity, concentration, and exposure time.3, 6 Our published data showed that BAs can induce and dictate the intracellular location of NUR77 to regulate proliferation or apoptosis of liver and colon cancer cells.55 Moreover, a long-term exposure to high concentrations of hydrophobic secondary BAs generates BA-resistant cells that are highly proliferative and have tumorigenic potential because of nuclear NUR77 overexpression.55 In the current study, our data revealed many free as well as conjugated secondary BAs, such as TLCA, DCA, GDCA, and hyodeoxycholic acid, that were enriched because of FXR inactivation and WD intake. Moreover, their hepatic concentrations were decreased because of reduced inflammation as a result of antibiotic treatment. These findings are in line with the data that show increased GDCA in FXR KO mice is associated with tumor growth and increased inflammatory cytokines.56 In contrast, taurine-α, β-MCA, CDCA, glycochenodeoxycholic acid, and ursodeoxycholic acid are likely to have a protective effect against inflammation because they were either reduced by WD or FXR KO in certain groups, and such reductions could be reversed by reduced hepatic inflammation after Abx or PolyB treatment. Consistently, it has been shown that ursodeoxycholic acid activates FXR and induces type 1 helper T-cell response via DCs to suppress airway inflammation.57 Together, it is clear that dysbiosis and dysregulated BAs are inseparable, and they jointly contribute to hepatic inflammation via the gut-liver axis. Moreover, in antibiotic-treated mice, the change in BA profile can also be primary as well as secondary to the alterations in gut microbiota because antibiotics can directly eliminate BA-generating bacteria, which, in turn, causes additional changes in BA composition.
Regarding microbiota, the biggest change due to FXR inactivation was reduction of Firmicutes and increase in Proteobacteria relative abundance in both sexes. Similar changes in Firmicutes and Proteobacteria were found in obesity and nonalcoholic steatohepatitis patients.58, 59 Proteobacteria are Gram-negative endotoxin producers, which can cause dysbiosis and contribute to chronic liver injury in nonalcoholic fatty liver disease.60 In the Firmicutes phylum, Erysipelotrichaceae, Clostridiaceae, Lachnospiraceae, Streptococcaceae, and Christensenellaceae families were reduced by FXR inactivation, suggesting the specific effect of BA production in reducing those beneficial bacteria. It has been shown that Erysipelotrichaceae is involved in BA metabolism and also has anti-inflammatory properties.54, 61, 62 Moreover, patients with Crohn disease and inflammatory bowel disease have reduced Erysipelotrichaceae and Clostridiaceae.61, 63 The abundance of genus Coprococcus under Lachnospiraceae family was enriched in PolyB-treated WD-fed FXR KO mice, revealing their potential beneficial effect. FXR KO mice also had reduced Streptococcaceae, which can be increased by probiotic VSL#3.64 Moreover, genus Lactococcus under Streptococcaceae might have the largest percentage increased because of the anti-inflammatory effect of Abx in CD-fed mice (from 3% to 80%). Lactococcus have many beneficial effects, ranging from metabolism improvement to anti-inflammation.48, 65 Moreover, increased Lactococcus and butyrate production were found in mice after vertical sleeve gastrectomy.66 However, WD intake substantially reduced the effect of Abx to enrich Lactococcus. This outcome might explain the presence of hepatic lymphocytes after Abx treatment in WD-fed mice.
The increase in relative abundance of Proteobacteria phylum in FXR KO mice was mainly because of an increased relative abundance of Desulfovibrionaceae and Helicobacteraceae families. Under these two families, FXR KO substantially increased Desulfovibrionaceae_g and Helicobacteraceae_g. Certain Desuflovibrionaceae can generate hydrogen sulfide via taurine respiration, which is a potent genotoxin and gut mucosal barrier breaker, leading to acute inflammation.67, 68 Moreover, mice with impaired glucose tolerance and ulcerative colitis also have increased Desulfovibrionaceae.69 In addition, different species of Desulfovibrionaceae are colonized in oral cavities and gastrointestinal tracts, which can have a systemic effect.70, 71, 72 Within the Helicobacteraceae family, Helicobacter pylori, Helicobacter aurati, and Helicobacter hepaticus are well-known for their roles in infection and carcinogenesis.73, 74 Moreover, Abx- and PolyB-reduced hepatic inflammation was accompanied by elimination of Desulfovibrionaceae_g and Helicobacteraceae_g. Furthermore, our novel data showed that Vcm-induced ALT in CD-fed FXR KO was associated with increased relative abundance of Helicobacteraceae_g.
Under Bacteroidetes phylum, Bacteroides and Parabacteroides genera, which are the predominant anaerobes in the gut (25%), increased as a result of FXR deactivation in both sexes. Bacteroides species are BA-resistant pathogens, which are found in most anaerobic infections, with a mortality of >19%.75 However, the percentage of Bacteroides increases due to lack of FXR were higher in females than males. Thus, the resistance to inflammation found in females might be due to having more beneficial microbiota rather than less proinflammatory bacteria. Our data revealed that genus Bacteroides was eliminated by Abx in CD-fed FXR KO mice. However, Bacteroides that persisted after Abx treatment might lead to persistent hepatic inflammation after Abx treatment. Together, these results suggested that decreased Bacteroides and Parabacteroides contribute to reduced hepatic inflammation.
In conclusion, using dietary modification and antibiotic treatments, our data unequivocally revealed the role of gut microbiota in control of hepatic inflammation. Moreover, BAs and their receptor FXR play a pivotal role in mediating the inflammatory and anti-inflammatory effects of microbiota in a sex-dependent manner. This scenario is supported by the findings that sex or diet effects are frequently abolished or enhanced because of lack of FXR. For example, the effect of diet on microbiota functions became apparent or was no longer found when FXR was inactivated in male and female mice, respectively. Moreover, there are similarities between the WD intake and FXR KO. This is shown by the reduction of the number of bacterial functional pathways differentially found between WT and FXR KO because of WD intake. Thus, probiotics and FXR agonists should be effective in the prevention and treatment of hepatic inflammation and progression into advanced liver diseases, such as cancer. The present study does not exclude the role of microbiota in regulating hepatic metabolism. Additional analyses are required to address the effect of diet and antibiotics in hepatic metabolism.
Acknowledgments
We thank Clarissa Rocha, Lyndsey Dobyins, and Nidhi Nagar for their contributions in preparing the manuscript.
Footnotes
See related Commentary on page 1658
Supported by NIH grant U01CA179582 (Y.-J.Y.W.).
P.K.J. and L.S. contributed equally to this work.
Disclosures: None declared.
Supplemental material for this article can be found at http://dx.doi.org/10.1016/j.ajpath.2017.04.019.
Supplemental Data
Supplemental Figure S1.
Hierarchical clustering analysis of hepatic bile acids and genes in WT and FXR KO mice. Heat map of Spearman correlation analysis between hepatic bile acids and gene expression (liver, ileum, and adipose tissue). ∗P < 0.05. CA, cholic acid; GCA, glycocholic acid; GCDCA, glycochenodeoxycholic acid; GHCA, glyohyocholic acid; GHDCA, glycohyodeoxycholic acid; GUDCA, glycoursodeoxycholic acid; HDCA, hyodeoxycholic acid; LCA, lithocholic acid; T-α, taurine-α; TCA, taurocholic acid; TCDCA, taurine-conjugated chenodeoxycholic acid; TDCA, taurodeoxycholic acid; UDCA, ursodeoxycholic acid.
Supplemental Figure S2.
Comparison in the relative abundance of phylogenetic investigation of communities by reconstruction of unobserved states–generated functional profile of gut microbiota. A: Heat map of the relative abundance changes of microbial functional pathways in control diet (CD)– and Western diet (WD)–fed wild-type (WT) and FXR knockout (KO) mice of both sexes. B: Heat map of relative abundance changes of microbial functional pathways after Abx, Vcm, and PolyB treatment in CD- and WD-fed FXR KO male mice. NOD, nucleotide-binding oligomerization; RIG, retinoic acid-inducible gene.
References
- 1.Wustefeld T., Rakemann T., Kubicka S., Manns M.P., Trautwein C. Hyperstimulation with interleukin 6 inhibits cell cycle progression after hepatectomy in mice. Hepatology. 2000;32:514–522. doi: 10.1053/jhep.2000.16604. [DOI] [PubMed] [Google Scholar]
- 2.Gabele E., Dostert K., Hofmann C., Wiest R., Scholmerich J., Hellerbrand C., Obermeier F. DSS induced colitis increases portal LPS levels and enhances hepatic inflammation and fibrogenesis in experimental NASH. J Hepatol. 2011;55:1391–1399. doi: 10.1016/j.jhep.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 3.Liu H.X., Keane R., Sheng L., Wan Y.J. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;63:1502–1510. doi: 10.1016/j.jhep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bechmann L.P., Hannivoort R.A., Gerken G., Hotamisligil G.S., Trauner M., Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. J Hepatol. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Xu R., Huang H., Zhang Z., Wang F.S. The role of neutrophils in the development of liver diseases. Cell Mol Immunol. 2014;11:224–231. doi: 10.1038/cmi.2014.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuei J., Chau T., Mills D., Wan Y.J. Bile acid dysregulation, gut dysbiosis, and gastrointestinal cancer. Exp Biol Med (Maywood) 2014;239:1489–1504. doi: 10.1177/1535370214538743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allen K., Jaeschke H., Copple B.L. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–186. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjursell M., Wedin M., Admyre T., Hermansson M., Bottcher G., Goransson M., Linden D., Bamberg K., Oscarsson J., Bohlooly Y.M. Ageing Fxr deficient mice develop increased energy expenditure, improved glucose control and liver damage resembling NASH. PLoS One. 2013;8:e64721. doi: 10.1371/journal.pone.0064721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang J.Y. Bile acid metabolism and signaling. Compr Physiol. 2013;3:1191–1212. doi: 10.1002/cphy.c120023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo C., Xie S., Chi Z., Zhang J., Liu Y., Zhang L., Zheng M., Zhang X., Xia D., Ke Y., Lu L., Wang D. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:944. doi: 10.1016/j.immuni.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Liu H.X., Rocha C.S., Dandekar S., Wan Y.J. Functional analysis of the relationship between intestinal microbiota and the expression of hepatic genes and pathways during the course of liver regeneration. J Hepatol. 2016;64:641–650. doi: 10.1016/j.jhep.2015.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Sayin S.I., Wahlstrom A., Felin J., Jantti S., Marschall H.U., Bamberg K., Angelin B., Hyotylainen T., Oresic M., Backhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225–235. doi: 10.1016/j.cmet.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L., Xie C., Nichols R.G., Chan S.H., Jiang C., Hao R., Smith P.B., Cai J., Simons M.N., Hatzakis E., Maranas C.D., Gonzalez F.J., Patterson A.D. Farnesoid X receptor signaling shapes the gut microbiota and controls hepatic lipid metabolism. mSystems. 2016;1 doi: 10.1128/mSystems.00070-16. e00070-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Torres J., Bao X., Iuga A.C., Chen A., Harpaz N., Ullman T., Cohen B.L., Pineton de Chambrun G., Asciutti S., Odin J.A., Sachar D.B., Gaskins H.R., Setchell K., Colombel J.F., Itzkowitz S.H. Farnesoid X receptor expression is decreased in colonic mucosa of patients with primary sclerosing cholangitis and colitis-associated neoplasia. Inflamm Bowel Dis. 2013;19:275–282. doi: 10.1097/MIB.0b013e318286ff2e. [DOI] [PubMed] [Google Scholar]
- 16.Huang X.F., Zhao W.Y., Huang W.D. FXR and liver carcinogenesis. Acta Pharmacol Sin. 2015;36:37–43. doi: 10.1038/aps.2014.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang F., Huang X., Yi T., Yen Y., Moore D.D., Huang W. Spontaneous development of liver tumors in the absence of the bile acid receptor farnesoid X receptor. Cancer Res. 2007;67:863–867. doi: 10.1158/0008-5472.CAN-06-1078. [DOI] [PubMed] [Google Scholar]
- 18.Li F., Jiang C., Krausz K.W., Li Y., Albert I., Hao H., Fabre K.M., Mitchell J.B., Patterson A.D., Gonzalez F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat Commun. 2013;4:2384. doi: 10.1038/ncomms3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang C., Xie C., Li F., Zhang L., Nichols R.G., Krausz K.W., Cai J., Qi Y., Fang Z.Z., Takahashi S., Tanaka N., Desai D., Amin S.G., Albert I., Patterson A.D., Gonzalez F.J. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim I., Morimura K., Shah Y., Yang Q., Ward J.M., Gonzalez F.J. Spontaneous hepatocarcinogenesis in farnesoid X receptor-null mice. Carcinogenesis. 2007;28:940–946. doi: 10.1093/carcin/bgl249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu N., Meng Z., Lou G., Zhou W., Wang X., Zhang Y., Zhang L., Liu X., Yen Y., Lai L., Forman B.M., Xu Z., Xu R., Huang W. Hepatocarcinogenesis in FXR-/- mice mimics human HCC progression that operates through HNF1alpha regulation of FXR expression. Mol Endocrinol. 2012;26:775–785. doi: 10.1210/me.2011-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinal C.J., Tohkin M., Miyata M., Ward J.M., Lambert G., Gonzalez F.J. Targeted disruption of the nuclear receptor FXR/BAR impairs bile acid and lipid homeostasis. Cell. 2000;102:731–744. doi: 10.1016/s0092-8674(00)00062-3. [DOI] [PubMed] [Google Scholar]
- 23.Committee for the Update of the Guide for the Care and Use of Laboratory Animals; National Research Council . ed 8. National Academies Press; Washington, DC: 2011. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 24.Ooi J.H., Li Y., Rogers C.J., Cantorna M.T. Vitamin D regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J Nutr. 2013;143:1679–1686. doi: 10.3945/jn.113.180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pappo I., Becovier H., Berry E.M., Freund H.R. Polymyxin B reduces cecal flora, TNF production and hepatic steatosis during total parenteral nutrition in the rat. J Surg Res. 1991;51:106–112. doi: 10.1016/0022-4804(91)90078-z. [DOI] [PubMed] [Google Scholar]
- 26.Frese S.A., Parker K., Calvert C.C., Mills D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome. 2015;3:28. doi: 10.1186/s40168-015-0091-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bokulich N.A., Thorngate J.H., Richardson P.M., Mills D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc Natl Acad Sci U S A. 2014;111:E139–E148. doi: 10.1073/pnas.1317377110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bokulich N.A., Collins T.S., Masarweh C., Allen G., Heymann H., Ebeler S.E., Mills D.A. Associations among wine grape microbiome, metabolome, and fermentation behavior suggest microbial contribution to regional wine characteristics. MBio. 2016;7 doi: 10.1128/mBio.00631-16. e00631-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caporaso J.G., Kuczynski J., Stombaugh J., Bittinger K., Bushman F.D., Costello E.K., Fierer N., Pena A.G., Goodrich J.K., Gordon J.I., Huttley G.A., Kelley S.T., Knights D., Koenig J.E., Ley R.E., Lozupone C.A., McDonald D., Muegge B.D., Pirrung M., Reeder J., Sevinsky J.R., Turnbaugh P.J., Walters W.A., Widmann J., Yatsunenko T., Zaneveld J., Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Langille M.G.I., Zaneveld J., Caporaso J.G., McDonald D., Knights D., Reyes J.A., Clemente J.C., Burkepile D.E., Vega Thurber R.L., Knight R., Beiko R.G., Huttenhower C. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31:814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox M.A., Jackson J., Stanton M., Rojas-Triana A., Bober L., Laverty M., Yang X., Zhu F., Liu J., Wang S., Monsma F., Vassileva G., Maguire M., Gustafson E., Bayne M., Chou C.C., Lundell D., Jenh C.H. Short-chain fatty acids act as antiinflammatory mediators by regulating prostaglandin E(2) and cytokines. World J Gastroenterol. 2009;15:5549–5557. doi: 10.3748/wjg.15.5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dvorak K., Chavarria M., Payne C.M., Ramsey L., Crowley-Weber C., Dvorakova B., Dvorak B., Bernstein H., Holubec H., Sampliner R.E., Bernstein C., Prasad A., Green S.B., Garewal H. Activation of the interleukin-6/STAT3 antiapoptotic pathway in esophageal cells by bile acids and low pH: relevance to barrett's esophagus. Clin Cancer Res. 2007;13:5305–5313. doi: 10.1158/1078-0432.CCR-07-0483. [DOI] [PubMed] [Google Scholar]
- 33.Ji Y.R., Kim H.J., Bae K.B., Lee S., Kim M.O., Ryoo Z.Y. Hepatic serum amyloid A1 aggravates T cell-mediated hepatitis by inducing chemokines via Toll-like receptor 2 in mice. J Biol Chem. 2015;290:12804–12811. doi: 10.1074/jbc.M114.635763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu L., Badolato R., Murphy W.J., Longo D.L., Anver M., Hale S., Oppenheim J.J., Wang J.M. A novel biologic function of serum amyloid A: induction of T lymphocyte migration and adhesion. J Immunol. 1995;155:1184–1190. [PubMed] [Google Scholar]
- 35.Badolato R., Wang J.M., Murphy W.J., Lloyd A.R., Michiel D.F., Bausserman L.L., Kelvin D.J., Oppenheim J.J. Serum amyloid A is a chemoattractant: induction of migration, adhesion, and tissue infiltration of monocytes and polymorphonuclear leukocytes. J Exp Med. 1994;180:203–209. doi: 10.1084/jem.180.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amoras Eda S., Gomes S.T., Freitas F.B., Santana B.B., Ishak G., Ferreira de Araujo M.T., Demachki S., Conde S.R., Ishak Mde O., Ishak R., Vallinoto A.C. Intrahepatic mRNA expression of FAS, FASL, and FOXP3 genes is associated with the pathophysiology of chronic HCV infection. PLoS One. 2016;11:e0156604. doi: 10.1371/journal.pone.0156604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Riezu-Boj J.I., Larrea E., Aldabe R., Guembe L., Casares N., Galeano E., Echeverria I., Sarobe P., Herrero I., Sangro B., Prieto J., Lasarte J.J. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol. 2011;54:422–431. doi: 10.1016/j.jhep.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Heiseke A.F., Faul A.C., Lehr H.A., Forster I., Schmid R.M., Krug A.B., Reindl W. CCL17 promotes intestinal inflammation in mice and counteracts regulatory T cell-mediated protection from colitis. Gastroenterology. 2012;142:335–345. doi: 10.1053/j.gastro.2011.10.027. [DOI] [PubMed] [Google Scholar]
- 39.Belperio J.A., Dy M., Murray L., Burdick M.D., Xue Y.Y., Strieter R.M., Keane M.P. The role of the Th2 CC chemokine ligand CCL17 in pulmonary fibrosis. J Immunol. 2004;173:4692–4698. doi: 10.4049/jimmunol.173.7.4692. [DOI] [PubMed] [Google Scholar]
- 40.Santulli-Marotto S., Boakye K., Lacy E., Wu S.J., Luongo J., Kavalkovich K., Coelho A., Hogaboam C.M., Ryan M. Engagement of two distinct binding domains on CCL17 is required for signaling through CCR4 and establishment of localized inflammatory conditions in the lung. PLoS One. 2013;8:e81465. doi: 10.1371/journal.pone.0081465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Affo S., Morales-Ibanez O., Rodrigo-Torres D., Altamirano J., Blaya D., Dapito D.H., Millan C., Coll M., Caviglia J.M., Arroyo V., Caballeria J., Schwabe R.F., Gines P., Bataller R., Sancho-Bru P. CCL20 mediates lipopolysaccharide induced liver injury and is a potential driver of inflammation and fibrosis in alcoholic hepatitis. Gut. 2014;63:1782–1792. doi: 10.1136/gutjnl-2013-306098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Loonen L.M., Stolte E.H., Jaklofsky M.T., Meijerink M., Dekker J., van Baarlen P., Wells J.M. REG3gamma-deficient mice have altered mucus distribution and increased mucosal inflammatory responses to the microbiota and enteric pathogens in the ileum. Mucosal Immunol. 2014;7:939–947. doi: 10.1038/mi.2013.109. [DOI] [PubMed] [Google Scholar]
- 43.Aminov R.I., Walker A.W., Duncan S.H., Harmsen H.J., Welling G.W., Flint H.J. Molecular diversity, cultivation, and improved detection by fluorescent in situ hybridization of a dominant group of human gut bacteria related to Roseburia spp. or Eubacterium rectale. Appl Environ Microbiol. 2006;72:6371–6376. doi: 10.1128/AEM.00701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plöger S., Stumpff F., Penner G.B., Schulzke J.D., Gäbel G., Martens H., Shen Z., Günzel D., Aschenbach J.R. Microbial butyrate and its role for barrier function in the gastrointestinal tract. Ann N Y Acad Sci. 2012;1258:52–59. doi: 10.1111/j.1749-6632.2012.06553.x. [DOI] [PubMed] [Google Scholar]
- 45.Chang P.V., Hao L., Offermanns S., Medzhitov R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A. 2014;111:2247–2252. doi: 10.1073/pnas.1322269111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Morotomi M., Nagai F., Watanabe Y. Description of Christensenella minuta gen. nov., sp. nov., isolated from human faeces, which forms a distinct branch in the order Clostridiales, and proposal of Christensenellaceae fam. nov. Int J Syst Evol Microbiol. 2012;62:144–149. doi: 10.1099/ijs.0.026989-0. [DOI] [PubMed] [Google Scholar]
- 47.Goodrich J.K., Waters J.L., Poole A.C., Sutter J.L., Koren O., Blekhman R., Beaumont M., Van Treuren W., Knight R., Bell J.T., Spector T.D., Clark A.G., Ley R.E. Human genetics shape the gut microbiome. Cell. 2014;159:789–799. doi: 10.1016/j.cell.2014.09.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Korpela K., Flint H.J., Johnstone A.M., Lappi J., Poutanen K., Dewulf E., Delzenne N., de Vos W.M., Salonen A. Gut microbiota signatures predict host and microbiota responses to dietary interventions in obese individuals. PLoS One. 2014;9:e90702. doi: 10.1371/journal.pone.0090702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smit B.A., van Hylckama Vlieg J.E., Engels W.J., Meijer L., Wouters J.T., Smit G. Identification, cloning, and characterization of a Lactococcus lactis branched-chain alpha-keto acid decarboxylase involved in flavor formation. Appl Environ Microbiol. 2005;71:303–311. doi: 10.1128/AEM.71.1.303-311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Gwaiz L.A., Babay H.H. The diagnostic value of absolute neutrophil count, band count and morphologic changes of neutrophils in predicting bacterial infections. Med Princ Pract. 2007;16:344–347. doi: 10.1159/000104806. [DOI] [PubMed] [Google Scholar]
- 51.Golden-Mason L., Palmer B.E., Kassam N., Townshend-Bulson L., Livingston S., McMahon B.J., Castelblanco N., Kuchroo V., Gretch D.R., Rosen H.R. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones R.B., Ndhlovu L.C., Barbour J.D., Sheth P.M., Jha A.R., Long B.R., Wong J.C., Satkunarajah M., Schweneker M., Chapman J.M., Gyenes G., Vali B., Hyrcza M.D., Yue F.Y., Kovacs C., Sassi A., Loutfy M., Halpenny R., Persad D., Spotts G., Hecht F.M., Chun T.W., McCune J.M., Kaul R., Rini J.M., Nixon D.F., Ostrowski M.A. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Massafra V., Ijssennagger N., Plantinga M., Milona A., Ramos Pittol J.M., Boes M., van Mil S.W.C. Splenic dendritic cell involvement in FXR-mediated amelioration of DSS colitis. Biochim Biophys Acta. 2016;1862:166–173. doi: 10.1016/j.bbadis.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 54.Vavassori P., Mencarelli A., Renga B., Distrutti E., Fiorucci S. The bile acid receptor FXR is a modulator of intestinal innate immunity. J Immunol. 2009;183:6251–6261. doi: 10.4049/jimmunol.0803978. [DOI] [PubMed] [Google Scholar]
- 55.Hu Y., Chau T., Liu H.X., Liao D., Keane R., Nie Y., Yang H., Wan Y.J. Bile acids regulate nuclear receptor (Nur77) expression and intracellular location to control proliferation and apoptosis. Mol Cancer Res. 2015;13:281–292. doi: 10.1158/1541-7786.MCR-14-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dai J., Wang H., Shi Y., Dong Y., Zhang Y., Wang J. Impact of bile acids on the growth of human cholangiocarcinoma via FXR. J Hematol Oncol. 2011;4:41. doi: 10.1186/1756-8722-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Willart M.A., van Nimwegen M., Grefhorst A., Hammad H., Moons L., Hoogsteden H.C., Lambrecht B.N., Kleinjan A. Ursodeoxycholic acid suppresses eosinophilic airway inflammation by inhibiting the function of dendritic cells through the nuclear farnesoid X receptor. Allergy. 2012;67:1501–1510. doi: 10.1111/all.12019. [DOI] [PubMed] [Google Scholar]
- 58.Ley R.E., Backhed F., Turnbaugh P., Lozupone C.A., Knight R.D., Gordon J.I. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turnbaugh P.J., Backhed F., Fulton L., Gordon J.I. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Minicis S., Rychlicki C., Agostinelli L., Saccomanno S., Candelaresi C., Trozzi L., Mingarelli E., Facinelli B., Magi G., Palmieri C., Marzioni M., Benedetti A., Svegliati-Baroni G. Dysbiosis contributes to fibrogenesis in the course of chronic liver injury in mice. Hepatology. 2014;59:1738–1749. doi: 10.1002/hep.26695. [DOI] [PubMed] [Google Scholar]
- 61.Chen J., Chia N., Kalari K.R., Yao J.Z., Novotna M., Soldan M.M., Luckey D.H., Marietta E.V., Jeraldo P.R., Chen X., Weinshenker B.G., Rodriguez M., Kantarci O.H., Nelson H., Murray J.A., Mangalam A.K. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci Rep. 2016;6:28484. doi: 10.1038/srep28484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Labbe A., Ganopolsky J.G., Martoni C.J., Prakash S., Jones M.L. Bacterial bile metabolising gene abundance in Crohn's, ulcerative colitis and type 2 diabetes metagenomes. PLoS One. 2014;9:e115175. doi: 10.1371/journal.pone.0115175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gevers D., Kugathasan S., Denson Lee A., Vázquez-Baeza Y., Van Treuren W., Ren B., Schwager E., Knights D., Song Se J., Yassour M., Morgan Xochitl C., Kostic Aleksandar D., Luo C., González A., McDonald D., Haberman Y., Walters T., Baker S., Rosh J., Stephens M., Heyman M., Markowitz J., Baldassano R., Griffiths A., Sylvester F., Mack D., Kim S., Crandall W., Hyams J., Huttenhower C., Knight R., Xavier Ramnik J. The treatment-naive microbiome in new-onset Crohn's disease. Cell Host Microbe. 2014;15:382–392. doi: 10.1016/j.chom.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Degirolamo C., Rainaldi S., Bovenga F., Murzilli S., Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7:12–18. doi: 10.1016/j.celrep.2014.02.032. [DOI] [PubMed] [Google Scholar]
- 65.Nishitani Y., Tanoue T., Yamada K., Ishida T., Yoshida M., Azuma T., Mizuno M. Lactococcus lactis subsp. cremoris FC alleviates symptoms of colitis induced by dextran sulfate sodium in mice. Int Immunopharmacol. 2009;9:1444–1451. doi: 10.1016/j.intimp.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 66.Ryan K.K., Tremaroli V., Clemmensen C., Kovatcheva-Datchary P., Myronovych A., Karns R., Wilson-Perez H.E., Sandoval D.A., Kohli R., Backhed F., Seeley R.J. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature. 2014;509:183–188. doi: 10.1038/nature13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ijssennagger N., Belzer C., Hooiveld G.J., Dekker J., van Mil S.W., Muller M., Kleerebezem M., van der Meer R. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon. Proc Natl Acad Sci U S A. 2015;112:10038–10043. doi: 10.1073/pnas.1507645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Attene-Ramos M.S., Wagner E.D., Plewa M.J., Gaskins H.R. Evidence that hydrogen sulfide is a genotoxic agent. Mol Cancer Res. 2006;4:9–14. doi: 10.1158/1541-7786.MCR-05-0126. [DOI] [PubMed] [Google Scholar]
- 69.Gibson G.R., Cummings J.H., Macfarlane G.T. Growth and activities of sulphate-reducing bacteria in gut contents of healthy subjects and patients with ulcerative colitis. FEMS Microbiol Ecol. 1991;9:103–111. [Google Scholar]
- 70.Loubinoux J., Bisson-Boutelliez C., Miller N., Le Faou A.E. Isolation of the provisionally named Desulfovibrio fairfieldensis from human periodontal pockets. Oral Microbiol Immunol. 2002;17:321–323. doi: 10.1034/j.1399-302x.2002.170510.x. [DOI] [PubMed] [Google Scholar]
- 71.Hagiwara S., Yoshida A., Omata Y., Tsukada Y., Takahashi H., Kamewada H., Koike S., Okuzumi K., Hishinuma A., Kobayashi K., Nakano M. Desulfovibrio desulfuricans bacteremia in a patient hospitalized with acute cerebral infarction: case report and review. J Infect Chemother. 2014;20:274–277. doi: 10.1016/j.jiac.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 72.Tee W., Dyall-Smith M., Woods W., Eisen D. Probable new species of Desulfovibrio isolated from a pyogenic liver abscess. J Clin Microbiol. 1996;34:1760–1764. doi: 10.1128/jcm.34.7.1760-1764.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nambiar P.R., Kirchain S., Fox J.G. Gastritis-associated adenocarcinoma and intestinal metaplasia in a Syrian hamster naturally infected with Helicobacter species. Vet Pathol. 2005;42:386–390. doi: 10.1354/vp.42-3-386. [DOI] [PubMed] [Google Scholar]
- 74.Fox J.G., Li X., Yan L., Cahill R.J., Hurley R., Lewis R., Murphy J.C. Chronic proliferative hepatitis in A/JCr mice associated with persistent Helicobacter hepaticus infection: a model of helicobacter-induced carcinogenesis. Infect Immun. 1996;64:1548–1558. doi: 10.1128/iai.64.5.1548-1558.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wexler H.M. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev. 2007;20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.