Abstract
Background
Capsular contracture is a devastating complication of post-mastectomy implant-based breast reconstruction. Unfortunately, capsular contracture rates are drastically increased by targeted radiotherapy, a standard post-mastectomy treatment. Thy1 (also called CD90) is important in myofibroblast differentiation and scar tissue formation. However, the impact of radiotherapy on Thy1 expression and the role of Thy1 in capsular contracture are unknown.
Methods
We analyzed Thy1 expression in primary human capsular tissue and primary fibroblast explants by RT-qPCR, Western blotting, and immunohistochemistry. Thy1 was depleted using RNA interference to determine if Thy1 expression was essential for the myofibroblast phenotype in capsular fibroblasts. Furthermore, human capsular fibroblasts were treated with a new anti-scarring compound, salinomycin to determine if Thy1 expression and myofibroblast formation were blocked by salinomycin.
Results
Herein, we show that radiation therapy significantly increased Thy1 mRNA and protein expression in peri-implant scar tissue. Capsular fibroblasts explanted from scar tissue retained the ability to make the myofibroblast produced scar forming components collagen I and α-smooth muscle actin (αSMA). Depletion of Thy1 decreased the fibrotic morphology of capsular fibroblasts and significantly decreased αSMA and collagen levels. Furthermore, we show for the first time that salinomycin decreased Thy1 expression and prevented myofibroblast formation in capsular fibroblasts.
Conclusions
These data reveal that ionizing radiation-induced Thy1 over-expression may contribute to increased capsular contracture severity, and fibroblast scar production can be ameliorated through targeting Thy1 expression. Importantly, our new results show promise for the anti-scarring ability of salinomycin in radiation-induced capsular contracture.
Introduction
Breast cancer is the most common malignancy affecting women, and results in the second highest female cancer mortality rate. Effective treatments have significantly prolonged survival. (1) Adjuvant radiation therapy may reduce local recurrence rates and improve long-term survival. However, radiation may induce exuberant scar formation. In light of increased patient survival, physicians now face the daunting task of managing unintended adverse effects associated with cancer treatments.
Over 80,000 women in the United States chose implant-based breast reconstruction during 2014.(2) A sequela of implant placement is the formation of a capsular scar composed of myofibroblasts embedded in a matrix of collagen, fibronectin, and glycosaminoglycans. Myofibroblasts are key effector cells in scar tissue formation and are characterized by alpha-smooth muscle actin (αSMA) expression.(3-5)
When the scarring process persists unabated, patients develop capsular contracture, a pathology marked by shrinkage of the capsule resulting in pain, breast disfigurement, a need for revision surgery, and, ultimately, possible reconstructive failure with implant loss.(6) Ionizing radiation, while beneficial in eliminating residual cancer cells, can exacerbate the scar response, resulting in increased rates of capsular contracture.(7-14). Ionizing radiation compounds the body's natural scarring inclination and increases the incidence of severe capsular contracture from 1.3-30% of non-irradiated reconstruction patients to 32-73% of irradiated, reconstructed patients.(6)
The development of radiation-induced hyperactive scar formation often necessitates surgical mammoplasty revision, straining the healthcare system, and subjecting patients to further morbidity and mortality.(9, 11) There are no effective strategies for preventing or treating radiation-induced scarring in breast tissue and important knowledge gaps remain regarding the specific mechanism underlying this phenomenon.(15-17)
Fibroblasts, sentinel cells that can form many effector cell types, comprise a heterogeneous population that can be characterized based on their expression of the membrane-anchored protein Thy1 (CD90). (18-21) Thy1 has been suggested as a potential marker for predicting myofibroblastic differentiation following exposure to pro-fibrotic stimuli.(18, 21, 22) Fibroblasts that lack surface expression of Thy1 cannot differentiate to myofibroblasts, even under pro-fibrotic stimulation.(21) Conversely, fibroblasts that express high levels of Thy1 can be induced to differentiate into contractile myofibroblasts.(4, 21, 23) We hypothesize that ionizing radiation increases Thy1 expression resulting in increased myofibroblast differentiation and resultant capsular contracture.
Methods
Biopsy Tissue
De-identified tissue was obtained from patients undergoing implant-based breast reconstruction revision surgeries for any reason, including scar contracture, implant exchange, or aesthetic revision, and was frozen at -80° C. A total of twenty-six samples were collected, nine of which were from irradiated breasts, and the remaining seventeen non-irradiated. All samples were handled with proper authorization from the University of Rochester IRB. Tissue was thawed, mechanically minced and incubated on ice in lysis buffer (60 mM Tris, pH 6.8, 2% SDS, and protease inhibitor (Sigma, St. Louis, Missouri)) for 30 minutes. The resulting mixtures were sonicated (Vibra Cell, Sonics & Materials Inc., Dansbury, CT, USA), and the supernatant was stored at -80°C.
Immunohistochemistry of Capsular Tissue
Paraffin embedded, formalin fixed capsular tissue was deparaffinized and treated with Universal antigen unmasking buffer following manufacturer's instructions (R&D Systems). Tissue sections were blocked and probed using sheep anti-Thy1 polyclonal antibody (R&D Systems) at 2.0 ug/mL overnight at 4°C. Anti-sheep HRP-DAB staining kit (R&D Systems) was used following manufacturer's protocol, and slides were counterstained with hematoxylin.
RT-qPCR, Western Blotting & Collagen Slot Blot
Capsular fibroblasts were lysed and passed through a 27 gauge needle to shear genomic DNA. RNA was isolated using the RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to manufacturer's protocol. RNA concentration was measured and analyzed by quantitative PCR using methods and primers described previously (24, 25). Western and collagen slot blots were performed as previously described using rabbit anti-β-tubulin (Cell Signaling), rabbit anti-Thy1 (9798, Cell Signaling), mouse anti-αSMA (A2547, Sigma), and goat anti-collagen 1 (8783, Santa Cruz) (24, 25).
Cell Culture
Primary fibroblast strains, each isolated from a different individual, were explanted from breast skin and peri-implant capsular tissue samples. Tissue was minced and fibroblasts grown and characterized as previously described.(26) Capsular fibroblasts were seeded onto six-well plates and at 80% confluence, were serum starved (MEM w/0.05% FBS) for 48 hours, changed to fresh media (0.05% FBS) and treated with 2.5 ng/mL transforming growth factor- β (TGF-β, R&D Systems) and/or 100-250 nM salinomycin (SNC).
Transduction-mediated Thy1 Knockdown
Capsular fibroblasts were seeded onto six-well plates at a density of 50% and then transduced with pGIPZ shControl or pGIPZ shThy1 lentiviral particles (Open Biosystems) with a multiplicity of infection of 10. Cells were selected for stable integration of transgene expression in 2 ug/mL puromycin. Validation of Thy1 knockdown was confirmed by Western blot.
Immunofluorescence
Capsular fibroblasts on 6-well culture dishes were washed with PBS and fixed with 2% paraformaldehyde for 10 minutes. Cells were rinsed in phosphate buffered saline (PBS), and then permeabilized and blocked with 0.25% Triton-X 100, 1% bovine serum antigen (BSA) in PBS for an additional 30 minutes. Cells were washed and incubated with anti-Thy1 (Brilliant Violet (BV) 421), phalloidin (Alexa Fluor (AF) 594), and the nucleic acid counterstain, SytoxGreen, for 30 minutes. Cells were washed twice and visualized on a ZOE Imager (BioRad).
Flow Cytometry
Cells were collected by trypsinization, washed in PBS, and then blocked with 5% normal mouse serum. Cells were incubated with anti-Thy1-PE conjugated antibody (BD Biosciences) and analyzed on a FACS Canto II flow cytometer (BD Biosciences).
Statistical Analysis
Data were analyzed using GraphPad Prism utilizing student's T-test, one-way and two-way analyses of variance (ANOVA) with Sidak's correction for multiple comparisons. P values < 0.05 were considered significant.
Results
Histological analysis of human breast tissue
The differentiation of fibroblasts into myofibroblasts with subsequent overproduction of extracellular matrix is the hallmark of fibrotic pathologies.(4, 5) To characterize the fibroblasts that contribute to breast implant capsules, we obtained de-identified biopsy samples from both irradiated (n=9) and non-irradiated (n=17) breasts of women undergoing surgery following implant-based breast reconstruction (Fig. 1A).
Figure 1. Capsular Contracture.
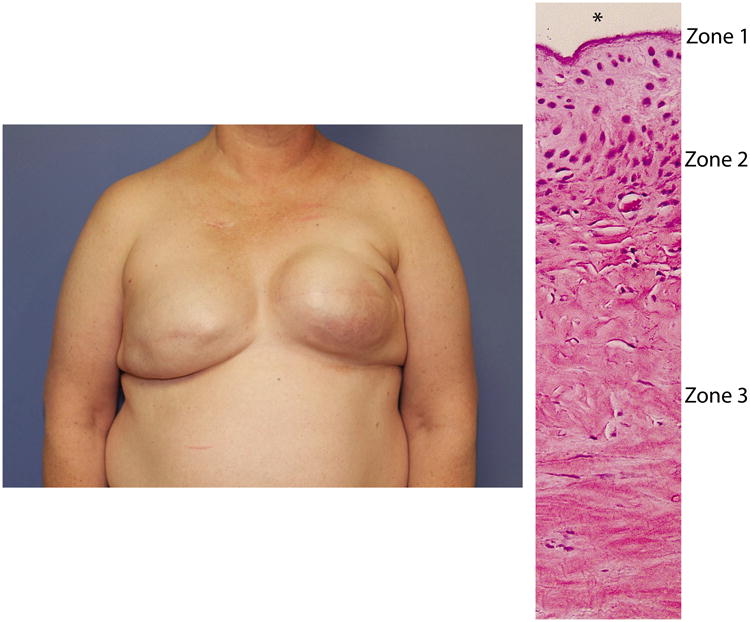
A) Classic retraction and loss of breast contour on left consistent with capsular contracture following implant-based reconstruction mammoplasty. B) Histological section of a peri-implant scar highlighting the zones of capsular contracture. * indicates implant location in fig. 1B.
Histologically, capsular contracture is marked by a hyper-proliferative cellular zone composed of fibroblasts and myofibroblasts that manufacture collagen and other matrix proteins that form the capsule (Fig. 1B).(27) The histopathology of peri-implant capsule is shown in the representative photomicrographs in Supplemental Figure 1(See Supplemental Digital Content 1. Figure shows the Biopsy tissue from non-irradiated peri-implant breast capsule. A and B) Capsular tissue entraps pre-existing adipose tissue (green asterisks) and consists of an innermost synovial-like Zone 1 lining the site of the implant (black asterisks) subtended by a cellular Zone 2 and a peripheral hypocellular Zone 3. C and D) Zone 2 contains numerous plump fibroblasts and myofibroblasts. E and F) Zone 3 fibroblasts are more slender and separated by a dense collagenous matrix with scattered small-caliber blood vessels (black arrows). G and H) Thy1 immunohistochemistry reveals moderate to strong positive immunoreactivity in capsular fibroblasts (red arrows) and endothelium (yellow arrows). H&E (A, C, E, G, and H) and Masson's Trichrome (B, D, and F). Bar = 550 uM (A and B) 150 uM (C-F), or 50 uM (G and H), INSERT LINK). Immunohistochemistry revealed Thy1 staining in the fibroblast.
Thy1 expression is greatest in irradiated capsule
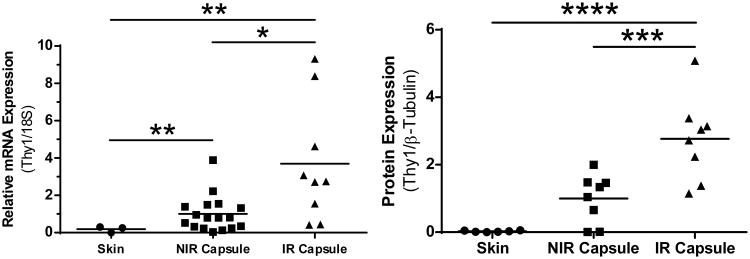
We next quantified and compared several key mRNAs from capsule scar and normal skin samples. There was not a significant difference in the amount of ACTA2 or COL1A mRNA in capsule tissue compared to normal skin despite the pathologic scar tissue present in the capsule. However, Thy1 mRNA in both non-irradiated (NIR) and irradiated (IR) capsule tissue were 5.3 and 18.7-fold increased over levels from normal skin (Fig. 2A). Further analysis confirmed the significance of the 3.7 fold increase in Thy1 in the IR vs. NIR capsules.
Figure 2. Thy1 is elevated in irradiated capsule.
Comparison of Thy1 in tissue samples taken from normal skin, radiation-naïve capsule (NIR capsule), and irradiated capsule (IR Capsule). A) NIR capsule and IR capsule THY1 mRNA is significantly elevated above skin. B) Elevated mRNA expression translated into increased Thy1 protein, with IR capsule significantly elevated above both NIR capsule and skin. Finally, while NIR capsule Thy1 trended higher than skin, this did not reach significance. * = p<0.05, ** = p<0.01. *** = p<0.001, **** = p<0.0001
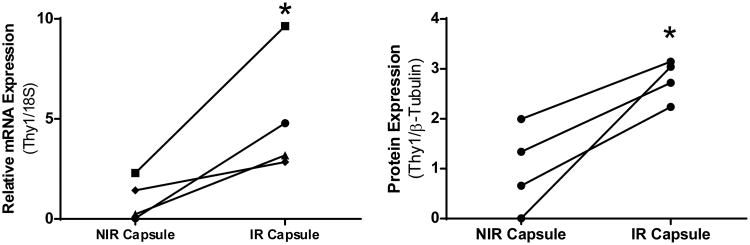
Evaluation of Thy1 protein levels by Western blotting showed that IR capsular lysates were over 100 fold greater than normal skin and 2.8 fold above non-IR capsule samples (Fig. 2B). The mean value for NIR capsule had ∼35-fold increase above normal skin (Fig. 2B). Next, we had the opportunity to compare Thy1 levels from capsule tissue taken from the same patient where one side was irradiated and the other side was not (Fig 3). In this patient matched analysis, IR tissue had 5.1 times greater Thy1 mRNA and 2.8 fold higher Thy1 protein compared to the same patients NIR capsule tissue (Fig. 3).
Figure 3. Radiation increases Thy1 mRNA and protein in patient matched samples.
Compared to their paired, contralateral breast, irradiated breast capsules contained significantly more A) THY1 mRNA, as well as B) Thy1 protein. THY1 mRNA values were normalized to 18S rRNA, and Thy1 protein values were normalized to β-tubulin. * = p<0.05
Explanted human capsular fibroblasts express Thy1
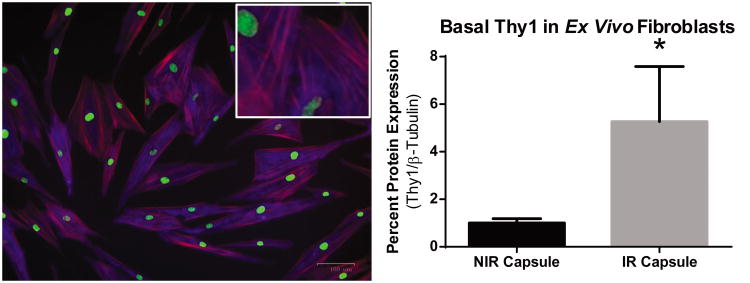
In order to test capsular fibroblasts in vitro, cells were explanted from biopsy samples. We observed that fibroblasts from NIR capsule could be grown, trypsinized and plated to expand the cultures up to at least 12 serial passages, however, IR cells assumed a flattened, spiculated morphology indicative of myofibroblast differentiation (28, 29) and failed to proliferate following explant from the primary tissue. Growth of IR cells was resistant to stimulation, either with fetal bovine serum or with the addition of specialized fibroblast growth medium. Irradiated capsule tissue fibroblasts may be more terminally differentiated into myofibroblasts thus blocking further proliferation and subsequent expansion of the cultures. By contrast, the NIR fibroblasts appeared as a heterogeneous population of cells, spanning the phenotypic spectrum from elongated, rounded fibroblasts to flatter, spiculated cells (Figure 4A). This morphology was maintained through serial passages, and Western blot (Fig. 4B) and flow cytometry (data not shown) confirmed preserved expression of Thy1 protein. Due to poor fibroblast growth from irradiated tissue, these cells could not be expanded; however, enough cells were initially grown to compare basal levels of Thy1. When compared against NIR capsule fibroblasts in vitro, IR fibroblasts demonstrated a 5.3-fold increase in Thy1 (Fig. 5B; p=0.04).
Figure 4. Explanted fibroblasts maintain Thy1 expression.
Thy1 expression is maintained in fibroblasts following explant. A) Fibroblasts were fixed and stained for Thy1 (blue), filamentous actin (red), and nucleic acid (green) to confirm presence of Thy1. B) Western analysis shows that, while all fibroblasts expressed Thy1 protein, fibroblasts explanted from IR Capsule exhibited a 5 -fold increase in Thy1 compared to NIR Capsule. C) Finally, flow cytometry analysis demonstrates a positive shift in signal intensity in Thy1 stained cells compared to unstained fibroblasts. Thy1 protein normalized to β-tubulin. * = p<0.05.
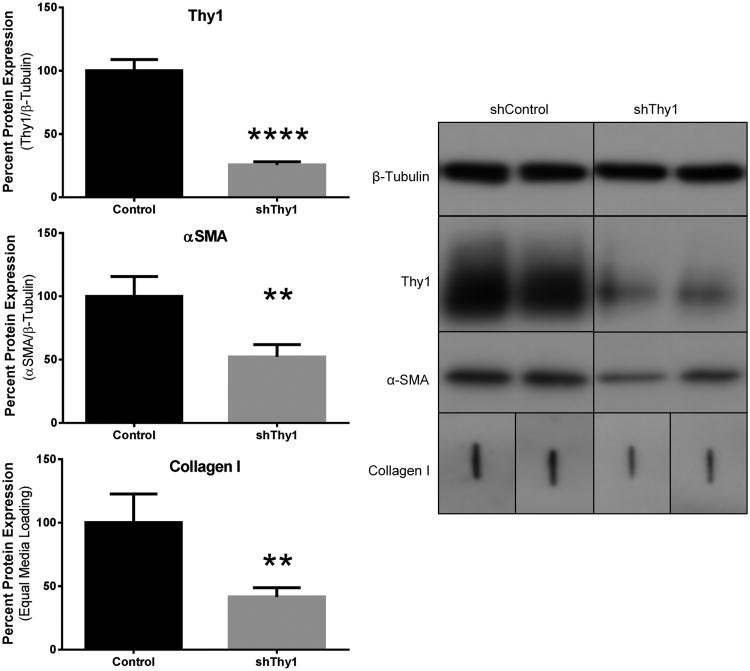
Figure 5. Thy1 depletion reduces αSMA and collagen.
A) Thy1 protein knockdown achieved via short hairpin RNA coding lentiviral vector (shThy1) also reduces αSMA and collagen compared to control shRNA lentiviral vector (Control). Thy1 and αSMA normalized to β-tubulin. Collagen quantification based on equal volume media loading. B) Representative Western blot showing Thy1 and αSMA normalized to β-tubulin. Collagen quantified via slot blot based on loading equal culture supernatant volume. ** = p<0.01; **** = p<0.0001.
Reduction of Thy1 expression using Thy1 shRNA decreases αSMA and collagen levels in capsular fibroblasts
To determine if expression of Thy1 was important for capsular fibroblast morphology, we used short-hairpin RNA (shRNA) to deplete cells of Thy1. Thy1 shRNA treated cells exhibited a 75% reduction in Thy1 expression and αSMA and collagen 1A levels also dropped by 48% and 58% respectively, compared to control fibroblasts (Fig. 5A). These findings were confirmed by Western blot (Fig. 5B). Immunofluorescent images also show the reduction of Thy1 expression and a decrease in parallel protein structure and organization in the shThy1 fibroblasts (Fig. 6). This structural change correlates with a greater proportion of cells assuming a rounded appearance with elongated stroma consistent with undifferentiated fibroblasts. By contrast, control cells appeared morphologically comparable to non-transduced cells, with a mixture of flattened and rounded fibroblasts.
Figure 6. Thy1 depletion affects cellular morphology.
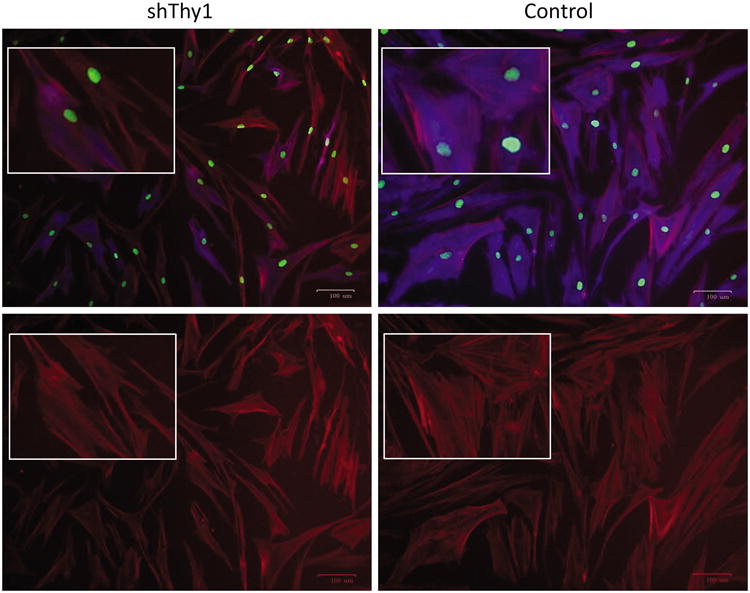
Qualitative changes observed in shThy1 fibroblasts (A&C) compared to control fibroblasts (B&C) include elongated soma with fewer cytoplasmic projections, rounded shape, and greater in vitro cell density. Cells were stained for DNA (green) using Sytox Green, filamentous actin (red) using phallodin-AF594, and Thy1 (blue) using anti-Thy1 antibody conjugated to BV421.
Salinomycin reduces Thy1 expression in human capsular fibroblasts
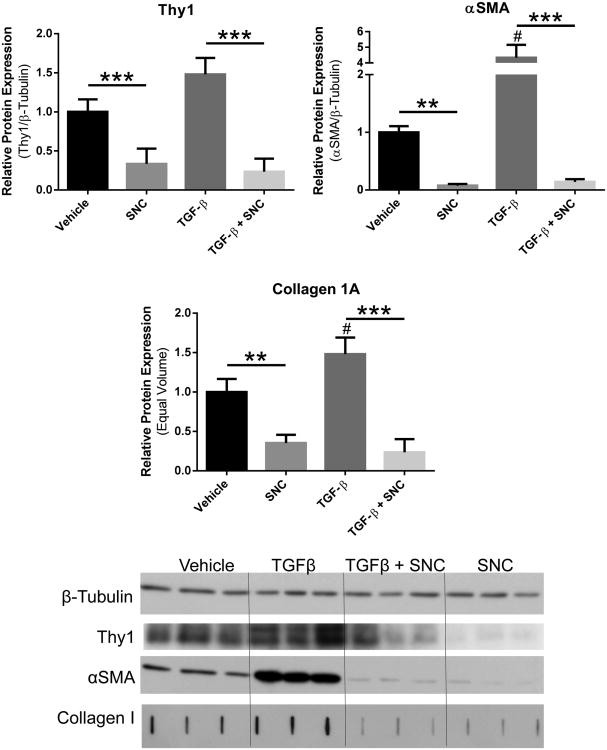
Having shown that fibroblasts maintained Thy1 expression following explant, we next sought to mimic tissue insult via in vitro activation of the scarring pathway. TGF-β, a fibrotic stimulus, induced a 4-fold increase in αSMA, as well as roughly a 50% increase in soluble collagen production (Fig. 7B-C). In a previous screen for anti-scarring drugs, we discovered the anti-scarring effects of an antibiotic, salinomycin (SNC).(25) However, it is unknown whether salinomycin affects Thy1 expression to mitigate myofibroblast formation. When treated with salinomycin, fibroblasts experienced a three-fold reduction in Thy1 expression, while also decreasing αSMA by 65% and collagen by 92% (Fig. 7A-C). Even in the presence of TGF-β, salinomycin dampened myofibroblast formation in human capsular fibroblasts, reducing αSMA by 86%, and collagen 1A by 74% below basal, untreated levels (Fig. 7B-C). Excitingly, salinomycin not only negated the pro-fibrotic effects of TGF- β, but this decrease in capsular fibroblast protein production was associated with a 76% reduction in Thy1 (Fig. 7A). These findings were confirmed by Western blot (Fig. 7D).
Figure 7. Salinomycin reduces basal and TGF-β-induced myofibroblast formation.
Fibroblasts increased production of α-smooth muscle actin (αSMA) by 334% and collagen 1A production by 48% when exposed to TGF-β. Treatment with salinomycin (SNC) not only reduces basal expression of A) Thy1, B) αSMA, and C) collagen 1A, but also prevents myofibroblast induction by TGF-β. D) Thy1 and αSMA levels normalized to β-tubulin. Collagen quantified via slot blot based on loading equal culture supernatant volume. # = p<0.05 compared to vehicle. ** = p<0.01; **** = p<0.0001.
Discussion
This study demonstrates that peri-implant capsules express significantly higher Thy1 levels than normal skin samples. Furthermore, radiated capsule showed higher levels of Thy1 mRNA and protein than non-irradiated capsule. Increased Thy1 may relate to a fibroblast cell population or population subset that is more responsive to chemoattraction and activation by pro-fibrotic cytokines. Fibroblasts that highly express Thy1 are present and/or recruited to the peri-implant site in order to respond to the inflammatory signaling caused by tissue injury that leads to wound healing and scarring. Normal skin was chosen as the control tissue as breast parenchyma is largely composed of adipocytes, which likely arise from Thy1 negative fibroblasts, and therefore do not possess the same scarring potential.(20, 30)
We further divided peri-capsular tissue into biopsies taken from breasts that had previously undergone radiation therapy, and those from radiation-naïve breasts. When compared, radiation treated tissue showed higher Thy1 mRNA and protein levels. Given the scope of our current study, and the fact that patient de-identification precluded us from doing so, we chose not to pursue correlation of Thy1 to factors such as time from implant placement, time from radiation treatment, Baker stage, or other variables. However, the acquisition of bilateral samples from four women post-unilateral radiation treatment enabled paired comparisons utilizing a patient's own NIR tissue as an internal control. This helped to control for the large degree of inter-human variability, as well as time from surgery to tissue harvest. These novel results show that a history of radiation treatment correlates with increased Thy1, indicating that radiotherapy up regulates locoregional protein expression. This finding is consistent with published data showing Thy1 up regulation following inflammatory stimulus.(18, 22, 31) The degree to which Thy1 is expressed in a given tissue may then correlate with the propensity of fibroblasts in that tissue to differentiate to myofibroblasts and increase their scar production. While our data show that radiation leads to increased expression of Thy1 in capsular scars, it is unknown if Thy1 expression would differ based on capsules that form due to other insults such as microbial biofilm, heme deposits, etc. Future work looking at capsules that form from other etiologies, as well as Thy1 kinetics in capsular tissue after irradiation, are required.
While the full extent of its physiologic role remains enigmatic, Thy1 does appear to play a role in fibroblast differentiation.(20, 21) We sought to isolate and reduce the Thy1 levels on capsular fibroblasts to determine downstream effects on collagen and αSMA production. Thy1 reduction translated into a concomitant reduction in both αSMA and collagen without any additional treatments, indicating that Thy1 expression is important in determining a cell's basal scar production ability. Furthermore, Thy1 reduction also altered the cellular phenotype of capsular fibroblasts. The differentiation of fibroblasts to myofibroblasts with increased matrix deposition and the generation of contractile forces in vivo requires multiple signaling mechanisms at the site of tissue injury.(32-34) Current understanding implicates a central role for TGF-β in both the inflammatory and fibrotic phases of capsular scar development.(35, 36) Acute injury at the site as a result of surgery, infection, radiation, mechanical stress, or other factors causes TGF-β release in conjunction with dense granule release from platelets.(37, 38) In this setting, the local TGF-β acts as a chemokine for monocyte recruitment and eventual differentiation to macrophages, thereby priming the chronic inflammatory phase.(39, 40) During this phase, macrophages continue production of TGF-β, which signals migration of either resident or circulating fibrocytes to the site of injury, wherein they produce the lattice of collagen, fibronectin, and αSMA that will eventually form a mature scar. (36)
Having established TGF-β-induced production of αSMA and collagen, we next sought to determine the effects of salinomycin on both untreated and induced cells. Salinomycin successfully reduced αSMA and collagen production as previously shown in other fibroblasts.(25) Excitingly, in this study, we show for the first time that salinomycin reduced expression of Thy1 in human capsular fibroblasts. Interestingly, a greater reduction in scar matrix protein production was seen with salinomycin treatment than with Thy1 reduction via lentiviral transduction alone, and likely occurs due the effect of salinomycin on both Thy1 expression, as well as its influence on the TGF-β-induced cellular response.(25)
Similar to Thy1 reduction via lentiviral vector, fibroblasts treated with salinomycin also exhibited morphologic changes. This may, in fact, represent a phenotypic reversal of a proportion of total cells from a myofibroblast state back to fibroblasts, as loss of Thy1 prevents these cells from being able to maintain their myofibroblast differentiation. The phenotypic change in these cells persisted despite co-culture with TGF-β, consistent with previous work showing Thy1 negative fibroblasts are resistant to pro-fibrotic influence.(21) Thus, salinomycin appears to have a dual mechanism of scar mitigation, both by 1) decreasing constitutive Thy1 expression and preventing associated myofibroblast differentiation, and 2) reducing the inherent cellular response to TGF-β.
The ability of salinomycin to blunt capsular myofibroblast formation reveals that future studies testing salinomycin efficacy in vivo are warranted. To treat capsular contracture, many delivery methods of salinomycin or other promising drug candidates are possible. Systemic routes such as oral dosing, intravenous injections or localized capsule coating or site injections could be considered. We recently reported coating a temporary wound dressing with salinomycin and demonstrated dramatic reduction in myofibroblast formation(41). Salinomycin is an antibiotic and has can kill many gram positive bacteria, thus an additional potential benefit could be its anti-microbial action if used in vivo (25). Furthermore, salinomycin is also being tested as a chemotherapy for breast cancer through injection and has so far proved promising (42-44). Future studies aimed at elucidating the best delivery methods for blocking capsular contracture are needed.
Conclusions
Increased Thy1 in fibroblasts from IR breast capsules suggests a possible mechanism for the increased incidence of capsular contracture in radiotherapy-treated patients. Radiated capsule showed higher Thy1 mRNA and protein levels, but in vivo blockade using Salinomycin resulted in reduced scar component production and a phenotypic disruption of myofibroblast transformation. Given our new understanding of Thy1's role in myofibroblast differentiation and scar production in capsular contracture, Thy1-targeting therapies such as Salinomycin could potentially reduce capsular scarring following radiation or inhibit its formation if preempted appropriately.
Supplementary Material
Supplemental Digital Content 1. Figure shows the Biopsy tissue from non-irradiated peri-implant breast capsule. A and B) Capsular tissue entraps pre-existing adipose tissue (green asterisks) and consists of an innermost synovial-like Zone 1 lining the site of the implant (black asterisks) subtended by a cellular Zone 2 and a peripheral hypocellular Zone 3. C and D) Zone 2 contains numerous plump fibroblasts and myofibroblasts. E and F) Zone 3 fibroblasts are more slender and separated by a dense collagenous matrix with scattered small-caliber blood vessels (black arrows). G and H) Thy1 immunohistochemistry reveals moderate to strong positive immunoreactivity in capsular fibroblasts (red arrows) and endothelium (yellow arrows). H&E (A, C, E, G, and H) and Masson's Trichrome (B, D, and F). Bar = 550 uM (A and B) 150 uM (C-F), or 50 uM (G and H), INSERT LINK.
Acknowledgments
This work generously supported by the Clinical and Translational Science Institute grant GR525581, a Wilmot Cancer Center Seed Grant, and NIH grant HL127001. We thank Katie Lannan and Amanda Croadsdell for discussions and help with preparing the manuscript.
Footnotes
Trevor C. Hansen: Performed and designed experiments, analyzed the data and wrote the paper.
Collynn F. Woeller: Performed and designed experiments, analyzed the data and wrote the paper.
Shannon H. Lacy: Performed and designed experiments, analyzed the data and wrote the paper.
Peter F. Koltz: Designed experiments, analyzed the data and wrote the paper.
Howard N. Langstein: Designed experiments, analyzed the data and wrote the paper.
Richard P. Phipps: Designed experiments, analyzed the data and wrote the paper.
References
- 1.Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2010. Bethesda, MD: National Cancer Institute; 2013. [Google Scholar]
- 2.Surgeons ASoP. 2014 Report of the 2013 Statistics: National Clearinghouse of Plastic Surgery Statistics. [Accessed March 12, 2015]; Available at: http://www.plasticsurgery.org.ezpminer.urmc.rochester.edu/Documents/news-resources/statistics/2012-Plastic-Surgery-Statistics/full-plastic-surgery-statistics-report.pdf.
- 3.Gabbiani G. The myofibroblast: a key cell for wound healing and fibrocontractive diseases. Progress in clinical and biological research. 1981;54:183–194. [PubMed] [Google Scholar]
- 4.Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. The Journal of pathology. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- 5.Sappino AP, Schürch W, Gabbiani G. Differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic modulations. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- 6.Araco A, Caruso R, Araco F, Overton J, Gravante G. Capsular contractures: a systematic review. Plast Reconstr Surg. 2009;124:1808–1819. doi: 10.1097/PRS.0b013e3181bf7f26. [DOI] [PubMed] [Google Scholar]
- 7.Cordeiro PG, McCarthy CM. A single surgeon's 12-year experience with tissue expander/implant breast reconstruction: part II. An analysis of long-term complications, aesthetic outcomes, and patient satisfaction. Plast Reconstr Surg. 2006;118:832–839. doi: 10.1097/01.prs.0000232397.14818.0e. [DOI] [PubMed] [Google Scholar]
- 8.Cordeiro PG, Albornoz CR, McCormick B, Hu Q, Van Zee K. The impact of postmastectomy radiotherapy on two-stage implant breast reconstruction: an analysis of long-term surgical outcomes, aesthetic results, and satisfaction over 13 years. Plast Reconstr Surg. 2014;134:588–595. doi: 10.1097/PRS.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 9.Hvilsom GB, Hölmich LR, Steding-Jessen M, et al. Delayed breast implant reconstruction: is radiation therapy associated with capsular contracture or reoperations? Ann Plast Surg. 2012;68:246–252. doi: 10.1097/SAP.0b013e318214e69c. [DOI] [PubMed] [Google Scholar]
- 10.McCarthy CM, Pusic AL, Disa JJ, McCormick BL, Montgomery LL, Cordeiro PG. Unilateral postoperative chest wall radiotherapy in bilateral tissue expander/implant reconstruction patients: a prospective outcomes analysis. Plast Reconstr Surg. 2005;116:1642–1647. doi: 10.1097/01.prs.0000187794.79464.23. [DOI] [PubMed] [Google Scholar]
- 11.Cordeiro PG, Pusic AL, Disa JJ, McCormick B, VanZee K. Irradiation after immediate tissue expander/implant breast reconstruction: outcomes, complications, aesthetic results, and satisfaction among 156 patients. Plast Reconstr Surg. 2004;113:877–881. doi: 10.1097/01.prs.0000105689.84930.e5. [DOI] [PubMed] [Google Scholar]
- 12.Fischer JP, Basta MN, Shubinets V, Serletti JM, Fosnot JA. Systematic Meta-analysis of Prosthetic-Based Breast Reconstruction in Irradiated Fields With or Without Autologous Muscle Flap Coverage. Ann Plast Surg. 2014 doi: 10.1097/SAP.0000000000000288. [DOI] [PubMed] [Google Scholar]
- 13.Kraemer O, Andersen M, Siim E. Breast reconstruction and tissue expansion in irradiated versus not irradiated women after mastectomy. Scand J Plast Reconstr Surg Hand Surg. 1996;30:201–206. doi: 10.3109/02844319609062815. [DOI] [PubMed] [Google Scholar]
- 14.Collier P, Williams J, Edhayan G, Kanneganti K, Edhayan E. The effect of timing of postmastectomy radiation on implant-based breast reconstruction: a retrospective comparison of complication outcomes. Am J Surg. 2014;207:408–411. doi: 10.1016/j.amjsurg.2013.09.016. discussion 410-401. [DOI] [PubMed] [Google Scholar]
- 15.Katzel EB, Koltz PF, Tierney R, et al. The impact of Smad3 loss of function on TGF-β signaling and radiation-induced capsular contracture. Plast Reconstr Surg. 2011;127:2263–2269. doi: 10.1097/PRS.0b013e3182131bea. [DOI] [PubMed] [Google Scholar]
- 16.Lipa JE, Qiu W, Huang N, Alman BA, Pang CY. Pathogenesis of radiation-induced capsular contracture in tissue expander and implant breast reconstruction. Plast Reconstr Surg. 2010;125:437–445. doi: 10.1097/PRS.0b013e3181c82d05. [DOI] [PubMed] [Google Scholar]
- 17.Katzel EB, Koltz PF, Tierney R, et al. A novel animal model for studying silicone gel-related capsular contracture. Plast Reconstr Surg. 2010;126:1483–1491. doi: 10.1097/PRS.0b013e3181ef8b8e. [DOI] [PubMed] [Google Scholar]
- 18.Jurisic G, Iolyeva M, Proulx ST, Halin C, Detmar M. Thymus cell antigen 1 (Thy1, CD90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Exp Cell Res. 2010;316:2982–2992. doi: 10.1016/j.yexcr.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baglole CJ, Ray DM, Bernstein SH, et al. More than structural cells, fibroblasts create and orchestrate the tumor microenvironment. Immunological investigations. 2006;35:297–325. doi: 10.1080/08820130600754960. [DOI] [PubMed] [Google Scholar]
- 20.Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1- subpopulations exhibit distinct phenotypes. European journal of immunology. 2002;32:477–485. doi: 10.1002/1521-4141(200202)32:2<477::AID-IMMU477>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 21.Koumas L, Smith TJ, Feldon S, Blumberg N, Phipps RP. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. The American journal of pathology. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mason JC, Yarwood H, Tarnok A, et al. Human Thy-1 is cytokine-inducible on vascular endothelial cells and is a signaling molecule regulated by protein kinase C. J Immunol. 1996;157:874–883. [PubMed] [Google Scholar]
- 23.Lehmann GM, Xi X, Kulkarni AA, et al. The aryl hydrocarbon receptor ligand ITE inhibits TGFβ1-induced human myofibroblast differentiation. Am J Pathol. 2011;178:1556–1567. doi: 10.1016/j.ajpath.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woeller CF, O'Loughlin CW, Pollock SJ, Thatcher TH, Feldon SE, Phipps RP. Thy1 (CD90) controls adipogenesis by regulating activity of the Src family kinase, Fyn. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2015;29:920–931. doi: 10.1096/fj.14-257121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woeller CF, O'Loughlin CW, Roztocil E, Feldon SE, Phipps RP. Salinomycin and other polyether ionophores are a new class of antiscarring agent. The Journal of biological chemistry. 2015;290:3563–3575. doi: 10.1074/jbc.M114.601872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lehmann GM, Woeller CF, Pollock SJ, et al. Novel anti-adipogenic activity produced by human fibroblasts. American journal of physiology Cell physiology. 2010 doi: 10.1152/ajpcell.00451.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prantl L, Schreml S, Fichtner-Feigl S, et al. Clinical and morphological conditions in capsular contracture formed around silicone breast implants. Plast Reconstr Surg. 2007;120:275–284. doi: 10.1097/01.prs.0000264398.85652.9a. [DOI] [PubMed] [Google Scholar]
- 28.Eyden B. The myofibroblast: a study of normal, reactive and neoplastic tissues, with an emphasis on ultrastructure part 2 - tumours and tumour-like lesions. Journal of submicroscopic cytology and pathology. 2005;37:231–296. [PubMed] [Google Scholar]
- 29.Baum J, Duffy HS. Fibroblasts and myofibroblasts: what are we talking about? J Cardiovasc Pharmacol. 2011;57:376–379. doi: 10.1097/FJC.0b013e3182116e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lehmann GM, Woeller CF, Pollock SJ, et al. Novel anti-adipogenic activity produced by human fibroblasts. Am J Physiol Cell Physiol. 2010;299:C672–681. doi: 10.1152/ajpcell.00451.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zimmermann T, Kunisch E, Pfeiffer R, et al. Isolation and characterization of rheumatoid arthritis synovial fibroblasts from primary culture--primary culture cells markedly differ from fourth-passage cells. Arthritis Res. 2001;3:72–76. doi: 10.1186/ar142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song C. Hypertrophic scars and keloids in surgery: current concepts. Ann Plast Surg. 2014;73(1):S108–118. doi: 10.1097/SAP.0000000000000256. [DOI] [PubMed] [Google Scholar]
- 33.Wick G, Grundtman C, Mayerl C, et al. The immunology of fibrosis. Annu Rev Immunol. 2013;31:107–135. doi: 10.1146/annurev-immunol-032712-095937. [DOI] [PubMed] [Google Scholar]
- 34.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kane CJ, Hebda PA, Mansbridge JN, Hanawalt PC. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991;148:157–173. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- 36.Leask A, Abraham DJ. TGF-beta signaling and the fibrotic response. FASEB J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 37.Harrison P, Cramer EM. Platelet alpha-granules. Blood Rev. 1993;7:52–62. doi: 10.1016/0268-960x(93)90024-x. [DOI] [PubMed] [Google Scholar]
- 38.Ashcroft GS. Bidirectional regulation of macrophage function by TGF-beta. Microbes Infect. 1999;1:1275–1282. doi: 10.1016/s1286-4579(99)00257-9. [DOI] [PubMed] [Google Scholar]
- 39.Wahl SM, McCartney-Francis N, Allen JB, Dougherty EB, Dougherty SF. Macrophage production of TGF-beta and regulation by TGF-beta. Ann N Y Acad Sci. 1990;593:188–196. doi: 10.1111/j.1749-6632.1990.tb16111.x. [DOI] [PubMed] [Google Scholar]
- 40.Johnston RB. Current concepts: immunology Monocytes and macrophages. N Engl J Med. 1988;318:747–752. doi: 10.1056/NEJM198803243181205. [DOI] [PubMed] [Google Scholar]
- 41.Woodroof A, Phipps R, Woeller C, et al. Evolution of a Biosynthetic Temporary Skin Substitute: A Preliminary Study. Eplasty. 2015;15:e30. [PMC free article] [PubMed] [Google Scholar]
- 42.Al Faraj A, Shaik AS, Ratemi E, Halwani R. Combination of drug-conjugated SWCNT nanocarriers for efficient therapy of cancer stem cells in a breast cancer animal model. J Control Release. 2016;225:240–251. doi: 10.1016/j.jconrel.2016.01.053. [DOI] [PubMed] [Google Scholar]
- 43.Kopp F, Hermawan A, Oak PS, Herrmann A, Wagner E, Roidl A. Salinomycin treatment reduces metastatic tumor burden by hampering cancer cell migration. Molecular cancer. 2014;13:16. doi: 10.1186/1476-4598-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gupta PB, Onder TT, Jiang G, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content 1. Figure shows the Biopsy tissue from non-irradiated peri-implant breast capsule. A and B) Capsular tissue entraps pre-existing adipose tissue (green asterisks) and consists of an innermost synovial-like Zone 1 lining the site of the implant (black asterisks) subtended by a cellular Zone 2 and a peripheral hypocellular Zone 3. C and D) Zone 2 contains numerous plump fibroblasts and myofibroblasts. E and F) Zone 3 fibroblasts are more slender and separated by a dense collagenous matrix with scattered small-caliber blood vessels (black arrows). G and H) Thy1 immunohistochemistry reveals moderate to strong positive immunoreactivity in capsular fibroblasts (red arrows) and endothelium (yellow arrows). H&E (A, C, E, G, and H) and Masson's Trichrome (B, D, and F). Bar = 550 uM (A and B) 150 uM (C-F), or 50 uM (G and H), INSERT LINK.