Abstract
The relevance of adiponectin to insulin sensitivity has been elucidated over the last two decades. As a promoter of ceramide degradation, it works through its cognate receptors, AdipoR1 and AdipoR2, to alter bioactive sphingolipid species. Adiponectin diminishes the accumulation of ceramide, a lipid metabolite which can play a causal role in obesity-induced insulin resistance. Concurrently, adiponectin stimulates the production of sphingosine-1-phosphate (S1P), a cyto-protective molecule that accentuates adiponectin’s positive metabolic effects. This review focuses on recent work that solidifies knowledge of the adiponectin signaling pathway, gives new insight into some notable characteristics of adiponectin’s receptors, and most importantly, affirms adiponectin receptor agonism as a viable therapeutic tool to combat elevated ceramide levels and improve insulin sensitivity in obese patients with type II diabetes.
Keywords: Ceramide, ceramidase, AMPK, S1P, insulin resistance
Introduction
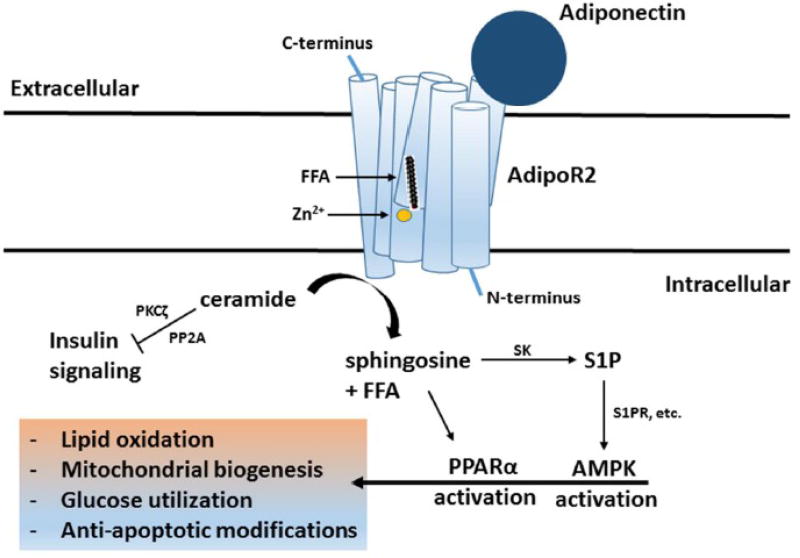
As obesity has become more common, its associated risks, such as insulin resistance and cardiovascular disease have followed its trajectory. This incredibly relevant relationship has been the focus of extensive research. Many mechanisms detailing this link have been presented and one compelling explanation involves ceramides, which are simple sphingolipids and act as precursors to other sphingolipids. Ceramide biosynthesis, which has been detailed comprehensively [1], involves the initial condensation of serine and palmitoyl-CoA by serine palmitoyltranferase (SPT) to produce 3-oxosphinganine. The abnormal accumulation of ceramides in various tissues has already been implicated in numerous pathologies, including but not limited to, atherosclerosis [2], cardiomyopathy [3], vascular dysfunction [4], lipotoxic cell death [5,6,7], and most importantly for the purposes of this review, insulin resistance [8,9]. Elevated circulating ceramides have also been reported in human subjects with type II diabetes [10,11,12], pointing either to a causative effect or implying at the very least that ceramide is an effective marker of insulin resistance. If it is an integral component of obesity’s connection to insulin resistance, ceramide presents itself as a viable target of therapeutic endeavors going forward. In recent years, adiponectin (Acrp30) and its signaling mechanism have become potential avenues through which these endeavors can be pursued (Figure 1).
Figure 1.
Adiponectin-mediated agonism of adipoR1 and adipoR2 enhances receptor-intrinsic ceramidase activity. This degrades ceramide, a bioactive sphingolipid that blunts insulin signaling via PKCζ and PP2A. Specifically, this catalytic activity is driven by the nucleophilic cleavage of ceramide’s defining amide bond by a zinc-stabilized hydroxide ion. The resulting sphingosine is available for conversion into S1P by sphingosine kinase (SK). S1P is further involved with AMPK activation, while sphingosine and free fatty acid (FFA) are available to serve as ligands for PPARα activation. Together, these activities promote lipid oxidation, mitochondrial biogenesis, glucose utilization, and other anti-apoptotic modifications to culminate in adiponectin’s metabolic benefits within target cells.
Adiponectin, first identified by Scherer et al. in 1995 [13], is an adipokine that has attracted significant attention in the past few decades [14]. Counterintuitively, it is secreted inversely with obesity-related adipose tissue expansion [15,16,17]. Adiponectin’s structure, secretion, and regulation have been described in depth by Scherer et al. in the 1990’s and reviewed extensively by Ye et al. and Yamauichi et al. [14,18]. Though numerous metabolic benefits have been ascribed to adiponectin, its protective nature, quite possibly, is its most important. As reviewed extensively in 2013 and 2014 by Holland and Tao respectively [19,20], adiponectin is anti-apoptotic [21], anti-fibrotic [22], anti-inflammatory [23,24], anti-lipotoxic [25], promotes ceramide reduction, and is insulin sensitizing [26]. In light of recent advances, we will look to summarize ceramide’s insulin desensitizing role, overview adiponectin’s ceramide lowering and insulin sensitizing effects, and bring attention to new knowledge that reveals the dynamic nature of adiponectin’s receptors, especially in regards to combatting insulin resistance.
Ceramides promote insulin resistance
Work by Holland et al. in 2007 demonstrated that atypical, elevated ceramide synthesis contributes to insulin resistance [8]. Dexamethasone is a synthetic glucocorticoid that stimulates the expression of ceramide synthesis genes and increases overall ceramide content in serum and tissues. It was shown to impair glucose homeostasis and insulin signaling via in vivo studies that involved measuring serum glucose levels, glucose/insulin tolerance tests, and insulin stimulated pSerine473-AKT/total AKT ratios. All parameters were markedly worse in dexamethasone treated rodents. Hyperinsulinemic-euglycemic clamps confirmed this impairment as well. Dexamethasone treatment led to a dramatic decline in the glucose infusion rate needed to maintain euglycemia (~150 mg/dL) under hyperinsulinemia; this difference was brought upon by insulin’s inability to effectively suppress hepatic glucose output and promote glucose uptake in the skeletal muscle of dexamethasone treated rodents. However, the concurrent administration of myriocin, a fungal antibiotic that potently inhibits serine palmitoyltransferase (SPT), significantly mitigated dexamethasone’s insulin desensitizing effects. Myriocin treatment neutralized dexamethasone-induced disruption in glucose homeostasis and restored all the previously mentioned metabolic parameters to levels representative of vehicle treatment. Further studies in Zucker diabetic fatty (ZDF) rats corroborated the anti-diabetic effects of myriocin, solidifying ceramide as central to lipotoxicity-mediated insulin resistance [8]. Myriocin’s insulin sensitizing effects have been affirmed by others as well [27,28,29].
The deleterious effects of ceramide were further delineated by Xia et al. in 2015. In their studies, they generated transgenic mice that overexpressed acid ceramidase (ASAH1) in a liver or white adipose tissue specific, titratable, and doxycycline-inducible manner. These mice demonstrated the effectiveness of localized ceramide degradation in protection against HFD-induced insulin resistance, metabolic dysfunction, and tissue lipotoxicity. Both transgenic models, which had significantly lower amounts of several ceramide species (namely C16:0, C18:0, and C20:0 ceramides) in both the targeted tissues and serum, demonstrated greatly improved glucose homeostasis/tolerance, enhanced whole body insulin sensitivity, and an overall decline in lipotoxicity triggered complications (such as fibrosis and inflammation) in comparison to their wildtype counterparts under an extended high fat diet (HFD) challenge. Most evaluations took place after at least 8 weeks on HFD (doxycycline was administered with the HFD after ~2 months of age) [9]. Interestingly, it seems that localized ceramide degradation in either the liver or white adipose tissue was sufficient to protect against hepatic steatosis and improve hepatic insulin sensitivity in transgenic mice under HFD challenge. This indicates, as Xia and colleagues put it, a “cross-talk” of sorts between the liver and white adipose tissue; degrading ceramides in one tissue significantly lowers ceramides in both tissues, with the white adipose tissue specific degradation acting as a far more potent regulator of ceramide content in both tissues [9]. In 2016, Chaurasia et al. also looked at the effects of lowering ceramides in white adipose tissue by knocking out SPT long chain base unit 1 (Sptlc1) in obese mice. They observed reduced levels of several ceramide species and changes in fat pad weight that mirrored observations made by Xia and colleagues in 2015. However, the Sptlc1 knockout mice also displayed improvements in white adipose tissue energy expenditure and overall beiging [30]. This tissue remodeling was not seen in the Art-AC (which overexpress ASAH1 in white adipose tissue) mice used by Xia et al. This is likely because the lysosomal degradation of ceramide does not produce sphingosine that can be phosphorylated by sphingosine kinase (SK). However, the accumulated sphingosine in the Sptlc1 knockout mice is available for phosphorylation. The sphingosine-1-phosphate (S1P) that results may play a role in the thermogenic differences between these two transgenic models.
Though the precise minutiae of ceramide’s insulin desensitizing effects are not completely resolved, there are some convincing suggestions. One argument indicates that ceramides blunt insulin signaling by impairing activation of Akt/PKB, a central mediator of insulin-mediated GLUT4 translocation and other anabolic effects. Dual mechanisms involving protein kinase C isoform ζ (PKCζ and protein phosphatase 2A (PP2A) prevent Akt/PKB activation and stimulate its inactivation, respectively. Specifically, ceramides block the translocation of Akt/PKB to the plasma membrane via an inhibitory phosphorylation mediated by PKCζ [31,32,33] and promote the inhibitory de-phosphorylation of Akt/PKB via PP2A activation [34,35,36,37].
Additionally, specific ceramide species have been identified as leading antagonists of insulin signaling [38,39,40,41,42,43,44]. In 2014, Raichur et al. showed that ceramide synthase 2 (CerS2) haploinsufficiency decreases glucose tolerance and insulin sensitivity. Paralleling these observations was a striking elevation in hepatic C16:0 ceramide levels. This was induced by an increased, likely compensatory, expression of synthases (such as CerS6) involved with the production of C16:0 ceramides [38]. Studies involving obese human subjects further implicate C16:0 ceramides. Compared to lean subjects, obese individuals with lowered glucose tolerance and insulin sensitivity, have greatly elevated C16:0 ceramide levels and increased CerS6 expression in visceral white adipose tissue [39]. In vivo deletion of CerS6 in mice also leads to decreased C16:0 ceramides and much improved glucose tolerance/insulin sensitivity under high fat diet challenge (HFD) challenge [39]. C18:0 ceramide has also been widely correlated with insulin resistance [40,41,42,43]. Taken together, the aberrant accumulation of ceramide (especially C16:0 and C18:0 ceramides) in tissues not primarily designed to store fats is critical for the development of obesity-induced type II diabetes. Ceramides help maintain the link between obesity and diabetes.
Adiponectin, an insulin-sensitizing “friendly adipokine”, stimulates ceramidase activity via adipoR1 and adipoR2
Adiponectin’s ceramide lowering and anti-diabetic effects were covered by Holland and colleagues in 2011 [26]. In that paper, acute administration of recombinant adiponectin to ob/ob mice, which are leptin deficient and normally display elevated ceramides due to fat overload, universally lowered all ceramide species in livers. Furthermore, when obese mice on a long term HFD were acutely given recombinant adiponectin, they displayed significant declines in hepatic ceramide content in comparison to obese mice that were administered PBS. These observations affirmed the connection between adiponectin and ceramide reduction. Evidence for adiponectin’s insulin sensitizing effects was also overwhelming. Adiponectin administered ob/ob mice displayed markedly improved insulin response during hyperinsulinemic-euglycemic clamps; they required a far greater glucose infusion rate to maintain euglycemia (~150 mg/dL) and exhibited increased suppression of hepatic glucose output under hyperinsulinemia. Overall, recombinant adiponectin treatment allowed ob/ob mice to have enhanced glucose homeostasis and whole body insulin sensitivity in comparison to control ob/ob mice.
The paper also described the potential manner in which adiponectin exerts its effects. This brings us to adiponectin’s cognate receptors, adipoR1 and adipoR2. Though both are ubiquitously expressed, AdipoR1 and adipoR2 are specifically abundant in skeletal muscle and liver, respectively. They are highly conserved members of the PAQR family and reverse g-protein coupled receptors with seven transmembrane domains [45,46]. For this review, their relevance is tied to their involvement in insulin sensitization via adiponectin’s ceramide lowering effects. Before 2011, various PAQRs had been implicated with enhancing ceramidase activity. In 2009, Villa et al. even went so far as to show that some have considerable sequence homology with alkaline ceramidases, implying that adipoR1 and adipoR2 may have intrinsic catalytic roles [47]. Other research showed that adiponectin acts through its receptors to boost AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor α (PPARα) activity [48]. This increase in AMPK and PPARα activity conveys adiponectin’s benefits in various tissues, specifically liver and skeletal muscle, leading to enhanced lipid oxidation, mitochondrial biogenesis, and other anti-apoptotic modifications [49,50,51]. Regarding improved insulin sensitivity, the implication is that an increase in AMPK-mediated lipid oxidation depletes the cellular availability of sphingolipid precursors and therefore, enhances insulin signaling due to reduced ceramide content [8]. This alternative, or perhaps coinciding, mechanism emphasizes a downstream elimination of ceramides brought upon by adiponectin’s interaction with its receptors.
Regardless, advancing previous research, Holland et al. (2011) argued that adiponectin promotes ceramidase activity via adipoR1 and adipoR2 [26,52] and in doing so, eliminates ceramide, which blunts insulin signaling, and generates sphingosine-1-phosphate (S1P), which has been shown to be cytoprotective and anti-apoptotic [53,54,55]. In these studies, the use of mouse embryonic fibroblasts (MEF) showed the dependence of adiponectin on its receptors for ceramidase activity. MEF cells that lacked adipoR1 and adipoR2 (double knockouts or DKO) were incredibly resistant to adiponectin’s stimulation of ceramidase activity. The DKO MEFs exhibited much lower levels of S1P and vastly increased levels of cell death, thwarting adiponectin’s well documented anti-apoptotic effects and further highlighting the importance of adiponectin’s receptors. They also documented in vivo stimulation of ceramidase activity in the presence of adipoR1 and adipoR2 overexpression within the liver. These overexpressing mice displayed significantly elevated hepatic ceramidase activity and dramatically reduced hepatic ceramide content after an extended HFD challenge.
While this work confirms adiponectin’s promotion of ceramidase activity and its mechanism of action, it also brings attention to a deeper discussion about the nature of adiponectin’s receptors and their molecular mechanisms. Do they have intrinsic ceramidase activity or do they accentuate adiponectin’s messages via a downstream pathway? Is there an adiponectin-independent degradation of ceramides by these receptors that adiponectin only serves to elevate? If there is a basal level of ceramidase activity, how strong is it? The work in Holland et al. (2011) indicates that adipoR1 and adipoR2 have intrinsic ceramidase activity that is initiated by adiponectin. They observed, in vitro, the effects of mutating conserved histidine residues that the receptors share with alkaline ceramidases [47]. These mutations caused a massive decline in overall ceramidase activity, one that adiponectin treatment could not rescue. All of these developments indicated a growing appreciation of both adiponectin and its receptors.
AdipoR1 and adipoR2, dependent on adiponectin, possess intrinsic ceramidase activity and are viable transgenic targets in the fight against insulin resistance
Recent work indicates that adiponectin’s uniqueness is matched by the extraordinary characteristics of its receptors, adipoR1 and adipoR2. In 2015, Tanabe and colleagues reported on the receptors’ structures. Using crystallization, they verified the seven transmembrane spans in both receptors, identified extensive structural differences between the two receptors, confirmed adipoRl’s role in AMPK activation, and most notably, identified several large cavities and zinc binding sites in the transmembrane domains of both receptors [56]. The incredible nature of this discovery cannot be understated. Prior to Tanabe et al., only the members of the site-2 protease family were known to contain zinc ions in their transmembrane domains [57]. Though Tanabe et al. did not fully specify the nature of these cavities and the zinc binding site, they had potentially ascribed an intrinsic catalytic role to the adiponectin receptors; zinc ions have been associated with ceramidase activity [58].
Even more recently, this discovery has been built upon by Vasiliauskaité-Brooks et al. Using in meso crystallization and fluorescent spectroscopy, they described the crystal structures of both receptors, validating previous knowledge and revealing new information [59]. Most importantly, they showed, using fluorescent spectroscopy and fluorescent size exclusion chromatography (FSEC), that adipoR2 can bind and hydrolyze C18:0 ceramides into free fatty acid and sphingosine. This observation was substantiated when the purified crystal structures of adipoR2 crystals grown in a ceramide-doped lipidic cubic phase had free fatty acid molecules located within their intermembrane zinc binding cavity [60]. Further computational simulations and analyses conveyed a cavity designed to facilitate hydrolytic activity, in particular the nucleophilic cleavage of ceramide’s defining amide bond by a zinc-stabilized hydroxide ion. Though the enzyme kinetics of adipoR2’s hydrolase activity are physiologically slow, they are consistent with those of other intramembrane proteases. This activity, as measured by spectroscopically detectable sphingosine, is also massively amplified in adiponectin’s presence (25-fold). AdipoRl’s crystal structure revealed a catalytic area that is surprisingly similar to adipoR2’s. This contrasts with the numerous other structural differences between the two. Though they were not able to show a bound free fatty acid molecule in the crystal structure, identical experiments and LC-MS analyses demonstrated adipoRl’s ability to bind and hydrolyze ceramide. This ceramidase activity, as measured by spectroscopically detectable sphingosine, was also greatly elevated in adiponectin’s presence. With their work, Vasiliauskaité-Brooks et al. have described the catalytic nature of adipoR1 and adipoR2, providing an assertive response to our earlier questions. Their data, though not completely conclusive, insinuate that there is not much independent, “basal” ceramidase activity; adiponectin’s presence prompts a massive, 20–25 fold increase in ceramidase activity from both receptors. Vasiliauskaité-Brooks et al. also indicate that though adipoR2 can universally hydrolyze various ceramide species, from C6:0 to C24:0, it seems to have a binding preference for C18:0 ceramides. As explained prior, this species plays a crucial role in the development of hepatic insulin resistance and non-alcoholic fatty liver disease (NAFLD) [40,41,42,43,44].
These revelations represent a sprouting interest in harnessing adiponectin receptor signaling for the treatment of diabetes. While these recent studies help to solidify a revised view of adiponectin and its ceramide-reducing effects, much of the earlier work on this subject pointed to AMPK and PPARα as the main envoys of adiponectin’s effects on lipid regulation within cells. Collectively, these studies place ceramidase activity further upstream in adiponectin’s accepted signaling pathway, in its membrane receptors, as the initiating event in adiponectin signaling. Notably, blocking ceramidase activity prevents downstream activation of AMPK and PPAR [26].
The elevated importance of adipoR1 and adipoR2 now make them the targets of transgenic manipulation and therapeutic intervention. A recent paper in Molecular Metabolism typifies this trend. Transgenic mice that overexpress either adipoR1 or adipoR2 in a tissue-specific, doxycycline-inducible, titratable manner were generated. Liver (Alb-R1/R2) and white adipose tissue (Art-R1/R2) were targeted in these studies. All mice were placed on HFD containing doxycycline after ~2 months of age. Under extended HFD challenge (all measurements were made after 8 weeks of HFD), all the mice became obese. Both Alb-R1/R2 mice displayed numerous physiological and metabolic advantages over their wildtype counterparts. They had better glucose homeostasis/tolerance and insulin tolerance, as measured by serum glucose levels, glucose/insulin tolerance tests, insulin stimulated pSerine473-AKT/total AKT ratios, and glucose infusion rates during hyperinsulinemic-euglycemic clamps [44]. During clamps, there was a dramatic suppression of hepatic glucose output in alb-R1/R2 mice, indicating improved hepatic insulin sensitivity in particular. These improvements were coupled with lowered hepatic steatosis and hepatic ceramide content (namely C16:0, C18:0, and C20:0 ceramides) in the alb-R1/R2 mice. The art-R1/R2 mice displayed the same differences. As in Xia et al., there is evidence of a “cross-talk”; degrading ceramides in one tissue lowers ceramide content in the other [44]. Though the reason for this “cross-talk” is not completely clear, it may involve ceramide transport between tissues.
It is difficult to know if overexpression of either receptor in both tissues better protects against HFD-induced insulin resistance and steatosis - titrating equivalent levels of receptor expression would be critical for such an analysis [44]. With recent work describing the various structural differences between the receptors [56,59], it would not be surprising if there is also a difference in the overall efficacy of ceramide degradation between the two receptors. Indeed, in Vasiliauskaité-Brooks et al., it is shown that adipoRl’s catalytic cavity is exposed to the cytoplasm in its open conformation. On the other hand, adipoR2’s open catalytic cavity is positioned farther within the plasma membrane [59]. Perhaps, the divergent abundance of the receptors may play a role in any supposed difference in catalytic efficacy. Though they are expressed ubiquitously, adipoR1 is abundant in skeletal muscle and adipoR2 is abundant in liver [45]. Maybe the receptors’ roles developed to cater to their specific environments.
Holland et al. (2017) also illustrated the importance of adiponectin to its receptors’ ceramide-hydrolase activities. This corroborates previous observations and affirms that adiponectin and its receptors are mutually dependent on each other for their activities [26,44,59]. Alb-R1/R2 mice were crossed with adiponectin KO mice (APNKO), which do not endogenously produce adiponectin. All of the aforementioned improvements were neutralized in the absence of adiponectin. Alb-R1/R2APNKO mice did not have lower ceramide levels of any species in the liver in comparison to control APNKO mice. Any differences in glucose homeostasis/tolerance, insulin sensitivity, or lipid homeostasis/tolerance were also negated. Speculation about significant receptor ceramidase activity in the absence of adiponectin agonism has been, for now, put to rest. The anti-diabetic potential of adipoR2 was also assessed [44]. These studies were conducted with ob/ob mice, which are leptin deficient, simulate type II diabetic conditions, and are known to have lowered expression of both adiponectin receptors [61]. Ob/ob mice were crossed with alb-R2, art-R2, alb-AC, and art-AC mice. For some reason, adipoR1 overexpression on an ob/ob background was not evaluated. Though all overexpressing cohorts displayed lowered ceramide levels in their specific target tissues, the degradation was different, specifically between the ob/obart-R2 and ob/obart-AC mice. Ob/ob/art-R2 targeted C16:0 ceramides at higher rates. Overall, the ob/obalb-R2/art-R2 mice had improved glucose homeostasis/tolerance when compared to their ob/ob counterparts. Ob/obalb-AC/art-AC had no such improvements. The reason behind this may be that acid ceramidase, a lysosomal hydrolase, does not promote S1P accrual as a byproduct of ceramide degradation. In contrast, the sphingosine produced by adipoR1 or adipoR2 ceramidase activity is available for phosphorylation by sphingosine kinase (SK). S1P is known to be anti-apoptotic, cytoprotective, and stimulate AMPK activity, which can enhance adiponectin’s anti-diabetic effects [48,53,54,55].
Clinical relevance of adiponectin and its cognate receptors and overall outlook
The past few decades have established adiponectin as a unique adipokine, one that is both a vital marker and a highly active, almost universally positive protein. Amongst its many benefits, the adiponectin: adipoR1/R2 interaction can, under proper stimulation, potently degrade a bioactive species that links obesity and insulin resistance in peripheral tissues, a hallmark of type II diabetes. Moreover, adiponectin induced ceramide degradation creates a pool of sphingosine that can be shunted into the production of S1P, a molecule with well documented anti-apoptotic, cytoprotective effects [53,54,55]. All these observations point to a novel and effective therapeutic means to combat obesity-driven type II diabetes. Efforts could involve replenishing plasma adiponectin levels, which are significantly reduced during obesity, and cultivating elevated adiponectin receptor agonism. However, as adiponectin is highly abundant, the use of recombinant adiponectin could never be a cost-effective solution to improve metabolism. Moreover, adiponectin’s complexity, size, and kinetics have made it difficult to produce en masse for therapeutic uses [62,63]. Small molecules may offer an alternative solution to propagate adiponectin signaling. AdipoRon, an orally-bioavailable adiponectin mimetic that can bind to and stimulate adipoR1 and adipoR2, was identified by Okada-Iwabu et al. in 2013 [64]. It is able to improve insulin sensitivity in vitro, improves lifespan in severely diabetic mice, and like adiponectin, promotes ceramidase activity (diabetic ob/ob mice given AdipoRon displayed reductions in hepatic ceramide levels) [44,64]. However, any translational effort should also proceed with caution. Data that correlates adiponectin with reduced bone density, infertility, and left ventricular hypertrophy has been produced [65,66,67,68] and must be taken into account as therapeutic advances are pursued. Still, adiponectin receptor agonism represents a fantastic opportunity to advance the clinical treatment of obesity-driven insulin resistance and type II diabetes.
Expression of adiponectin receptor in mammalian cells, offers minimal signal-to-noise for the evaluation of receptor agonism. In HEK293T cells it is difficult to achieve more than a 3-fold change in ceramidase activity. This is likely due to two reasons. First, culture of mammalian cells with serum additives provides a high level of contaminating adiponectin with other growth factors. Second, all mammalian cells generate endogenous ceramides that will be difficult to isolate from a transmembrane receptor without losing receptor function. The production of adiponectin receptor from an Sf9 insect expression system appears to mitigate these problems, as Vasiliauskaité-Brooks and colleagues used serum free (adiponectin free) growth conditions [59]. Moreover, insect cells predominantly produce short chain (C14) ceramides [69]. These may be less likely to compete with exogenous ceramides that are added during ceramidase activity assays. Ultimately, this source of receptors yields an 8-fold greater signal to noise ratio than mammalian cells, which should aid screening efforts for adiponectin receptor agonists.
In the end, the recent advancements in understanding adiponectin signaling through activation of its hydrolase receptors have created a momentous opportunity for clinical advancements and opened the door for exciting new research that broadens our knowledge about obesity and diabetes.
Acknowledgments
The authors wish to acknowledge support from the American Heart Association 15UFEL25090280 (AXS) and 12BGIA-8910006 (WLH) as well as the NIH grants R00DK094973 and R01DK108833 to WLH.
Footnotes
Conflict of Interest: No conflicts declared.
References
- 1.Merrill AH., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J Biol Chem. 2002;277:25843–25846. doi: 10.1074/jbc.R200009200. [DOI] [PubMed] [Google Scholar]
- 2.Schissel SL, Tweedie-Hardman J, Rapp JH, Graham G, Williams KJ, Tabas I. Rabbit aorta and human atherosclerotic lesions hydrolyze the sphingomyelin of retained low-density lipoprotein. Proposed role for arterial-wall sphingomyelinase in subendothelial retention and aggregation of atherogenic lipoproteins. J Clin Invest. 1996;98:1455–1464. doi: 10.1172/JCI118934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ. Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res. 2008;49:2101–2112. doi: 10.1194/jlr.M800147-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bharath LP, Ruan T, Li Y, Ravindran A, Wan X, Nhan JK, Walker ML, Deeter L, Goodrich R, Johnson E, Munday D, Mueller R, Kunz D, Jones D, Reese V, Summers SA, Babu PV, Holland WL, Zhang QJ, Abel ED, Symons JD. Ceramide-Initiated Protein Phosphatase 2A Activation Contributes to Arterial Dysfunction In Vivo. Diabetes. 2015;64:3914–3926. doi: 10.2337/db15-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maedler K, Oberholzer J, Bucher P, Spinas GA, Donath MY. Monounsaturated fatty acids prevent the deleterious effects of palmitate and high glucose on human pancreatic β-cell turnover and function. Diabetes. 2003;52:726–733. doi: 10.2337/diabetes.52.3.726. [DOI] [PubMed] [Google Scholar]
- 6.Boslem E, MacIntosh G, Preston AM, Bartley C, Busch AK, Fuller M, Laybutt DR, Meikle PJ, Biden TJ. A lipidomic screen of palmitate-treated MIN6 β-cells links sphingolipid metabolites with endoplasmic reticulum (ER) stress and impaired protein trafficking. Biochem J. 2003;435:267–276. doi: 10.1042/BJ20101867. [DOI] [PubMed] [Google Scholar]
- 7.Lei X, Zhang S, Emani B, Barbour SE, Ramanadham S. A link between endoplasmic reticulum stress-induced β-cell apoptosis and the group VIA Ca2+-independent phospholipase A2 (iPLA2β) Diabetes Obes Metab. 2010;12:93–98. doi: 10.1111/j.1463-1326.2010.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Holland WL, Brozinick JT, Wang LP, Hawkins ED, Sargent KM, Liu Y, Narra K, Hoehn KL, Knotts TA, Siesky A, Nelson DH, Karathanasis SK, Fontenot GK, Birnbaum MJ, Summers SA. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Xia JY, Holland WL, Kusminski CM, Sun K, Sharma AX, Pearson MJ, Sifuentes AJ, McDonald JG, Gordillo R, Scherer PE. Targeted Induction of Ceramide Degradation Leads to Improved Systemic Metabolism and Reduced Hepatic Steatosis. Cell Metab. 2015;22:266–278. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boon J, Hoy AJ, Stark R, Brown RD, Meex RC, Henstridge DC, Schenk S, Meikle PJ, Horowitz JF, Kingwell BA, Bruce CR, Watt MJ. Ceramides contained in LDL are elevated in type 2 diabetes and promote inflammation and skeletal muscle insulin resistance. Diabetes. 2013;62:401–410. doi: 10.2337/db12-0686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haus JM, Kashyap SR, Kasumov T, Zhang R, Kelly KR, Defronzo RA, Kirwan JP. Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes. 2009;58:337–343. doi: 10.2337/db08-1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez X, Goldfine AB, Holland WL, Gordillo R, Scherer PE. Plasma ceramides are elevated in female children and adolescents with type 2 diabetes. J Pediatr Endocrinol Metab. 2013;26:995–998. doi: 10.1515/jpem-2012-0407. [DOI] [PubMed] [Google Scholar]
- 13.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- 14.Ye R, Scherer PE. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol Metab. 2013;2(3):133–141. doi: 10.1016/j.molmet.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J-i. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochemical and Biophysical Research Communications. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 16.Turer AT, Khera A, Ayers CR, Turer CB, Grundy SM, Vega GL. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54:2515–2524. doi: 10.1007/s00125-011-2252-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi T, Kadowaki T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013;17:185–196. doi: 10.1016/j.cmet.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Holland WL, Scherer PE. Ronning after the Adiponectin Receptors. Science. 2013;342:1460–1461. doi: 10.1126/science.1249077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tao C, Sifuentes AJ, Holland WL. Regulation of Glucose and Lipid Homeostasis by Adiponectin: Effects on Hepatocytes, Pancreatic β Cells and Adipocytes. Best Pract Res Clin Endocrinol Metab. 2014;28:43–58. doi: 10.1016/j.beem.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye R, Holland WL, Gordillo R, Wang M, Wang QA, Shao M, Morley TS, Gupta RK, Stahl A, Scherer PE. Adiponectin is essential for lipid homeostasis and survival under insulin deficiency and promotes β-cell regeneration. eLife. 2014;3 doi: 10.7554/eLife.03851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shafiei MS, Shetty S, Scherer PE, Rockey DC. Adiponectin regulation of stellate cell activation via PPARγ-dependent and -independent mechanisms. Am J Pathol. 2011;178:2690–2699. doi: 10.1016/j.ajpath.2011.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang H, Park PH, McMullen MR, Nagy LE. Mechanisms for the anti-inflammatory effects of adiponectin in macrophages. J Gastroenterol Hepatol. 2008;23:S50–S53. doi: 10.1111/j.1440-1746.2007.05284.x. [DOI] [PubMed] [Google Scholar]
- 24.Mandal P, Park PH, McMullen MR, Pratt BT, Nagy LE. The anti-inflammatory effects of adiponectin are mediated via a heme oxygenase-1-dependent pathway in rat Kupffer cells. Hepatology. 2010;51:1420–1429. doi: 10.1002/hep.23427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo M-s, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. The Pleiotropic Actions of Adiponectin are Initiated via Receptor-Mediated Activation of Ceramidase Activity. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ussher JR, Koves TR, Cadete VJJ, Zhang L, Jaswal JS, Swyrd SJ, Lopaschuk DG, Proctor SD, Keung W, Muoio DM, Lopaschuk GD. Inhibition of de novo ceramide synthesis reverses diet-induced insulin resistance and enhances whole-body oxygen consumption. Diabetes. 2010;59:2453–2464. doi: 10.2337/db09-1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang G, Badeanlou L, Bielawski J, Roberts AJ, Hannun YA, Samad F. Central role of ceramide biosynthesis in body weight regulation, energy metabolism, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2009;297:E211–E224. doi: 10.1152/ajpendo.91014.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frangioudakis G, Garrard J, Raddatz K, Nadler JL, Mitchell TW, Schmitz-Peiffer C. Saturated and n-6 polyunsaturated fat diets each induce ceramide accumulation in mouse skeletal muscle: reversal and improvement of glucose tolerance by lipid metabolism inhibitors. Endocrinology. 2010;151:4187–4196. doi: 10.1210/en.2010-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chaurasia B, Kaddai VA, Lancaster GI, Henstridge DC, Sriram S, Galam DL, Gopalan V, Prakash KN, Velan SS, Bulchand S, Tsong TJ, Wang M, Siddique MM, Yuguang G, Sigmundsson K, Mellet NA, Weir JM, Meikle PJ, Bin M, Yassin MS, Shabbir A, Shayman JA, Hirabayashi Y, Shiow ST, Sugii S, Summers SA. Adipocyte Ceramides Regulate Subcutaneous Adipose Browning, Inflammation, and Metabolism. Cell Metab. 2016;24:820–834. doi: 10.1016/j.cmet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Stratford S, DeWald DB, Summers SA. Ceramide dissociates 3′-phosphoinositide production from pleckstrin homology domain translocation. Biochem J. 2001;354:359–368. doi: 10.1042/0264-6021:3540359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Powell DJ, Turban S, Gray A, Hajduch E, Hundal HS. Intracellular ceramide synthesis and protein kinase Cζ activation play an essential role in palmitate-induced insulin resistance in rat L6 skeletal muscle cells. Biochem J. 2004;382:619–629. doi: 10.1042/BJ20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chavez JA, Knotts TA, Wang LP, Li G, Dobrowsky RT, Florant GL, Summers SA. A role for ceramide, but not diacylglycerol, in the antagonism of insulin signal transduction by saturated fatty acids. J Biol Chem. 2003;278:10297–10303. doi: 10.1074/jbc.M212307200. [DOI] [PubMed] [Google Scholar]
- 35.Salinas M, López-Valdaliso R, Martín D, Alvarez A, Cuadrado A. Inhibition of PKB/Akt1 by C2-ceramide involves activation of ceramide-activated protein phosphatase in PC 12 cells. Mol Cell Neurosci. 2000;15:156–169. doi: 10.1006/mcne.1999.0813. [DOI] [PubMed] [Google Scholar]
- 36.Teruel T, Hernandez R, Lorenzo M. Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes. 2001;50:2563–2571. doi: 10.2337/diabetes.50.11.2563. [DOI] [PubMed] [Google Scholar]
- 37.Zinda MJ, Vlahos CJ, Lai MT. Ceramide induces the dephosphorylation and inhibition of constitutively activated Akt in PTEN negative U87mg cells. Biochem Biophys Res Commun. 2001;280:1107–1115. doi: 10.1006/bbrc.2000.4248. [DOI] [PubMed] [Google Scholar]
- 38.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Öhman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, Summers SA. CerS2 haploinsufficiency inhibits β-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 39.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Brönneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Blüher M, Krönke M, Brüning JC. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 40.Blachnio-Zabielska AU, Chacinska M, Vendelbo MH, Zabielski P. The Crucial Role of C18-Cer in Fat-Induced Skeletal Muscle Insulin Resistance. Cell Physiol Biochem. 2016;40:1207–1220. doi: 10.1159/000453174. [DOI] [PubMed] [Google Scholar]
- 41.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Wei T, Thomas MK, Kuo MS, Perreault L. Muscle sphingolipids during rest and exercise: a C18:0 signature for insulin resistance in humans. Diabetologia. 2016;59:785–798. doi: 10.1007/s00125-015-3850-y. [DOI] [PubMed] [Google Scholar]
- 42.Bergman BC, Brozinick JT, Strauss A, Bacon S, Kerege A, Bui HH, Sanders P, Siddall P, Kuo MS, Perreault L. Serum sphingolipids: relationships to insulin sensitivity and changes with exercise in humans. Am J Physiol Endocrinol Metab. 2015;309:E398–E408. doi: 10.1152/ajpendo.00134.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kasumov T, Li L, Li M, Gulshan K, Kirwan JP, Liu X, Previs S, Willard B, Smith JD, McCullough A. Ceramide as a mediator of non-alcoholic fatty liver disease and associated atherosclerosis. PLoS One. 2015;10:e0126910. doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holland WL, Xia JY, Johnson JA, Sun K, Pearson MJ, Sharma AX, Quittner-Strom E, Tippetts TS, Gordillo R, Scherer PE. Inducible overexpression of adiponectin receptors highlight the roles of adiponectin-induced ceramidase signaling in lipid and glucose homeostasis. Mol Metab. 2017;6:267–275. doi: 10.1016/j.molmet.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 46.Lyons TJ, Villa NY, Regalla LM, Kupchak BR, Vagstad A, Eide DJ. Metalloregulation of yeast membrane steroid receptor homologs. Proc Natl Acad Sci USA. 2004;101(15):5506–5511. doi: 10.1073/pnas.0306324101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Villa NY, Kupchak BR, Garitaonandia I, Smith JL, Alonso E, Alford C, Cowart LA, Hannun YA, Lyons TJ. Sphingolipids function as downstream effectors of a fungal PAQR. Mol Pharmacol. 2009;75:866–875. doi: 10.1124/mol.108.049809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwabu M, Yamauchi T, Okada-Iwabu M, Sato K, Nakagawa T, Funata M, Yamaguchi M, Namiki S, Nakayama R, Tabata M, Ogata H, Kubota N, Takamoto I, Hayashi YK, Yamauchi N, Waki H, Fukayama M, Nishino I, Tokuyama K, Ueki K, Oike Y, Ishii S, Hirose K, Shimizu T, Touhara K, Kadowaki T. Adiponectin and AdipoR1 regulate PGC-1alpha and mitochondria by Ca (2+) and AMPK/SIRT1. Nature. 2010;464:1313–1319. doi: 10.1038/nature08991. [DOI] [PubMed] [Google Scholar]
- 49.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nature Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 50.Tomas E, Tsao TS, Saha AK, Murrey HE, Zhang CcCc, Itani SI, Lodish HF, Ruderman NB. Enhanced muscle fat oxidation and glucose transport by ACRP30 globular domain: acetyl-CoA carboxylase inhibition and AMP-activated protein kinase activation. Proc. Natl Acad. Sci. USA. 2002;99:16309–16313. doi: 10.1073/pnas.222657499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. doi: 10.1016/j.cmet.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 52.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 53.Zhang H, Desai NN, Olivera A, Seki T, Brooker G, Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Olivera A, Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 55.Cuvillier O, Pirianov G, Kleuser B, Vanek PG, Coso OA, Gutkind S, Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 56.Tanabe H, Fujii Y, Okada-Iwabu M, Iwabu M, Nakamura Y, Hosaka T, Motoyama K, Ikeda M, Wakiyama M, Terada T, Ohsawa N, Hato M, Ogasawara S, Hino T, Murata T, Iwata S, Hirata K, Kawano Y, Yamamoto M, Kimura-Someya T, Shirouzu M, Yamauchi T, Kadowaki T, Yokoyama S. Crystal structures of the human adiponectin receptors. Nature. 2015;520:312–316. doi: 10.1038/nature14301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Feng L, Yan H, Wu Z, Yan N, Wang Z, Jeffrey PD, Shi Y. Structure of a site-2 protease family intramembrane metalloprotease. Science. 2007;318:1608–1612. doi: 10.1126/science.1150755. [DOI] [PubMed] [Google Scholar]
- 58.Airola MV, Allen WJ, Pulkoski-Gross MJ, Obeid LM, Rizzo RC, Hannun YA. Structural basis for ceramide recognition and hydrolysis by human neutral ceramidase. Structure. 2015;23:1482–1491. doi: 10.1016/j.str.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vasiliauskaite-Brooks I, Sounier R, Rochaix P, Bellot G, Fortier M, Hoh F, Colibus L, Bechara C, Saied EM, Arenz C, Leyrat C, Granier S. Structural insights into adiponectin receptors suggest ceramidase activity. Nature. 2017;544:120–123. doi: 10.1038/nature21714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Landau EM, Rosenbusch JP. Lipidic cubic phases: A novel concept for the crystallization of membrane proteins. PNAS. 1996;93:14532–14535. doi: 10.1073/pnas.93.25.14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wade TE, Mathur A, Lu D, Swartz-Basile DA, Pitt HA, Zyromski NJ. Adiponectin receptor-1 expression is decreased in the pancreas of obese mice. J Surg Res. 2009;154:78–84. doi: 10.1016/j.jss.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 62.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications for metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 63.Halberg N, Schraw TD, Wang ZV, Kim JY, Yi J, Hamilton MP, Luby-Phelps K, Scherer PE. Systemic fate of the adipocyte-derived factor adiponectin. Diabetes. 2009;58:1961–1970. doi: 10.2337/db08-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okada-Iwabu M, Yamauchi T, Iwabu M, Honma T, Hamagami K, Matsuda K, Yamaguchi M, Tanabe H, Kimura-Someya T, Shirouzu M, Ogata H, Tokuyama K, Ueki K, Nagano T, Tanaka A, Yokoyama S, Kadowaki T. A small-molecule AdipoR agonist for type 2 diabetes and short life in obesity. Nature. 2013;503:493–499. doi: 10.1038/nature12656. [DOI] [PubMed] [Google Scholar]
- 65.Ealey KN, Kaludjerovic J, Archer MC, Ward WE. Adiponectin is a negative regulator of bone mineral and bone strength in growing mice. Exp Biol Med (Maywood) 2008;233:1546–1553. doi: 10.3181/0806-RM-192. [DOI] [PubMed] [Google Scholar]
- 66.Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, Mangelsdorf DJ, Kliewer SA, Wan Y. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor γ. Proc Natl Acad Sci U S A. 2012;109:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, Ezaki O, Akanuma Y, Gavrilova O, Vinson C, Reitman ML, Kagechika H, Shudo K, Yoda M, Nakano Y, Tobe K, Nagai R, Kimura S, Tomita M, Froguel P, Kadowaki T. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- 68.Combs TP, Pajvani UB, Berg AH, Lin Y, Jelicks LA, Laplante M, Nawrocki AR, Rajala MW, Parlow AF, Cheeseboro L, Ding YY, Russell RG, Lindemann D, Hartley A, Baker GR, Obici S, Deshaies Y, Ludgate M, Rossetti L, Scherer PE. A transgenic mouse with a deletion in the collagenous domain of adiponectin displays elevated circulating adiponectin and improved insulin sensitivity. Endocrinology. 2004;145:367–383. doi: 10.1210/en.2003-1068. [DOI] [PubMed] [Google Scholar]
- 69.Fyrst H, Herr DR, Harris GL, Saba JD. Characterization of free endogenous C14 and C16 sphingoid bases from Drosophila melanogaster. J Lipid Res. 2004;45:54–62. doi: 10.1194/jlr.M300005-JLR200. [DOI] [PubMed] [Google Scholar]