Abstract
Anopheles gambiae is a major vector of human malaria and its immune system in part determines the fate of ingested parasites. Proteins, hemocytes and fat body in hemolymph are critical components of this system, mediating both humoral and cellular defenses. Here we assessed differences in the hemolymph proteomes of water- and E. coli-pricked mosquito larvae by a gel-LC-MS approach. Among the 1,756 proteins identified, 603 contained a signal peptide but accounted for two-third of the total protein amount on the quantitative basis. The sequence homology search indicated that 233 of the 1,756 may be related to defense. In general, we did not detect substantial differences between the control and induced plasma samples in terms of protein numbers or levels. Protein distributions in the gel slices suggested post-translational modifications (e.g. proteolysis) and formation of serpin-protease complexes and high Mr immune complexes. Based on the twenty-five most abundant proteins, we further suggest that major functions of the larval hemolymph are storage, transport, and immunity. In summary, this study provided first data on constitution, levels, and possible functions of hemolymph proteins in the mosquito larvae, reflecting complex changes occurring in the fight against E. coli infection.
Keywords: insect immunity, hemolymph proteins, LC-MS/MS, label-free quantification, serine protease, gel mobility shift
Graphical Abstract
1. Introduction
The mosquito Anopheles gambiae is one of the major vector species transmitting deadly diseases, which impact millions of human lives each year (World Malaria Report 2015). Since its genome sequence became available (Holt et al. 2002), continuous efforts have been made to improve gene annotation, profile transcript levels, and elucidate protein functions, especially those related to immunity (Yassine and Osta, 2010; Clayton et al., 2014). The genetic makeup of the mosquito innate immune system is considered to be similar to those of other model insects such as Drosophila melanogaster. In these models, molecular patterns on the pathogen surface are recognized by pattern recognition receptors (PRRs) of the host to trigger serine protease (SP) cascades, melanization, antimicrobial protein (AMP) synthesis, and hemocyte responses. Families of defense proteins are implicated in parasite resistance, including thioester proteins (e.g. TEP1) (Blandin et al., 2004), Leu-rich repeat (LRR) proteins (e.g. Leu-rich immune molecule-1 (LRIM1), A. plasmodium-responsive LRR protein-1C (APL1C)) (Povelones et al., 2009), C-type lectins (CTLs) (e.g. CTL4, CTLMA2) (Osta et al., 2004), fibrinogen-related proteins (FREP1, FBN30) (Dong and Dimopoulos, 2009; Li et al., 2013), and clip-domain serine proteases (SPs) and serine protease homologs (SPHs) (e.g. CLIPs B3, B4, B8, B14, B15, B17, A2, A5, A7, A8 and SPCLIP1) (Volz et al., 2005 and 2006; Povelones et al., 2013; Barillas-Mury, 2007), serpins (e.g. serpin-2, 6) (Abraham et al., 2005; Michel et al., 2006; Suwanchaichinda and Kanost, 2009), and caspase-S2 (Ramphul et al., 2015). Four of these genes (APL1C, CLIPB15, serpin-2, caspase-S2) were also identified in an expression pattern analysis, along with TEP2, TEP4 and TEP15, LRR-7060, CTL2, CTLMA3, FBN9, peptidoglycan recognition protein (PGRP) S1, CLIPs B5, B7 and C2, SCRA-SPH3, Spätzle3 and 4, Toll6, 10 and 11, inhibitor of apoptosis (IAP) 5, caspase-S4, and adenosine deaminase genes (Li et al., 2013).
Previous proteomic studies of A. gambiae characterized peritrophic matrix, head, eggshell, saliva and salivary gland (Dinglasan et al. 2009; Lefevre et al., 2007; Amenya et al., 2010; Francischetti et al., 2002; Kalume et al., 2005), identifying peritrophins, digestive and metabolic enzymes, vitelline membrane and chorion proteins, oxidases, odorant binding proteins, and SG and D7 proteins. In the hemolymph of adult mosquitoes, Paskewitz and Shi (2005) described 280 spots on 2-D PAGE gels and identified 28 including those related to immunity (e.g. TEP15, CLIPB4, CLIPA6, serpin-2, serpin-15, phenoloxidase-6 (PO6), proPO2), lipid binding (e.g. apolipophorin III, MD2-like protein-3 (MDL3)), and iron metabolism (e.g. ferritin). Eight of the 28 proteins were differentially regulated upon wounding (e.g. glutathione S transferase-S1) or bacterial infection (PO6, chitinase-like BR-1 and BR-2). While these studies provided useful information about proteins in tissues or body fluids in the mosquito, the numbers of identified proteins were dwarfed by those of mRNAs detected in the corresponding tissue transcriptomes, due to limitations of the proteomic techniques previously used. To better understand the roles of hemolymph in physiological processes such as innate immunity, we chose the E. coli infection model which had been used in A. gambiae adults to show lethality at high dosage and induced production of defensin (Blandin et al., 2002; Coggins et al., 2012) and characterized A. gambiae larval hemolymph using a gel-LC-MS approach (Blagoev et al., 2004). We report the identification and quantification of plasma proteins, some of which showed challenge-associated changes in abundance or gel mobility, suggesting proteolytic processing and immune complex formation.
2. Materials and Methods
2.1. Mosquito rearing
A colony of A. gambiae G3 strain was obtained from Malaria Research and Reference Reagent Resource Center and maintained in an incubator at 27.5°C with 80% relative humidity in a 12 h light-dark cycle with gradual sunset and sunrise light transitions. As described previously (Benedict, 1997), newly hatched larvae (day 1–2) were fed in suspension of baker’s yeast, the older larvae were fed a 1:2 (w/w) mixture of baker’s yeast and ground fish food (Mike Reed Enterprises) in distilled water. Pupae were picked and placed side by side in cups with water prior to emergence. Newly emerged adults were maintained by 10% sucrose solution. To trigger embryonic development, adult females (days 6–10) were fed heparinized sheep blood (HemoStat Laboratories) using a membrane feeder (Hemotek). Laid eggs were collected on wet filter papers and transferred to distilled water for hatching.
2.2. Preparation of cell-free hemolymph from water- and bacteria-pricked larvae
Fourth instar larvae (20–25/group, 8 groups) were transferred to new cups with distilled water and placed on a filter paper to remove excess water. They were individually pricked in the thorax with a glass capillary tube dipped in distilled water (C for control) or a pellet of live Escherichia coli cells (I for induced). To prepare the bacterial pellet, a single fresh colony of E. coli BL21 was grown in 3.0 ml of LB medium at 37°C with shaking at 220 rpm until OD600 reached 0.7–0.9. The cells were harvested by centrifugation at 4,500g for 20 min and resuspended in 1 ml of distilled water. This step was repeated twice to remove the medium. After being transferred back to the same cup, the wounded larvae were provided with a small volume of the food suspension and incubated for 24 h. For hemolymph extraction, five insects from the same group (C or I) were blotted with a filter paper and placed together on a piece of paraffin film. Five microliters of a cocktail of protease inhibitors (cOmplete ULTRA, Roche Diagnostics) supplemented with 0.1% 1-phenyl-2-thiourea was added to the larvae. Each larva was torn slightly with forceps in the thorax and gently pressed with a pipette tip in the abdomen, so that released hemolymph instantly encountered the inhibitors. Pooled plasma (P) samples (c.a. 20 μl per tube, 20–25 insects per pool) were centrifuged at 2000×g for 5 min to remove tissues and cell debris. Protein concentrations of the samples and biological replicates (CP1–CP4, IP1–IP4 from different cohorts of the mosquito larvae) were determined by a modified Bradford assay using BSA as a standard.
2.3. SDS-PAGE separation of plasma proteins, in-gel trypsinolysis, and MS sample preparation
The eight protein samples (4 CPs and 4 IPs) were treated as described previously (He et al., 2016). Briefly, 40 μg of each sample were separately on a 4–15% gradient SDS polyacrylamide gel (Bio-Rad), followed by staining with Coomassie blue. Subsequently, each of the eight lanes was divided into 12 gel slices based on the band patterns, generating a total of 96 gel samples. The gel pieces were reduced with tris(2-carboxyethyl)phosphine, alkylated with iodoacetamide, and digested with sequencing grade trypsin. Resulting trypsinolytic peptides were extracted from the gel pieces with 1% trifluoroacetic acid for LC-MS/MS analysis at Oklahoma State University Recombinant DNA/Protein Core Facility.
2.4. LC-MS/MS analysis
Each of the 40 samples from gel slices 1 through 5 (i.e. >80 kDa, Fig. 1A) was subjected to a single individual LC-MS/MS analysis on an LTQ-Orbitrap mass spectrometer (Thermo Fisher Scientific) as described previously (He et al., 2016), but using a 4-hr chromatography gradient of 2–40% ACN. The other 56 samples (gel slices 6 through 12, Fig. 1A) were each subjected to two LC-MS/MS analyses on an Orbitrap Fusion Tribrid mass spectrometer (Thermo Fisher Scientific), wherein peptides were separated (He et al., 2016) using a 100-min chromatography gradient of 2–40% ACN. The Fusion analyses employed a “Top Speed” data-dependent MS/MS strategy, wherein survey scans were performed in FT mode (R=120,000) at least every three seconds. In the interval between survey scans, up to 20 data-dependent MS/MS scans were conducted in the ion trap. The Fusion scan settings also included selection of precursor ions via the quadrupole mass filter, dynamic exclusion (n = 1), and monoisotopic precursor selection.
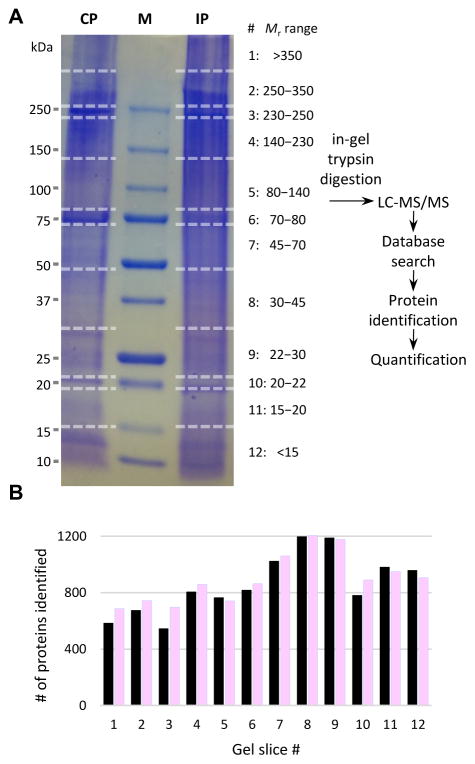
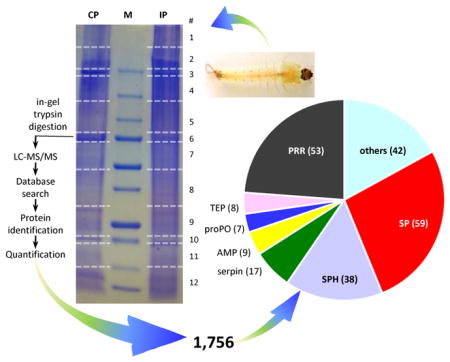
Fig. 1. Schematic overview of the sample treatment and data analysis.
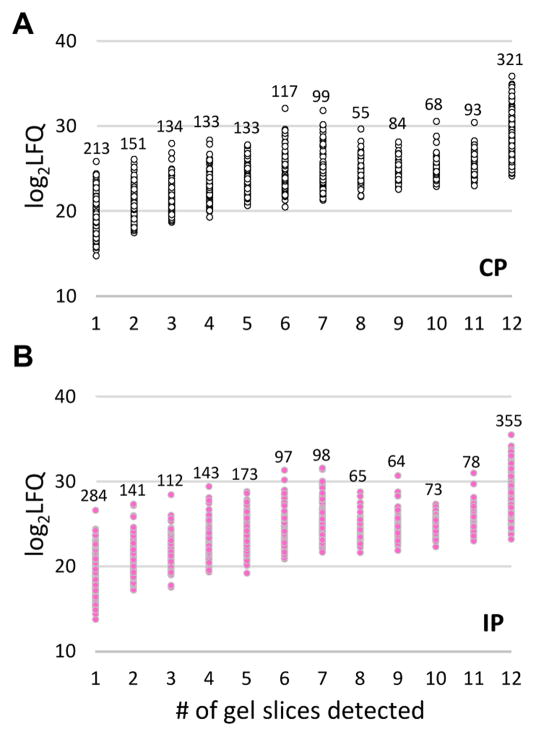
(A) Hemolymph from the larvae challenged with E. coli and water was separately pooled (20–25 insects per group) to generate four induced (IP) and four control (CP) plasma samples (see Materials and Methods). The eight plasma samples were separated by SDS-PAGE on a 4–15% gradient gel that was later cut into 96 pieces. After in-gel trypsinolysis, peptides were separately extracted and analyzed by LC-MS/MS. Protein identification and quantification were performed as described in Materials and Methods. Sizes and positions of the Mr markers (lane M) are labeled on the left, while gel slice numbers (#’s) and corresponding Mr ranges of the gel slices are indicated on the right. (B) Distributions of the number of proteins identified in each gel slice. Black = CP; pink = IP.
2.5. Protein identification, quantification, and mobility examination
The spectra were searched against an A. gambiae protein database (PEST Peptides_AgamP4.2) downloaded on 9/1/2015 from VectorBase (http://www.vectorbase.org/). MaxQuant version 1.5.2.28 (Cox and Mann, 2008) was used to process raw data from the Orbitrap and Fusion spectrometers for database searching. In setting up the MaxQuant search, all of the MS files obtained for individual gel slices from a single lane were designated as “fractions” describing that lane’s sample. The parameters used in the search were: no charge state deconvolution or deisotoping, trypsin digestion, maximum missed cleavage of 2, parental ion mass tolerance of 20 PPM, fragment ion mass tolerance of 0.50 Da, formylation or acetylation of protein N-termini, Met oxidization, cyclization of Gln to pyroGlu, iodoacetamide or acrylamide derivatization of Cys, no fixed modification, and all peptides (with or without modification) included in search. False discovery rates for proteins and peptides were set at 1%.
Proteins were quantified on the basis of their normalized label-free quantification (LFQ) intensities as a proxy for their relative abundances (Cox et al., 2014; Ahrné et al., 2013). The four samples from the challenged insects were designated as one protein group (i.e. “IP”), whereas the four samples from the control insects were designated as another (i.e. “CP”). The average LFQ intensity value for each protein’s expression in either group was calculated as the mean, and used to calculate the log2(I/C) expression ratio. Individual protein LFQ intensities among the four biological replicates were also used to calculate the extent to which differences in protein abundance were significant, using Student’s t test and a significance threshold of 0.05.
To examine the electrophoretic mobility of individual proteins, samples were quantified as described above, but instead designating each gel slice as an individual sample when setting up the MaxQuant search (that is, individual gel slices were not designated as collective sample “fractions”). Changes in electrophoretic mobility were analyzed by comparing protein LFQ protein intensities among gel slices.
2.6. Protein nomenclature
Most of the identified protein sequences from the database already had names that suggest function. For uncharacterized or hypothetical proteins, their sequences were used as queries to search non-redundant protein sequences at NCBI by BLASTP (E-value < 10−10). For sequences that still remained uncharacterized, BLAST2GO searches were performed using default settings. Based on the results, some were named after the domain(s) they contain and the rest referred by their VectorBase IDs (i.e. AGAP numbers). Signal peptides were predicted by SignalP 4.0 (Petersen et al., 2011) and Signal-3L (Shen and Chou, 2007).
2.7. Effects of sterile and septic wounding
To confirm E. coli infection, 4th instar larvae of A. gambiae were individually pricked in the thorax as described in Section 2.2 with two minor modifications. E. coli BL21 strain carrying a GFP expression plasmid was used to prepare bacterial pellet. The bacteria were washed in sterile phosphate-buffered saline (PBS, 138 mM NaCl, 2.7 mM KCl, 10 mM phosphate, pH 7.4). The control larvae were pricked with the same buffer. The live and dead larvae were counted at 24 h after treatment to calculate survival rates of the PBSp and E. colip groups. To compare survival and AMP expression caused by pricking andinjection, two more groups (PBSi and E. colii) of larvae at the same stage were injected at 69 nl/larva with PBS or E.coli suspended in PBS (OD600 = 1.0, c.a. 5.5×104 cells/insect), respectively. After scanning for E. coli GFP signals under a BX51 fluorescence microscope, 24 total RNA samples were prepared from live whole larvae (10 insects per sample, 2 PBS pricked and 2 E. coli pricked, 2 PBS injected, and 2 E. coli injected) at 2, 6 and 24 h using TRIZOL Reagent (Life Technologies, Inc). Two total RNA samples were isolated from naïve larvae (10 insects per sample) and used as negative control. cDNA synthesis and quantitative real-time PCR were performed with three technical replicates for each of the 26 samples according to Yang et al (2016). Each cDNA sample was equivalent to starting with 250 ng total RNA. The primers were: j1723 (5′-AAGAAGCTGACTGGCCGTGA) and j1724 (5′-GTAGCTGCTGCAAACTTCGG) for rpS7; j1737 (5′-TCTGCTGGAACCATCATCGG) and j1738 (5′-ATCTCGTAAACTGCACCGCA) for gambicin-1; j1733 (5′-ATCTTTGTCGTGCTGGCAGT) and j1734 (5′-CTGCCTTGAACACTCGCTTG) for cecropin A. Relative mRNA levels were calculated as: (1 + ErpS7)Ct, rpS3/(1 + EX)Ct,X (Rieu and Powers, 2009), where ErpS7 =107.8%, Egambicin-1 = 109.3%, and Ececropin A = 99.3%. The living and dead larvae pricked with PBS or E. coli were examined at 24 h by bright-field and fluorescence microscopy under the microscope with a DP-71 imaging system (Olympus).
3. Results and Discussion
3.1. Overview of the proteomics analysis
To identify and quantify proteins in A. gambiae larval plasma, we collected hemolymph samples from the larvae pricked with water or live E. coli and separated them by SDS-PAGE. There was no clear difference in band pattern between the CP and IP lanes (Fig. 1A). After gel cutting, in-gel trypsinolysis and LC-MS/MS analysis, we found the numbers of proteins identified in 12 groups of gel slices were comparable between CP and IP (Fig. 1B). These slices contained similar amounts of proteins (based on the staining pattern), ensuring that abundant proteins do not overwhelm signals of scarce ones and apparent Mr ranges of the identified proteins are available. To assess the samples’ reproducibility, pairwise Pearson correlation coefficients of protein LFQ values were examined, yielding r2 values of 0.897–0.978 (0.937 ± 0.029) among pairs of the CP samples and 0.959–0.991 (0.976 ± 0.011) among pairs of the IPs. Correlations between the CP and IP samples were 0.908–0.954 (0.941 ± 0.020), indicative of a minor global change in protein amounts after the immune challenge.
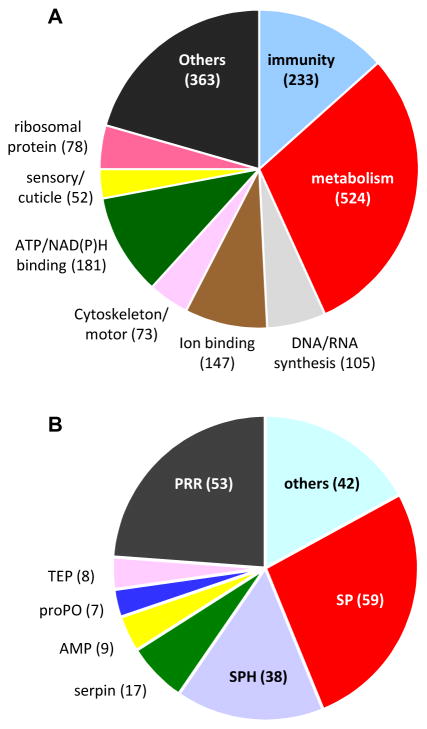
We identified a total of 1,756 proteins: 1688 from CP, 1695 from IP, and 1627 common to both conditions. Each protein had two or more matching MS/MS spectra (Table S1). Among them, 1,361 (78%) had descriptive names, 305 (17%) were named after their homologs found in the BLASTP search, 18 (1%) were designated with the domain(s) they contain, and the other 72 (4%) were represented by their VectorBase IDs. We manually divided the proteins into nine categories: immunity (233), metabolism (524), DNA/RNA synthesis (105), ion binding (147), cytoskeleton/motor (73), ATP/NAD(P)H binding (181), sensory and cuticle (52), ribosomal (78), and others (363) (Fig. 2A). Among the total, 603 (34.3%) were predicted to have a signal peptide (Table S1); the other 1,153 (65.7%) were predicted to be intracellular but detected in the plasma. This apparent contradiction was also observed in Manduca sexta plasma but the percentage (38.7%) was lower (He et al., 2016). We ascribed this anomaly to hemocyte rupture, tissue tearing, and incomplete removal of cellular debris. The contamination was substantial, as the LFQ sums of the extracellular proteins (34.3%) only accounted for 69.7% (CP) and 64.6% (IP) of the grand LFQ totals. An alternative explanation for these hemolymph proteins lacking an apparent signal peptide is that some of them might be secreted into plasma via unconventional pathways (Nickel, 2010).
Fig. 2. Categorization of the total (1,756) and putative defense (233) proteins.
(A) The 1,756 proteins classified into nine arbitrary groups with their names and number of proteins in each group indicated. (B) The 233 defense proteins were subdivided into seven groups: pattern recognition receptor (PRR), serine protease (SP), serine protease homolog (SPH), serpin, antimicrobial protein (AMP), prophenoloxidase (proPO), thioester protein (TEP), and others, with their counts marked.
3.2. Abundant proteins and their functions in the larval hemolymph
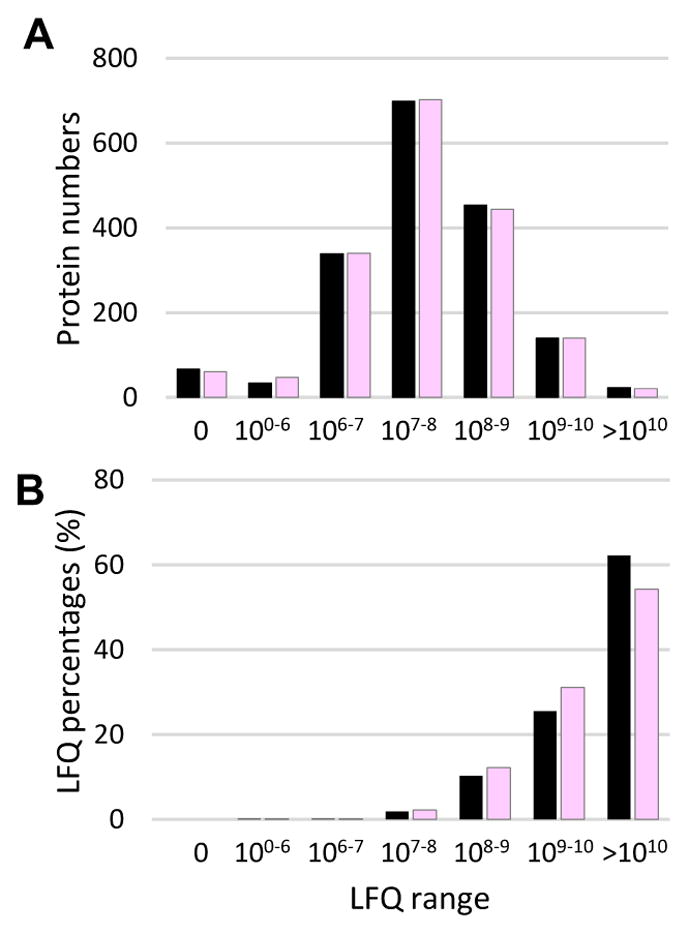
We examined protein distributions based on their relative abundances (Fig. 3A). There were 61 proteins identified in CP but not IP (LFQ = 0) and 68 other were only found in IP. Most proteins (88% in both CP and IP) fell into the LFQ range of 106 to 109. However, when total abundances of all proteins within the individual LFQ ranges were compared (Fig. 3B), we found that 23 CP and 21 IP proteins (LFQ >1010) accounted for 62.2% and 54.3% of the LFQ grand totals, respectively. Similarly, 140 CP and 140 IP proteins in the LFQ range of 109–1010 represented 25.5% and 31.1% of the total protein amounts. Note that LFQ percentages presented here should only be treated as estimates and large deviations may exist, especially when individual proteins are compared.
Fig. 3. Distributions of protein numbers (A) and LFQ percentages (B) in various LFQ ranges.
Within each range, sums of LFQs of the proteins identified in CP (black) and IP (pink) are used to calculate their percentages in the grand totals (ΣLFQCP and ΣLFQIP), respectively.
We examined 25 most abundant proteins (Table 1). Five hexamerins accounted for 27.8% and 24.5% of the total protein amounts (ΣLFQ1-1,756) in the CP and IP samples on average. These storage proteins support metamorphosis in the pupal stage. Lipophorins (9.6% CP; 8.3% IP) and vitellogenin (4.7% CP; 4.1% IP) transport lipids; larval serum protein-1β, chemosensory protein and odorant binding protein-9 (5.9% CP and 4.7% IP) may also bind lipids. Ferritins (3.2% CP; 2.3% IP) store and transport iron (Larade and Storey, 2004; Ong et al., 2005). To our surprise, proPO2 and proPO3 (3.3% CP; 3.1%) are among the top 25 most abundant proteins, along with imaginal disc growth factor (a homolog of hemocyte aggregation inhibitor protein), TEP15 and secreted gelsolin (a component of hemolymph clot) (3.3% CP; 2.8% IP). These defense proteins may participate in melanization, pathogen recognition, coagulation, and hemocyte aggregation (Pesch et al., 2016; Blandin et al., 2004; Levashina et al., 2001; Karlsson et al., 2004). We also correlated the intense bands (Fig. 1A) with the most abundant protein(s) in the corresponding gel slices. Slice 3 (230–250 kDa) contained mainly hexamerins (57% CP and 47% IP), and so did slice 6 (70–80 kDa, 53% CP and 34% IP). We understood the dissociation of hexamerins to 80 kDa monomers, but not why some of the 480 kDa complexes migrated to 240 kDa position. Functions of these covalently linked trimers are unclear. Apolipophorin III (74% CP and 67% IH) represented the intense band in slice 10 (20–22 kDa). In summary, identification of the abundant proteins suggests that the major functions of hemolymph are storage, transport, and immunity. This agrees with the findings on the adult hemolymph proteome (Paskewitz and Shi, 2005).
Table 1.
A list of 25 most abundant proteins
| ID | Name | Mr (kDa) | CP%* | IP%* | IP/CP |
|---|---|---|---|---|---|
| AGAP001826-PA | Apolipophorin-I&II | 371 | 2.50 | 1.10 | 0.38 |
| AGAP013365-PA | Apolipophorin-III | 22 | 7.07 | 7.16 | 0.86 |
| AGAP008369-PA | Vitellogenin | 170 | 4.71 | 4.06 | 0.74 |
| AGAP008054-PD | Chemosensory protein | 15 | 1.68 | 1.23 | 0.63 |
| AGAP000278-PA | Odorant binding protein-9 | 16 | 2.98 | 1.83 | 0.52 |
|
| |||||
| AGAP002464-PA | Ferritin G subunit | 26 | 2.05 | 1.45 | 0.6 |
| AGAP002465-PA | Ferritin heavy chain | 25 | 1.13 | 0.8 | 0.61 |
| AGAP001659-PA | Hexamerin | 84 | 7.99 | 6.41 | 0.69 |
| AGAP001657-PA | Hexamerin | 84 | 9.10 | 6.36 | 0.6 |
| AGAP005768-PA | Hexamerin | 82 | 1.15 | 2.75 | 2.04 |
|
| |||||
| AGAP005766-PA | Hexamerin A | 83 | 2.71 | 3.70 | 1.17 |
| AGAP001345-PA | Hexamerin A | 83 | 6.8 | 5.28 | 0.66 |
| AGAP010657-PA | Larval serum protein 1β | 24 | 1.26 | 1.68 | 1.14 |
| AGAP008060-PA | Imaginal disc growth factor | 48 | 0.88 | 0.94 | 0.91 |
| AGAP006258-PA | proPO2 | 78 | 1.19 | 1.18 | 0.85 |
|
| |||||
| AGAP004975-PA | proPO3 | 79 | 2.10 | 1.96 | 0.8 |
| AGAP008364-PA | TEP15 | 164 | 1.66 | 1.41 | 0.73 |
| AGAP011369-PA | Gelsolin | 43 | 0.78 | 0.49 | 0.54 |
| AGAP007059-PA | LRR-7059 | 124 | 0.72 | 0.67 | 0.79 |
| AGAP000651-PC | Actin | 42 | 0.64 | 0.84 | 1.12 |
|
| |||||
| AGAP005627-PD | Creatine kinase | 40 | 1.09 | 1.37 | 1.07 |
| AGAP002564-PE | Fructose-1,6BP aldolase, class I | 39 | 0.97 | 1.43 | 1.26 |
| AGAP009623-PA | GAP dehydrogenase | 36 | 0.77 | 0.72 | 0.8 |
| AGAP013400-PA | Fatty acid-binding protein | 15 | 0.67 | 1.34 | 1.72 |
| AGAP012057-PA | RNA polymerase-associated RTF1 | 88 | 0.93 | 0.49 | 0.45 |
|
| |||||
| Σ | 63.53 | 56.65 | |||
: average percentage of each protein’s LFQs in the total LFQ protein intensity for the four CP or IP samples. Proteins shaded light blue are intracellular proteins. The IP/CP ratios shaded green are statistically significant (p < 0.05).
3.3. Hemolymph response to the bacterial treatment
Among the 1,756 proteins identified, 158 or 9% demonstrated significant (p < 0.05) changes in abundance between CP and IP. As a part of the response, 71 proteins were up-regulated more than 1.5 fold in insects infected with E. coli (Table 2). We classified them into 6 groups: immunity (4), cytoskeleton (3), DNA/RNA synthesis (13), metabolism (24), ATP/NAD(P)H binding (5), and others (22). In contrast, 30 proteins were down-regulated (IP/CP < 0.67) after the bacterial challenge (Table 3). They belong to three groups: immunity (9), metabolism (10), and others (11). Increases in the 67 immunity-unrelated proteins might reflect a higher demand for transcription, translation, and energy production after exposure to the bacteria, decreases in the 21 immunity-unrelated ones suggest a down-regulation of other cellular processes.
Table 2.
A list of 71 up-regulated proteins (I/C > 1.5, p < 0.05)
| Group | ID | Name | RAC* | RAi* | IP/CP |
|---|---|---|---|---|---|
| Immunity | AGAP002982-PA | E3 SUMO-protein ligase RanBP2 | 14 | 32 | 2.00 |
| AGAP001212-PB | PGRP-LB | 27 | 72 | 2.24 | |
| AGAP005246-PD | SRPN10 | 435 | 983 | 1.93 | |
| AGAP008366-PA | TEP2 | 4 | 6 | 1.51 | |
|
| |||||
| Cytoskeleton/motor | AGAP012185-PA | Erythrocyte membrane protein band 4.1 | 52 | 109 | 1.80 |
| AGAP001315-PE | Microtubule-associated protein 7 family | 11 | 26 | 2.10 | |
| AGAP001799-PA | Tropomyosin 1 | 79 | 141 | 1.52 | |
|
| |||||
| DNA/RNA synthesis | AGAP002945-PA | Bifunctional glutamyl/prolyl-tRNA synthetase | 14 | 44 | 2.61 |
| AGAP006125-PA | Density-regulated protein | 6 | 13 | 1.78 | |
| AGAP001883-PA | ELAV-like 1 | 4 | 10 | 2.30 | |
| AGAP004725-PA | Eukaryotic translation initiation factor 3C | 11 | 27 | 2.16 | |
| AGAP003486-PA | General transcriptional corepressor trfa | 13 | 28 | 1.9 | |
| AGAP005015-PA | Heterogeneous nuclear ribonucleoprotein K | 5 | 11 | 1.75 | |
| AGAP007299-PA | Importin-7 | 23 | 43 | 1.64 | |
| AGAP012013-PA | Nuclear factor of activated T-cells 5 | 6 | 13 | 1.69 | |
| AGAP002351-PA | Nuclear pore complex protein Nup98-Nup96 | 2 | 10 | 4.67 | |
| AGAP002654-PB | Poly(A)-binding protein 1 | 9 | 20 | 1.87 | |
| AGAP010553-PA | Poly(U)-binding-splicing factor PUF60 | 2 | 4 | 1.63 | |
| AGAP002655-PA | RNA binding protein | 2 | 7 | 2.88 | |
| AGAP010640-PA | Translation initiation factor | 186 | 367 | 1.68 | |
|
| |||||
| Metabolism | AGAP006227-PA | Alpha esterase | 0 | 7 | ∞ |
| AGAP004236-PA | Beta-lactamase-like protein 2 homolog | 0 | 2 | ∞ | |
| AGAP009405-PA | CPAP3-E | 8 | 14 | 1.59 | |
| AGAP005627-PE | Creatine kinase | 1 | 6 | 10.27 | |
| AGAP003124-PA | Dihydropyrimidinase | 2 | 6 | 2.47 | |
| AGAP000513-PB | Dipeptidase E | 47 | 99 | 1.81 | |
| AGAP013400-PA | Fatty acid-binding protein | 6664 | 13411 | 1.72 | |
| AGAP004071-PB | Fimbrin | 59 | 113 | 1.63 | |
| AGAP006670-PA | Gamma-glutamyl hydrolase | 109 | 244 | 1.91 | |
| AGAP003077-PB | Glutamyl aminopeptidase | 80 | 161 | 1.72 | |
| AGAP004383-PA | GSTD10 | 9 | 32 | 3.07 | |
| AGAP003257-PA | GSTU2 | 20 | 46 | 2.03 | |
| AGAP006353-PA | Histidine triad nucleotide binding protein 1 | 156 | 289 | 1.58 | |
| AGAP004747-PA | Ion binding and proteolysis | 60 | 111 | 1.58 | |
| AGAP012008-PA | Na+/H+ exchange regulatory cofactor NHE-RF1 | 20 | 48 | 2.05 | |
| AGAP000500-PD | NADPH-ferrihemoprotein reductase | 0 | 1 | ∞ | |
| AGAP009172-PA | Prolyl oligopeptidase | 101 | 185 | 1.56 | |
| AGAP004758-PB | Proteasomal ubiquitin receptor adrm1 homolog | 65 | 136 | 1.80 | |
| AGAP006171-PA | Protein phosphatase | 0 | 6 | ∞ | |
| AGAP004093-PA | Sterol carrier protein-2 | 910 | 2399 | 2.25 | |
| AGAP003052-PA | Tetratricopeptide repeat-containing protein α | 116 | 219 | 1.62 | |
| AGAP011872-PA | Ubiquitin-activating enzyme E1 | 256 | 481 | 1.61 | |
| AGAP009841-PA | UBX domain-containing protein 1 | 15 | 30 | 1.67 | |
| AGAP009648-PA | Ureidoimidazoline decarboxylase | 219 | 408 | 1.59 | |
|
| |||||
| ATP/NAD(P)H binding | AGAP005981-PA | DnaJ homolog subfamily A | 10 | 23 | 1.87 |
| AGAP001690-PA | Regulating synaptic exocytosis protein 2 | 0 | 4 | ∞ | |
| AGAP003153-PD | V-type proton ATPase catalytic subunit A | 591 | 1315 | 1.90 | |
| AGAP009486-PA | V-type proton transporting ATPase 54 kDa | 58 | 135 | 1.97 | |
| AGAP002884-PA | V-type proton transporting ATPase subunit B | 348 | 718 | 1.76 | |
|
| |||||
| Other | AGAP001467-PA | AGAP001467-PA | 9 | 20 | 1.87 |
| AGAP013060-PA | AGAP013060-PA | 1380 | 4381 | 2.71 | |
| AGAP011762-PA | BAG domain-containing protein Samui | 10 | 19 | 1.59 | |
| AGAP010557-PA | B-cell receptor-associated protein 31 | 13 | 26 | 1.66 | |
| AGAP005316-PA | Charged multivesicular body protein 4 | 36 | 71 | 1.71 | |
| AGAP010251-PA | Coatomer protein complex alpha subunit | 4 | 10 | 2.37 | |
| AGAP010900-PA | Cuticular protein 1 from fifty-one aa family | 43 | 87 | 1.72 | |
| AGAP006103-PA | Farnesoic acid o-methyl transferase-like | 81 | 173 | 1.81 | |
| AGAP009738-PA | Glutaredoxin | 93 | 181 | 1.66 | |
| AGAP000941-PB | Heat shock protein beta-1 isoform x2 | 182 | 376 | 1.77 | |
| AGAP000941-PA | Heat shock protein beta-1 isoform x2 | 15 | 31 | 1.76 | |
| AGAP007310-PA | Klaroid | 5 | 10 | 1.59 | |
| AGAP005291-PA | Lupus la ribonucleoprotein | 44 | 84 | 1.63 | |
| AGAP003238-PC | N-myc downstream regulated protein | 49 | 92 | 1.62 | |
| AGAP005369-PA | NOLC1-like isoform x2 | 4 | 14 | 3.27 | |
| AGAP008747-PA | Nsp1p | 5 | 12 | 2.02 | |
| AGAP008046-PA | PACSIN2 | 1 | 5 | 3.91 | |
| AGAP004310-PA | Perq amino acid-rich protein 2 | 8 | 14 | 1.59 | |
| AGAP012746-PA | Phyhd1 protein | 17 | 32 | 1.58 | |
| AGAP004520-PA | Ran-binding protein 3 | 8 | 17 | 1.83 | |
| AGAP004273-PB | Synapse-associated protein | 1 | 4 | 2.96 | |
| AGAP000626-PA | Vesicle-associated membrane protein B | 10 | 19 | 1.67 | |
: Relative abundance (RAc or RAi) is defined as each protein’s LFQ × 1,000,000 ÷ total LFQs for the CP or IP samples.
Table 3.
A list of 30 down-regulated proteins (I/C < 0.67, p < 0.05)
| Group | IDs | Names | RAC* | RAi* | IP/CP |
|---|---|---|---|---|---|
| Immunity | AGAP009110-PA | GNBP | 302 | 185 | 0.52 |
| AGAP005663-PA | SP-5663 | 394 | 280 | 0.61 | |
| AGAP011792-PA | CLIPA7-like | 1548 | 944 | 0.52 | |
| AGAP003251-PA | CLIPB1 | 210 | 123 | 0.50 | |
| AGAP003057-PA | CLIPB8 | 1012 | 620 | 0.52 | |
| AGAP005072-PA | SP-IgG-2LamD | 35 | 22 | 0.53 | |
| AGAP007385-PA | Lysozyme 4 (c-type) | 9 | 4 | 0.37 | |
| AGAP002857-PB | MDL2 | 58 | 34 | 0.50 | |
| AGAP002825-PA | proPO1 | 110 | 43 | 0.34 | |
|
| |||||
| Metabolism | AGAP003490-PA | Alanine-glyoxylate aminotransferase | 15 | 11 | 0.64 |
| AGAP008783-PA | Arginase | 12 | 8 | 0.57 | |
| AGAP002465-PA | Ferritin heavy chain | 11332 | 8040 | 0.61 | |
| AGAP008798-PA | Guanine nucleotide exchange factor MSS4 | 5 | 1 | 0.16 | |
| AGAP007237-PA | Heme peroxidase | 11 | 0 | 0.00 | |
| AGAP009033-PA | Heme peroxidase | 272 | 178 | 0.56 | |
| AGAP001826-PA | Apolipophorin-I&II | 24991 | 11018 | 0.38 | |
| AGAP004654-PA | Phosphoadenylate 3′-nucleotidase | 9 | 4 | 0.39 | |
| AGAP000439-PA | Tetrahydrobiopterin dehydratase | 187 | 139 | 0.63 | |
| AGAP008064-PA | Uroporphyrinogen-III synthase | 6 | 5 | 0.64 | |
|
| |||||
| Other | AGAP010846-PA | AGAP010846 | 18 | 6 | 0.29 |
| AGAP028095-PC | AGAP028095 | 42 | 23 | 0.47 | |
| AGAP004108-PB | Amalgam | 311 | 213 | 0.59 | |
| AGAP008052-PA | Chemosensory protein | 1072 | 760 | 0.61 | |
| AGAP002822-PA | Condensin-2 complex subunit H2 | 12 | 2 | 0.16 | |
| AGAP008013-PA | Filaggrin-2 isoform x1 | 5122 | 3798 | 0.63 | |
| AGAP001768-PB | Gamma-interferon-inducible protein IP-30 | 37 | 21 | 0.49 | |
| AGAP001657-PA | Hexamerin | 90951 | 63631 | 0.60 | |
| AGAP001127-PA | P37NB protein | 16 | 8 | 0.42 | |
| AGAP012057-PA | RNA polymerase-associated protein RTF1 | 9333 | 4944 | 0.45 | |
| AGAP001989-PA | Secreted salivary gland protein | 1525 | 737 | 0.41 | |
: Relative abundance (RAc or RAi) is defined as each protein’s LFQs × 1000,000 ÷ total LFQs for the CP or IP samples.
Surprisingly, only four defense proteins (PGRP-LB, TEP2, SRPN10, E3 SUMO-protein ligase RanBP2) were up-regulated 1.5–2.2 fold (Table 2). Seven (Gram-negative bacteria-binding protein (GNBP), CLIPs B1, B8 & A7, SP219, GP71, MDL2) were down-regulated to 50–61% of the control levels (p < 0.05) (Table 3). Lysozyme-4 and proPO1–3 levels significantly reduced to 37, 34, 85, and 80% of the control, respectively. While the ratios were high for proPO2 and 3, the absolute amounts of reduction based on LFQs were 25- and 59-fold higher than that of proPO1 (Table S1). These observations in the larval hemolymph are drastically different from the transcriptome data of adults, which indicate that more immunity-related genes are up-regulated than down-regulated after an immune challenge (Aguilar et al., 2005). One possible explanation is that certain immune molecules (e.g. proPOs) have been heavily used during defense responses of the larvae but not fully replenished at 24 h after E. coli inoculation.
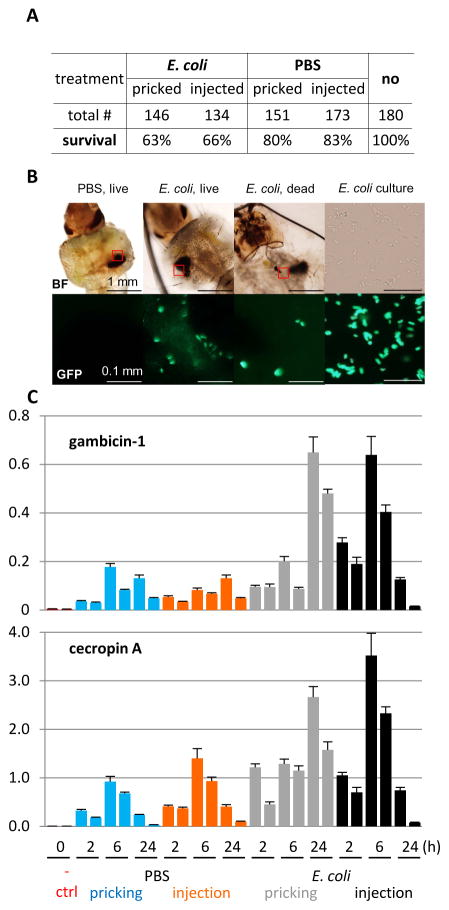
To further explain the disparity, we repeated the experiment using the same bacterial strain but carrying a GFP expression plasmid to test if the bacteria were still alive at 24 h after pricking, whether there was a difference in survival between the control and test groups, and what the dynamics of AMP gene expression was during the experiment. We found 80% of the PBS-pricked larvae survived whereas 63% of E. coli-pricked ones lived (Fig. 4A). This result suggests that E. coli pricking caused infection and infection led to more deaths than the buffer control did. In the other two groups, survival rates for PBS- and E. coli-injection were 83% and 66%, respectively, showing small variations between pricking and injection. The latter is a well-established infection model for adult mosquitos. Consistent with active infection, some of the bacteria were alive and mostly localized around the melanized wound site in the live larvae, as revealed by their movement during imaging (Fig. 4B). There was no fluorescent bacterium in the control larvae. In some dead larvae pricked with E. coli, GFP signals were distributed in various parts of the body, some near melanin tumors. The induced gambicin-1 expression peaked at 6 h in the E. coli-injected larvae and higher than those in the PBS-injected ones (Fig. 4C). The mRNA peak appeared at about 24 h in E. coli-pricked ones and, again, higher than those pricked with PBS. Similar expression patterns were observed for cecropin A. The gambicin and cecropin transcripts were hardly detected in the naïve larvae. Taken together, sterile and septic wounding both increased the AMP expression to various levels with different dynamics in larvae of this aquatic species. The survival of larvae was governed mainly by the presence/absence of E. coli in thousands and there was no clear difference between pricking and injection under the current conditions. The AMP transcript levels in E. coli-pricked larvae were only a few fold higher than PBS-pricked ones. In contrast to sterile wounding, the newly synthesized AMPs and other defense proteins (e.g. PPOs) were mostly consumed in the ongoing battle against E. coli, alive even at 24 h after inoculation. Based on the consistent results from these control experiments, we are confident that the proteomic data have faithfully reflected the complex changes in plasma proteins on the battleground. It would be interesting to compare hemolymph samples from naïve larvae and larvae pricked/injected with killed bacteria to confirm a consistent increase in mRNA and protein levels of defense molecules.
Fig. 4. Effects of sterile and septic wounding and injection on A. gambiae larvae.
(A) Survival statistics; (B) Localization of GFP-labeled bacteria in the living and dead larvae 24 h after pricking with PBS or E. coli. Blurring of the signals was caused by their movements during imaging. upper: bright field; lower: fluorescence. Due to movement of E. coli cells in the culture (right), their positions in the bright field no longer corresponded to those in the fluorescence microscope image obtained a few minutes later. (C) Expression profiles of gambicin-1 (upper) and cecropin A (lower) genes after PBS and E. coli pricking and injection. Two pools of naïve larvae (10 insect per group) were as used as negative controls. The AMP mRNA levels relative to A. gambiae rpS7 are shown for each sample as mean ± standard deviation (n = 3). Two biological replicates were analyzed for all these samples.
3.4. Immunity-related proteins
We have identified a total of 233 putative defense proteins in the larval hemolymph (Table 4), 166 of which are probably secreted. The percentage (71%), which is much higher than 29% (or 437) of the 1,521 other proteins, features the role of humoral responses. Besides, some defense proteins (e.g. proPOs) lack a signal peptide and may come from ruptured cells or by other unconventional paths (Kanost and Gorman, 2008; Nickel et al., 2010). We divide them into eight subgroups: 53 PRRs, 8 TEPs, 9 AMPs, 59 SPs, 38 SPHs, 17 SP inhibitors, 7 proPOs, and 42 others (Fig. 2B).
Table 4.
A list of 233 defense proteins
| Group | ID | Name | Mr (kDa) | RAC* | RAI* | IP/CP | p-value |
|---|---|---|---|---|---|---|---|
| PRR | AGAP007036-PA | APL1A | 49.4 | 15 | 0 | 0 | 0.356 |
| AGAP007035-PA | APL1B | 63.9 | 26 | 22 | 0.74 | 0.342 | |
| AGAP007033-PA | APL1C | 82.4 | 1577 | 1018 | 0.55 | 0.137 | |
| AGAP004811-PA | CTL1 | 21.8 | 31 | 29 | 0.79 | 0.402 | |
| AGAP004810-PA | CTL3 | 20.8 | 213 | 179 | 0.72 | 0.276 | |
| AGAP005335-PA | CTL4 | 19.8 | 13 | 4 | 0.28 | 0.103 | |
| AGAP003625-PA | CTL8 | 21.5 | 1160 | 1345 | 0.99 | 0.981 | |
| AGAP006430-PB | CTL-GA2 | 24.9 | 13 | 21 | 1.43 | 0.396 | |
| AGAP010193-PA | CTL-GA3 | 27 | 142 | 170 | 1.02 | 0.929 | |
| AGAP007412-PA | CTL-MA1 | 20.1 | 21 | 14 | 0.57 | 0.097 | |
| AGAP007411-PA | CTL-MA3 | 19.4 | 119 | 94 | 0.68 | 0.075 | |
| AGAP002911-PA | CTL-MA9 | 17.5 | 2 | 2 | 0.89 | 0.939 | |
| AGAP010021-PA | Dumpy | 172.4 | 13 | 71 | 4.71 | 0.414 | |
| AGAP010024-PA | Dumpy | 345.1 | 26 | 115 | 3.75 | 0.411 | |
| AGAP003027-PA | Dumpy-like | 43.6 | 11 | 22 | 1.63 | 0.31 | |
| AGAP009106-PA | GNBP | 32.1 | 249 | 155 | 0.53 | 0.064 | |
| AGAP009110-PA | GNBP | 42 | 302 | 185 | 0.52 | 0.004 | |
| AGAP009146-PA | GNBP | 33.5 | 122 | 47 | 0.33 | 0.084 | |
| AGAP006761-PA | GNBP-A1 | 55.7 | 9 | 7 | 0.67 | 0.176 | |
| AGAP004455-PA | GNBP-B1 | 44.1 | 5003 | 4409 | 0.75 | 0.338 | |
| AGAP002798-PA | GNBP-B2 | 43.7 | 211 | 265 | 1.07 | 0.829 | |
| AGAP002799-PA | GNBP-B3 | 43.1 | 0 | 5 | 146.34 | 0.356 | |
| AGAP002796-PA | GNBP-B4 | 46.7 | 21 | 41 | 1.63 | 0.232 | |
| AGAP006327-PA | LRIM (short) | 39.6 | 89 | 74 | 0.71 | 0.095 | |
| AGAP006348-PA | LRIM1 | 57.3 | 2032 | 1324 | 0.56 | 0.138 | |
| AGAP007039-PA | LRIM4 | 59.9 | 21 | 12 | 0.46 | 0.172 | |
| AGAP005693-PA | LRIM17 | 48.8 | 1784 | 1307 | 0.63 | 0.081 | |
| AGAP006644-PA | LRRP | 77 | 4 | 2 | 0.5 | 0.359 | |
| AGAP011503-PA | LRRP | 32 | 29 | 27 | 0.79 | 0.392 | |
| AGAP005962-PA | LRRP shoc-2 | 90.7 | 49 | 46 | 0.79 | 0.242 | |
| AGAP004832-PA | LRRP-1 | 117.8 | 875 | 719 | 0.7 | 0.03 | |
| AGAP003878-PA | LRRP-15 | 63.2 | 59 | 93 | 1.34 | 0.013 | |
| AGAP007030-PA | LRRP-7030 | 115.4 | 1 | 0 | 0 | 0.356 | |
| AGAP007059-PA | LRRP-7059 | 124 | 7232 | 6670 | 0.79 | 0.106 | |
| AGAP007060-PA | LRRP-7060 | 132.9 | 4086 | 3653 | 0.76 | 0.251 | |
| AGAP009762-PA | Nimrod | 141.6 | 614 | 545 | 0.76 | 0.497 | |
| AGAP001212-PB | PGRP-LB | 23.3 | 27 | 72 | 2.24 | 0.04 | |
| AGAP000536-PA | PGRP-S1 | 22.4 | 51 | 34 | 0.58 | 0.078 | |
| AGAP006343-PA | PGRP-S2 | 20 | 6 | 0 | 0 | 0.356 | |
| AGAP006342-PA | PGRP-S3 | 20 | 290 | 334 | 0.98 | 0.942 | |
| AGAP011239-PA | FBN7 | 30.4 | 80 | 76 | 0.81 | 0.468 | |
| AGAP009184-PA | FBN8 | 35.9 | 635 | 361 | 0.49 | 0.146 | |
| AGAP010775-PA | FBN8 | 23.3 | 0 | 0 | 0 | 0.356 | |
| AGAP011223-PA | FBN8 | 24.8 | 33 | 21 | 0.53 | 0.067 | |
| AGAP011225-PA | FBN8 | 34.5 | 60 | 37 | 0.52 | 0.084 | |
| AGAP009556-PA | FBN8 | 22.4 | 270 | 242 | 0.76 | 0.161 | |
| AGAP004918-PA | Fibrinogen | 35 | 32 | 39 | 1.03 | 0.835 | |
| AGAP004996-PA | Fibrinogen | 46.8 | 44 | 36 | 0.7 | 0.609 | |
| AGAP006743-PA | Fibrinogen | 37.4 | 34 | 31 | 0.79 | 0.042 | |
| AGAP006790-PA | Fibrinogen | 30.8 | 4 | 1 | 0.29 | 0.165 | |
| AGAP011197-PA | Fibrinogen | 32.3 | 97 | 111 | 0.98 | 0.941 | |
| AGAP004917-PA | Fibrinogen-related pr. 1 | 34.2 | 71 | 69 | 0.83 | 0.251 | |
| AGAP006914-PA | Fibrinogen-related pr. 1 | 31.3 | 77 | 76 | 0.84 | 0.343 | |
|
| |||||||
| TEP | AGAP010815-PA | TEP1 | 152.1 | 908 | 428 | 0.4 | 0.071 |
| AGAP008366-PA | TEP2 | 154.6 | 4 | 6 | 1.51 | 0.021 | |
| AGAP010812-PA | TEP4 | 149.4 | 237 | 278 | 1 | 0.993 | |
| AGAP010814-PA | TEP6 | 151.3 | 30 | 4 | 0.12 | 0.108 | |
| AGAP010830-PA | TEP9 | 151.5 | 5 | 2 | 0.31 | 0.537 | |
| AGAP008654-PA | TEP12 | 96.3 | 47 | 17 | 0.32 | 0.127 | |
| AGAP008368-PA | TEP14 | 139.4 | 5 | 1 | 0.17 | 0.091 | |
| AGAP008364-PA | TEP15 | 163.6 | 16555 | 14077 | 0.73 | 0.045 | |
|
| |||||||
| AMP | AGAP004632-PA | Defensin | 10 | 0 | 1 | 27.53 | 0.356 |
| AGAP007199-PA | Defensin | 7 | 4 | 9 | 2.08 | 0.341 | |
| AGAP008645-PA | Gambicin | 8.8 | 9 | 29 | 2.67 | 0.089 | |
| AGAP007347-PA | Lysozyme 1 (c-type) | 15.3 | 14 | 13 | 0.76 | 0.64 | |
| AGAP007345-PA | Lysozyme 3 (c-type) | 16.6 | 103 | 134 | 1.12 | 0.817 | |
| AGAP007385-PA | Lysozyme 4 (c-type) | 17.4 | 9 | 4 | 0.37 | 0.046 | |
| AGAP007344-PA | Lysozyme 8 (c-type) | 16.5 | 0 | 0 | 13.93 | 0.356 | |
| AGAP011119-PA | Lysozyme 3 | 18 | 93 | 106 | 0.98 | 0.916 | |
| AGAP000376-PA | Transferrin precursor | 69.2 | 4208 | 7912 | 1.61 | 0.055 | |
|
| |||||||
| proPO | AGAP002825-PA | proPO1 | 79.3 | 110 | 43 | 0.34 | 0.031 |
| AGAP006258-PA | proPO2 | 78.1 | 11866 | 11762 | 0.85 | 0.045 | |
| AGAP004975-PA | proPO3 | 78.6 | 21025 | 19615 | 0.8 | 0.024 | |
| AGAP004981-PA | proPO4 | 78.5 | 448 | 442 | 0.84 | 0.357 | |
| AGAP004977-PA | proPO6 | 79 | 710 | 880 | 1.06 | 0.827 | |
| AGAP004980-PA | proPO7 | 79.6 | 4 | 19 | 3.66 | 0.414 | |
| AGAP004976-PA | proPO8 | 79.3 | 521 | 629 | 1.03 | 0.914 | |
|
| |||||||
| SP | AGAP001798-PA | SP217 | 72.6 | 2 | 4 | 1.31 | 0.464 |
| AGAP005625-PA | SP213 | 146.8 | 128 | 154 | 1.03 | 0.903 | |
| AGAP001365-PA | SP208 | 68.6 | 19 | 15 | 0.68 | 0.149 | |
| AGAP005072-PA | SP219 | 96.4 | 35 | 22 | 0.53 | 0.002 | |
| AGAP003686-PA | CLIPB47 | 39.8 | 165 | 160 | 0.83 | 0.315 | |
| AGAP003251-PA | CLIPB1 | 40.9 | 210 | 123 | 0.5 | 0.004 | |
| AGAP003246-PA | CLIPB2 | 38.4 | 21 | 9 | 0.35 | 0.078 | |
| AGAP013487-PA | CLIPB3a | 34.2 | 101 | 170 | 1.44 | 0.331 | |
| AGAP003249-PA | CLIPB3b | 40.1 | 218 | 173 | 0.68 | 0.087 | |
| AGAP003250-PA | CLIPB4 | 39.4 | 4906 | 4007 | 0.7 | 0.076 | |
| AGAP004148-PA | CLIPB5 | 41.3 | 147 | 175 | 1.02 | 0.891 | |
| AGAP003057-PA | CLIPB8 | 44.8 | 1012 | 620 | 0.52 | 0.048 | |
| AGAP013442-PB | CLIPB9,10 | 42.0 | 351 | 276 | 0.67 | 0.054 | |
| AGAP009214-PA | CLIPB11 | 39.8 | 6 | 7 | 0.95 | 0.824 | |
| AGAP004855-PA | CLIPB13 | 44.8 | 303 | 263 | 0.74 | 0.036 | |
| AGAP009844-PA | CLIPB15 | 40.6 | 54 | 51 | 0.82 | 0.182 | |
| AGAP011325-PA | CLIPB45 | 34.2 | 46 | 41 | 0.76 | 0.131 | |
| AGAP008835-PA | CLIPC1 | 42.6 | 83 | 80 | 0.83 | 0.182 | |
| AGAP004317-PA | CLIPC2 | 41.5 | 49 | 46 | 0.8 | 0.346 | |
| AGAP004318-PA | CLIPC3 | 43.1 | 97 | 99 | 0.87 | 0.296 | |
| AGAP000573-PB | CLIPC4 | 39.4 | 180 | 166 | 0.79 | 0.086 | |
| AGAP000315-PA | CLIPC6 | 39.6 | 106 | 100 | 0.81 | 0.316 | |
| AGAP004719-PA | CLIPC9 | 40.6 | 76 | 77 | 0.87 | 0.354 | |
| AGAP000572-PA | CLIPC10 | 40.9 | 33 | 37 | 0.96 | 0.821 | |
| AGAP002422-PA | CLIPD1 | 48.5 | 48 | 53 | 0.94 | 0.581 | |
| AGAP002813-PA | CLIPD6 | 52.8 | 30 | 53 | 1.51 | 0.222 | |
| AGAP012022-PA | CLIPE24 | 97 | 8 | 13 | 1.41 | 0.146 | |
| AGAP008403-PA | CLIPE15 | 99.3 | 97 | 99 | 0.87 | 0.593 | |
| AGAP012614-PA | CLIPB3b-like | 43.4 | 103 | 186 | 1.55 | 0.261 | |
| AGAP001198-PA | GP13 | 29.4 | 3 | 0 | 0 | 0.078 | |
| AGAP005663-PA | GP71 | 33.8 | 394 | 280 | 0.61 | 0.026 | |
| AGAP005670-PA | GP49 | 32.2 | 866 | 746 | 0.74 | 0.277 | |
| AGAP005671-PA | GP65 | 32.2 | 2575 | 2765 | 0.92 | 0.709 | |
| AGAP005686-PA | SP31 | 31.9 | 95 | 109 | 0.98 | 0.95 | |
| AGAP007252-PA | GP51 | 32.9 | 43 | 37 | 0.74 | 0.266 | |
| AGAP009121-PA | GP92 | 27.7 | 48 | 52 | 0.93 | 0.785 | |
| AGAP005687-PA | SP33 | 32.1 | 14 | 16 | 1.01 | 0.968 | |
| AGAP006674-PA | GP66 | 32.4 | 790 | 793 | 0.86 | 0.444 | |
| AGAP006675-PA | GP60 | 32.1 | 9 | 4 | 0.41 | 0.214 | |
| AGAP001246-PA | GP72 | 30.3 | 33 | 45 | 1.14 | 0.792 | |
| AGAP001248-PA | GP70 | 28.9 | 12 | 16 | 1.12 | 0.814 | |
| AGAP001249-PA | GP52 | 27.1 | 1283 | 1331 | 0.89 | 0.666 | |
| AGAP006539-PA | SP63 | 28.8 | 37 | 67 | 1.54 | 0.369 | |
| AGAP011920-PA | GP59 | 26.3 | 522 | 323 | 0.53 | 0.11 | |
| AGAP012946-PA | SP53 | 35.5 | 285 | 271 | 0.81 | 0.359 | |
| AGAP013221-PA | SP7 | 35 | 101 | 74 | 0.63 | 0.085 | |
| AGAP004566-PA | SP25 | 35.7 | 11 | 13 | 0.95 | 0.837 | |
| AGAP006673-PA | GP81 | 33.1 | 21 | 21 | 0.88 | 0.535 | |
| AGAP002543-PA | SP71 | 29.8 | 1 | 0 | 0 | 0.142 | |
| AGAP012328-PA | SP45 | 36.5 | 7 | 7 | 0.77 | 0.636 | |
| AGAP008808-PA | SP130 | 67.5 | 67 | 79 | 1.02 | 0.784 | |
| AGAP010240-PA | GP97 | 28.2 | 161 | 110 | 0.59 | 0.063 | |
| AGAP003960-PA | SP122 | 64.6 | 391 | 340 | 0.74 | 0.171 | |
| AGAP013252-PA | SP134 | 66.6 | 111 | 121 | 0.94 | 0.398 | |
| AGAP011427-PA | SP111 | 96.2 | 60 | 28 | 0.39 | 0.061 | |
| AGAP012504-PA | CLIPE33 | 93.9 | 53 | 80 | 1.28 | 0.283 | |
| AGAP027981-PA | CLIPE33-like | 98 | 19 | 41 | 1.91 | 0.09 | |
| AGAP012269-PA | SP127 | 72.3 | 2 | 4 | 1.69 | 0.139 | |
| AGAP007043-PA | SP112 | 59.9 | 186 | 148 | 0.68 | 0.166 | |
|
| |||||||
| SPH | AGAP001979-PA | SPH220 | 226 | 4 | 10 | 2.21 | 0.422 |
| AGAP012505-PA | CLIPE23-like | 31.5 | 0 | 1 | 24.8 | 0.356 | |
| AGAP003691-PA | CLIPE12 | 94.4 | 533 | 335 | 0.54 | 0.088 | |
| AGAP000290-PA | CLIPA27 | 54 | 1 | 2 | 1.07 | 0.938 | |
| AGAP009216-PA | CLIPB43 | 33.9 | 0 | 0 | 0 | 0.356 | |
| AGAP010730-PA | CLIPA28 | 28.2 | 865 | 701 | 0.69 | 0.072 | |
| AGAP011791-PA | CLIPA1 | 48.4 | 582 | 392 | 0.58 | 0.112 | |
| AGAP011790-PB | CLIPA2 | 55.9 | 701 | 669 | 0.82 | 0.508 | |
| AGAP011780-PA | CLIPA4 | 45.9 | 1140 | 1127 | 0.84 | 0.473 | |
| AGAP011789-PA | CLIPA6 | 45.7 | 4565 | 4961 | 0.93 | 0.561 | |
| AGAP011792-PA | CLIPA7 | 80.9 | 1548 | 944 | 0.52 | 0.011 | |
| AGAP010731-PA | CLIPA8 | 40.9 | 143 | 117 | 0.7 | 0.248 | |
| AGAP011781-PA | CLIPA12 | 40.9 | 157 | 163 | 0.88 | 0.458 | |
| AGAP011788-PA | CLIPA14 | 30.4 | 2296 | 2343 | 0.87 | 0.34 | |
| AGAP002270-PA | CLIPB7 | 43.6 | 51 | 68 | 1.12 | 0.741 | |
| AGAP013184-PA | CLIPB36 | 42.5 | 20 | 21 | 0.9 | 0.573 | |
| AGAP003689-PA | CLIPC7 | 67 | 282 | 311 | 0.94 | 0.695 | |
| AGAP005642-PA | GPH46 | 33 | 30 | 34 | 0.97 | 0.916 | |
| AGAP011608-PA | GPH48 | 36.4 | 6 | 9 | 1.38 | 0.417 | |
| AGAP011919-PA | GPH76 | 28.1 | 244 | 156 | 0.55 | 0.401 | |
| AGAP011917-PA | GPH64 | 26.2 | 10 | 10 | 0.89 | 0.812 | |
| AGAP005707-PA | GPH77 | 32.3 | 44 | 28 | 0.54 | 0.201 | |
| AGAP005709-PA | GPH90 | 28.7 | 2 | 2 | 0.88 | 0.864 | |
| AGAP006676-PA | GPH50 | 28.6 | 452 | 578 | 1.09 | 0.719 | |
| AGAP004740-PA | GPH47 | 27.8 | 28 | 23 | 0.71 | 0.489 | |
| AGAP005703-PA | GPH61 | 31.4 | 1 | 0 | 0 | 0.356 | |
| AGAP005708-PA | GPH69 | 29.6 | 3 | 0 | 0 | 0.165 | |
| AGAP003248-PA | SPH6 | 33.2 | 1 | 1 | 1.02 | 0.986 | |
| AGAP004638-PA | SPH47 | 37.3 | 281 | 260 | 0.79 | 0.41 | |
| AGAP006486-PA | GPH53 | 30.8 | 43 | 45 | 0.9 | 0.817 | |
| AGAP006485-PA | GPH55 | 30.5 | 584 | 621 | 0.91 | 0.858 | |
| AGAP013117-PA | SPH48 | 33.5 | 48 | 36 | 0.64 | 0.105 | |
| AGAP001245-PA | GPH57 | 28.7 | 283 | 244 | 0.74 | 0.326 | |
| AGAP006677-PA | GPH67 | 29.6 | 29 | 35 | 1.01 | 0.969 | |
| AGAP009122-PA | GPH93 | 29.2 | 34 | 64 | 1.62 | 0.084 | |
| AGAP006487-PA | GPH54 | 30.4 | 429 | 486 | 0.97 | 0.945 | |
| AGAP013164-PA | SPH34 | 28.2 | 373 | 516 | 1.18 | 0.659 | |
| AGAP003626-PA | GPH36 | 34.5 | 2511 | 2094 | 0.71 | 0.215 | |
|
| |||||||
| Serpin | AGAP006909-PA | SRPN1 | 47.7 | 40 | 72 | 1.56 | 0.234 |
| AGAP006911-PA | SRPN2 | 46.5 | 2162 | 1987 | 0.79 | 0.199 | |
| AGAP006910-PA | SRPN3 | 47.1 | 520 | 720 | 1.18 | 0.373 | |
| AGAP009670-PA | SRPN4 | 68.9 | 338 | 340 | 0.86 | 0.596 | |
| AGAP009670-PB | SRPN4 | 61.8 | 1341 | 1253 | 0.8 | 0.314 | |
| AGAP007693-PA | SRPN7 | 44.3 | 368 | 408 | 0.95 | 0.792 | |
| AGAP003194-PA | SRPN8 | 48.8 | 95 | 98 | 0.88 | 0.383 | |
| AGAP003139-PA | SRPN9 | 50.4 | 2009 | 2956 | 1.26 | 0.365 | |
| AGAP005246-PD | SRPN10 | 42.6 | 435 | 983 | 1.93 | 0.044 | |
| AGAP005246-PE | SRPN10 | 42.2 | 0 | 4 | 124.84 | 0.356 | |
| AGAP001377-PA | SRPN11 | 57.1 | 2342 | 2844 | 1.04 | 0.905 | |
| AGAP001375-PA | SRPN12 | 64.8 | 891 | 1445 | 1.39 | 0.467 | |
| AGAP009213-PA | SRPN16 | 61.1 | 1471 | 1349 | 0.78 | 0.314 | |
| AGAP001376-PA | SRPN17 | 53.7 | 60 | 71 | 1.01 | 0.939 | |
| AGAP008968-PA | Kazal domain protein | 6.5 | 337 | 346 | 0.88 | 0.757 | |
| AGAP011482-PA | Kazal domain protein | 8.5 | 23 | 34 | 1.25 | 0.514 | |
| AGAP006813-PA | SP inhibitor | 13.4 | 2 | 4 | 1.5 | 0.377 | |
|
| |||||||
| Others | AGAP002585-PA | Cys-rich protein | 175.6 | 6 | 15 | 2.35 | 0.34 |
| AGAP004631-PA | Clotting factor deficiency 2 | 26.1 | 1 | 2 | 1.4 | 0.553 | |
| AGAP003987-PA | C1q binding protein | 29.6 | 38 | 22 | 0.48 | 0.209 | |
| AGAP002878-PA | Cystatin-like protein | 11 | 66 | 83 | 1.08 | 0.49 | |
| AGAP011460-PA | Cys-rich protein (salivary) | 11.2 | 258 | 119 | 0.39 | 0.11 | |
| AGAP006253-PA | Cys-rich venom protein | 9.5 | 323 | 431 | 1.14 | 0.761 | |
| AGAP012970-PA | Cys-rich venom protein | 8.8 | 5 | 3 | 0.64 | 0.45 | |
| AGAP011832-PA | Death-associated protein 1 | 10.3 | 97 | 68 | 0.6 | 0.395 | |
| AGAP008878-PA | Defense protein | 17.7 | 484 | 529 | 0.93 | 0.799 | |
| AGAP010884-PA | DsCAM A | 214.7 | 74 | 41 | 0.47 | 0.645 | |
| AGAP000025-PA | E3 SUMO-pr. ligase 2 | 150.4 | 0 | 0 | 0 | 0.356 | |
| AGAP002982-PA | E3 SUMO-pr. Lig. RanBP2 | 308.1 | 14 | 32 | 2 | 0.021 | |
| AGAP010822-PA | Fasciclin | 26.3 | 294 | 319 | 0.93 | 0.749 | |
| AGAP010823-PA | Fasciclin isoform c | 52.4 | 1 | 0 | 0 | 0.356 | |
| AGAP001708-PA | Gd-domain protein | 30.9 | 68 | 65 | 0.82 | 0.44 | |
| AGAP008797-PA | Ig (CD79A) binding pr. 1 | 42.2 | 0 | 0 | 14.42 | 0.356 | |
| AGAP000032-PA | Integrin alpha-ps2 x1 | 166.7 | 7 | 10 | 1.36 | 0.279 | |
| AGAP007629-PB | Laminin gamma 1 | 179.6 | 10 | 11 | 1.01 | 0.965 | |
| AGAP004993-PA | Laminin subunit alpha | 412.1 | 13 | 12 | 0.76 | 0.207 | |
| AGAP002857-PB | MDL2 | 18.1 | 58 | 34 | 0.5 | 0.02 | |
| AGAP011319-PA | Pacifastin-related peptide | 25.3 | 1342 | 1150 | 0.73 | 0.105 | |
| AGAP008804-PB | Peroxin-19 | 33.2 | 3 | 1 | 0.36 | 0.25 | |
| AGAP001325-PA | Peroxiredoxin 5, atypical | 20.6 | 429 | 428 | 0.85 | 0.438 | |
| AGAP004674-PA | Phenoloxidase inhibitor | 36.3 | 1199 | 1294 | 0.92 | 0.727 | |
| AGAP010477-PB | Phosducin-like 3 | 26.3 | 6 | 8 | 1.25 | 0.179 | |
| AGAP005531-PA | PCD6-interacting protein | 94.1 | 25 | 36 | 1.26 | 0.091 | |
| AGAP000378-PA | PCD protein 4 | 47.4 | 23 | 31 | 1.15 | 0.857 | |
| AGAP005432-PA | PCD protein 5 | 14.8 | 9 | 12 | 1.23 | 0.642 | |
| AGAP003476-PA | Protein BCP1 | 33.6 | 26 | 29 | 0.93 | 0.772 | |
| AGAP004333-PA | Multidomain TMP | 173.7 | 45 | 14 | 0.27 | 0.125 | |
| AGAP003012-PA | 3PAN-ZP-TM | 78.6 | 9 | 15 | 1.42 | 0.61 | |
| AGAP000305-PA | SPARC | 22.2 | 18 | 24 | 1.16 | 0.579 | |
| AGAP011765-PA | Spondin-1 | 87 | 153 | 145 | 0.81 | 0.403 | |
| AGAP003338-PA | Thioredoxin | 15.5 | 3 | 1 | 0.32 | 0.384 | |
| AGAP007201-PA | Thioredoxin | 15.6 | 24 | 35 | 1.27 | 0.468 | |
| AGAP009584-PA | Thioredoxin | 12.1 | 361 | 270 | 0.64 | 0.119 | |
| AGAP000396-PA | Thioredoxin peroxidase | 26 | 25 | 24 | 0.82 | 0.433 | |
| AGAP011054-PA | Thioredoxin peroxidase | 22 | 747 | 949 | 1.09 | 0.209 | |
| AGAP011824-PA | Thioredoxin peroxidase | 25 | 777 | 865 | 0.95 | 0.703 | |
| AGAP005462-PA | Thioredoxin-like pr. 1 | 31.6 | 18 | 19 | 0.89 | 0.528 | |
| AGAP001613-PA | Thioredoxin-like TMP1 | 38.9 | 7 | 11 | 1.38 | 0.455 | |
| AGAP003615-PA | Toll-interacting protein | 30.4 | 3 | 5 | 1.42 | 0.516 | |
: Relative abundance (RAc or RAi) is defined as each protein’s LFQs × 1000,000 ÷ total LFQs for the CP or IP samples.
The PRRs include 15 LRR proteins, 9 CTLs, 8 GNBPs, 4 PGRPs, and 13 FBNs among others. APL1C and LRIM1 are critical determinants of the mosquito antimalarial resistance (Povelones et al., 2009) and are more abundant than the other LRR proteins, except for LRR-7059 and LRR-7060. We detected low CTL4 and no CTLMA2 in the larval plasma. These two lectins positively impact the parasite development in adult females (Osta et al., 2004). CTL8, PGRP-S3, Nimrod, and GNBP-B1, most abundant in their respective families, may be important for antibacterial immunity in this stage. TEP1, TEP4 and TEP15 existed at higher levels than the other TEPs and, along with LRR-7060, may participate in antiparasitic responses (Blandin et al., 2004; Li et al., 2013). We did not detect any TEP in the proteomic analysis of M. sexta larval hemolymph (He et al., 2016). In contrast, the mosquito TEPs account for about 1% of total LFQs.
The detection of 59 SPs and 38 SPHs in these samples (Table 4) indicates a contamination of the plasma samples by gut contents. To address this problem, we examined their domain structures and expression profiles and considered 14 SPs and 17 SPHs as midgut proteins (data not shown). The GPs (i.e. gut SPs) likely digest dietary proteins but the GPHs’ function is unclear. It is common that catalytically inactive SPHs are expressed in the digestive tract (Cao et al., 2015; Lin et al., 2015; Zhao et al., 2010).
Most of the other 45 SPs and 21 SPHs may participate in processes unrelated to digestion, as additional structural modules are present in these proteins for interacting with other molecules and their expression is not as synchronized as the digestive enzymes (data not shown). For instance, SP208, SP213/GRAAL, SP217, SP219, CLIPB47, and CLIPD6 contain multiple regulatory domains; 13 CLIPBs, 7 CLIPCs, 2 CLIPDs and 3 CLIPEs have a clip domain and a protease domain; 14 SPHs contain a clip domain and a protease-like domain (Table 4). CLIPs B4, B8, B13, B9, B10, B3, B5, and C4 were more abundant than the other clip-domain SPs. We detected low levels of three multi-domain SPHs (SPH220, CLIPA2 and CLIPE12) and found ten of the fifteen clip-domain SPHs were more abundant, representing 0.02–0.50% of the LFQ totals. Identification and quantification of the nondigestive SPs and SPHs in the larval plasma provided useful clues for studying a putative SP-SPH network that coordinates some of the defense mechanisms (Barillas-Mury, 2007).
Biochemical elucidation of the mosquito SP-SPH system poses a fierce challenge, since less than 0.5 μl of hemolymph can be collected from one adult. Identification of the 66 nondigestive SPs and SPHs in larval plasma hints at a similarly complex system that regulates immune responses in adult hemolymph (Volz et al., 2005 and 2006; Povelones et al., 2013). Another problem is that fifteen of the identified 97 SPs and SPHs had minor to major flaws in their sequences (data not shown). Nonetheless, the proteome analysis at least validate them as authentic gene products. More notably, this study greatly confines the system exploration from nearly 350 SP-related genes in the genome (Christophides et al., 2002). Data on their levels should allow us to further focus on the abundant ones with complex domain structures as candidates of the immune SP-SPH pathways.
We have identified 14 serpins (including two variants of SRPN4 and SRPN10). SRPN2, 3, 4, 7, 9, 10A, 11, 12 and 16 were relatively abundant (0.03–0.30%). Biological functions of several A. gambiae serpins have been characterized in the adult, which may regulate proPO activation (Danielli et al., 2005; Abraham et al., 2005; Michel et al., 2006; Suwanchaichinda and Kanost, 2009). Together, nondigestive SPs, SPHs and serpins are speculated to constitute an enzyme-cofactor-inhibitor system to coordinate humoral and cellular immunity, as demonstrated in other insects (Jiang et al., 2010; Park et al., 2010; Kanost and Jiang, 2015).
As putative substrates of clip-domain SPs, seven of the nine proPOs were detected at different levels in the larval plasma. ProPO2 and 3 were most abundant (1.2% and 2.0% of the total LFQs), followed by proPO6, 8, 4 (0.04–0.09%), and then proPO1 and 7 (≤0.01%). Based on their sheer amounts, we suggest that proPO2 and proPO3 are more important in antimicrobial responses of the larvae. Other immune effectors such as defensins, gambicin and lysozymes existed at ≤0.01%. The high basal level of transferrin (0.42%) had a 1.61-fold increase to 0.79% (p = 0.055) in plasma.
3.5. Gel distribution of the defense proteins
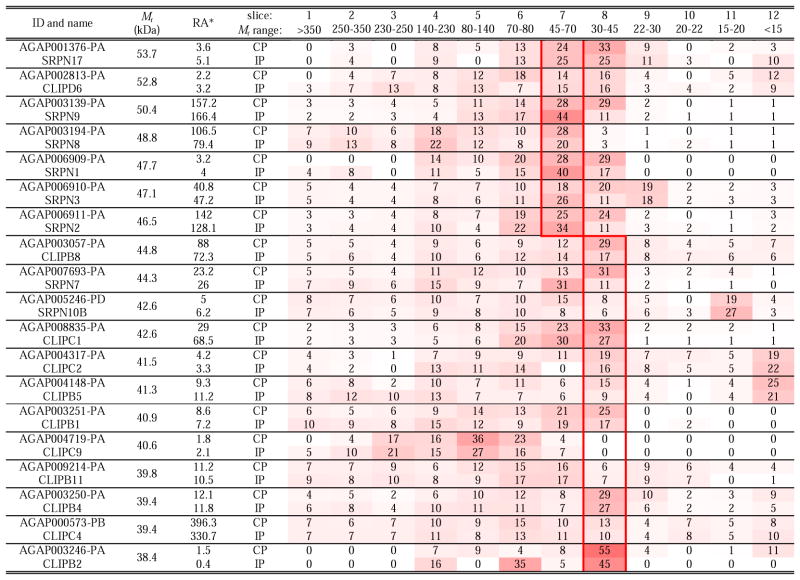
SDS-treated proteins should migrate in reducing gel to positions corresponding to their theoretical masses (Mr’s). However, post-translational modifications (e.g. glycosylation, proteolysis, covalent crosslinking) alters Mr’s and such changes are common during insect defense responses (He et al., 2016). Thus, to characterize the extent to which this might occur in the mosquito as a result of immune challenge, we examined distributions of the 233 defense proteins in the gel slices and identified fifteen showing major Mr decreases consistent with proteolytic processing (Table 5). While nature of these cleavages (e.g. sites and accountable enzymes) is unclear, these altered mobilities are notable because of their potential importance.
Table 5.
A list of fifteen proteins likely cleaved by proteases
: Relative abundance (RA) is defined as each protein’s LFQs × 1000,000 ÷ total LFQs of all proteins for the CP or IP samples. Value for a protein in a gel slice is the average percentage of that protein’s total LFQ in the four CP or IP samples. These values are also shown in the gradient heat map from white (0) to red (100). The red boxes indicate the positions of the proteins based on their calculated Mr’s.
Due to the importance of CLIPs and their inhibitory regulation by serpins, we closely examined the gel slices containing them, and found eleven clip-domain SPs and eight serpins in gel slice 6 (70–80 kDa). Since these proteins are typically 45–55 kDa, the observed mobility suggests the formation of SDS-stable serpin-protease complexes (Table 6). Such acyl-enzyme complexes usually contain a 40–45 kDa serpin fragment and a 30 kDa SP catalytic domain, consistent with the previous observations (An et al., 2011; Tong et al., 2005). Interestingly, SRPN1, 2, 7, 8, 10B, CLIPs B1, B2, B4, B5, B8, B11, C2, C4, C9 and D6 were also detected at a higher Mr range (80–350 kDa) (Table 7). This result suggests the proteins are parts of larger covalent complexes, which may contain other defense proteins.
Table 6.
Components of hypothesized high Mr serpin-protease complexes
: Relative abundance (RA) is defined as each protein’s LFQs × 1000,000 ÷ total LFQs of all proteins for the CP or IP samples. Value for a protein in a gel slice is the average percentage of that protein’s total LFQ in the four CP or IP samples. These values are also shown in the gradient heat map from white (0) to red (100). The red boxes indicate the positions of the proteins based on their calculated Mr’s.
Table 7.
Components of hypothesized high Mr immune complexes
: Relative abundance (RA) is defined as each protein’s LFQs × 1000,000 ÷ total LFQs of all proteins for the CP or IP samples. Value for a protein in a gel slice is the average percentage of that protein’s total LFQ in the four CP or IP samples. These values are also shown in the gradient heat map from white (0) to red (100). The red boxes indicate the positions of the proteins based on their calculated Mr’s.
Our previous study of M. sexta larval hemolymph (He et al., 2016) revealed that, in general, abundant proteins are distributed among more gel slices than scarce ones do. As shown in Fig. 5, the same correlation was observed in this project and applied in studying protein spreading in the gel slices. We found, like the CLIPs and serpins, 24 other defense proteins displayed substantial decreases in gel mobility (Table 7). They were GNBPA1, TEP6, FBN, 5 LRRs, Trynity, 6 SPs, 2 SPHs, and 7 proPOs. Since most of them were not abundant, their low-mobility species did not seem to be artifacts. We interpret these and some other slow migrating proteins (Table 6) as components of high Mr immune complexes. Covalent crosslinking by PO-generated compounds may have stabilized them under the reducing and denaturing conditions of SDS-PAGE; such formation of PO-generated macromolecular complexes has been previously observed in several studies (Yu et al., 2003; Zou and Jiang, 2005). Recently the role of POs in this process was clearly demonstrated (Clark and Strand, 2013). We found that 32 of the 66 proteins (10–80 kDa) identified in >80 kDa positions were immunity-related, at a ratio twice as high as 171 of the 702 total proteins identified in the M. sexta plasma proteome (He et al., 2016). Based on these, we are generally confident about the model of formation of high Mr complexes enriched with defense proteins in A. gambiae.
Fig. 5. Relationships of protein abundances and number of the gel slices they were identified.
Relative abundances, log2LFQ, for proteins identified in CP (open circles, panel A) or IP (pink dots, panel B) and numbers of gel slices they were detected were plotted. The number of proteins within each group is marked above the data series.
4. Conclusions
In this study, we have categorized 1,756 proteins in hemolymph of A. gambiae larvae, which represents a major increase in the hemolymph proteome coverage. In spite of the contaminating intracellular and gut enzymes, identification of bona fide plasma proteins provides an overview of the physiological functions of the mosquito larval hemolymph. Future studies of adult hemolymph samples should yield insights into composition changes in plasma proteins, which may directly interact with malaria parasites. There were no global differences between the control and induced samples and, instead of detecting increases in defense proteins, we observed a few more cases of relative decrease, possibly as a result of the imbalance between higher protein consumption and higher protein synthesis after bacterial infection than after sterile wounding. The active infection after pricking with E. coli, similar survival rates between pricking and injection, and transcription up-regulation of the AMP genes supported that the observed proteomic changes were not artifacts. The comparison of theoretical and observed Mr’s allowed us to detect possible posttranslational modifications of proteins (e.g. proteolysis and serpin-protease complex formation), occurring in the control and induced samples. Assembling and crosslinking of macromolecular complexes appears to be a common feature of innate immunity, now supported at the level of proteome in an important vector of human malaria.
Supplementary Material
Table S1. The 1,756 proteins identified in the larval hemolymph samples of A. gambiae
Identify 1,756 proteins in the larval hemolymph of Anopheles gambiae
Indicate storage, transport, and immunity as major functions of the hemolymph
Detect 233 proteins for immune recognition, signaling, regulation, and execution
Suggest immune complex formation and other dynamic changes during infection
Acknowledgments
This work was supported by National Institutes of Health Grants AI112662 and GM58634 (to HJ). We would like to express our gratitude to Dr. Li Ma at National Institute for Microbial Forensics & Food and Agricultural Biosecurity, who kindly provided the GFP expressing plasmid. Mass spectrometry analyses were performed in the DNA/Protein Resource Facility at Oklahoma State University, wherein the OrbitrapXL instrument was supported by the NSF MRI and EPSCoR programs (award DBI/0722494). This article was approved for publication by the Director of the Oklahoma Agricultural Experiment Station and supported in part under project OKL02450.
Abbreviations
- CP
control plasma or cell-free hemolymph from sterilely pricked larvae
- IP
induced plasma from E. coli pricked larvae
- AMP
antimicrobial protein
- APL1C
Anopheles plasmodium-responsive LRR protein-1C
- CTL
C-type lectin
- FBN
fibrinogen
- GFP
green fluorescent protein
- GNBP
Gram-negative bacteria-binding protein
- IAP
inhibitor of apoptosis
- iBAQ
intensity-based absolute quantification
- LDLp
low density lipophorin
- LFQ
label-free quantification intensity
- LRIM1
Leu-rich immune molecule-1
- LRR
leucine-rich repeat
- MDL
myeloid differentiation factor-2 (MD2) like protein
- MS
mass spectrometry
- PGRP
peptidoglycan recognition protein
- PRR
pattern recognition receptor
- PO and proPO
phenoloxidase and its precursor
- SP and SPH
serine protease and its non-catalytic homolog
- CLIP
clip-domain SP or SPH, SRPN, serpin
- TEP
thioester protein or its homolog
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham EG, Pinto SB, Ghosh A, Vanlandingham DL, Budd A, Higgs S, Kafatos FC, Jacobs-Lorena M, Michel K. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proc Natl Acad Sci USA. 2005;102:16327–16332. doi: 10.1073/pnas.0508335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar R, Jedlicka AE, Mintz M, Mahairaki V, Scott AL, Dimopoulos G. Global gene expression analysis of Anopheles gambiae responses to microbial challenge. Insect Biochem Mol Biol. 2005;35:709–719. doi: 10.1016/j.ibmb.2005.02.019. [DOI] [PubMed] [Google Scholar]
- Ahrné E, Molzahn L, Glatter T, Schmidt A. Critical assessment of proteome-wide label-free absolute abundance estimation strategies. Proteomics. 2013;13:2567–2578. doi: 10.1002/pmic.201300135. [DOI] [PubMed] [Google Scholar]
- Amenya DA, Chou W, Li J, Yan G, Gershon PD, James AA, Marinotti O. Proteomics reveals novel components of the Anopheles gambiae eggshell. J Insect Physiol. 2010;56:1414–1419. doi: 10.1016/j.jinsphys.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, Budd A, Kanost MR, Michel K. Characterization of a regulatory unit that controls melanization and affects longevity of mosquitoes. Cell Mol Life Sci. 2011;68:1929–1939. doi: 10.1007/s00018-010-0543-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asara JM, Christofk HR, Freimark LM, Cantley LC. A label-free quantification method by MS/MS TIC compared to SILAC and spectral counting in a proteomics screen. Proteomics. 2008;8:994–999. doi: 10.1002/pmic.200700426. [DOI] [PubMed] [Google Scholar]
- Barillas-Mury C. CLIP proteases and Plasmodium melanization in Anopheles gambiae. Trends Parasitol. 2007;23:297–299. doi: 10.1016/j.pt.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Benedict MQ. Care and maintenance of Anopheline mosquito colonies. In: Crampton JM, Beard CB, Louis C, editors. Molecular Biology of Insect Disease Vectors: A Methods Manual. Chapman & Hall; New York: 1997. pp. 3–12. [Google Scholar]
- Blandin S, Moita LF, Kocher T, Wilm M, Kafatos FC, Levashina EA. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Cao X, He Y, Hu Y, Zhang X, Wang Y, Zou Z, Chen Y, Blissard GW, Kanost MR, Jiang H. Sequence conservation, phylogenetic relationships, and expression profiles of nondigestive serine proteases and serine protease homologs in Manduca sexta. Insect Biochem Mol Biol. 2015;62:51–63. doi: 10.1016/j.ibmb.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christophides GK, Zdobnov E, Barillas-Mury C, Birney E, Blandin S, et al. Immunity-related genes and gene families in Anopheles gambiae. Science. 2002;298:159–165. doi: 10.1126/science.1077136. [DOI] [PubMed] [Google Scholar]
- Clark KD, Strand MR. Hemolymph melanization in the silkmoth Bombyx mori involves formation of a high molecular mass complex that metabolizes tyrosine. J Biol Chem. 2013;288:14476–14487. doi: 10.1074/jbc.M113.459222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton AM, Dong Y, Dimopoulos G. The Anopheles innate immune system in the defense against malaria infection. J Innate Immun. 2014;6:169–181. doi: 10.1159/000353602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggins SA, Estévez-Lao TY, Hillyer JF. Increased survivorship following bacterial infection by the mosquito Aedes aegypti as compared to Anopheles gambiae correlates with increased transcriptional induction of antimicrobial peptides. Dev Comp Immunol. 2012;37:390–401. doi: 10.1016/j.dci.2012.01.005. [DOI] [PubMed] [Google Scholar]
- Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J, Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- Danielli A, Barillas-Mury C, Kumar S, Kafatos FC, Loukeris TG. Overexpression and altered nucleocytoplasmic distribution of Anopheles ovalbumin-like SRPN10 serpins in Plasmodium-infected midgut cells. Cell Microbiol. 2005;7:181–190. doi: 10.1111/j.1462-5822.2004.00445.x. [DOI] [PubMed] [Google Scholar]
- Dinglasan RR, Devenport M, Florens L, Johnson JR, McHugh CA, Donnelly-Doman M, Carucci DJ, Yates JR, 3rd, Jacobs-Lorena M. The Anopheles gambiae adult midgut peritrophic matrix proteome. Insect Biochem Mol Biol. 2009;39:125–134. doi: 10.1016/j.ibmb.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Dimopoulos G. Anopheles fibrinogen-related proteins provide expanded pattern recognition capacity against bacteria and malaria parasites. J Biol Chem. 2009;284:9835–9844. doi: 10.1074/jbc.M807084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischetti IM, Valenzuela JG, Pham VM, Garfield MK, Ribeiro JM. Toward a catalog for the transcripts and proteins (sialome) from the salivary gland of the malaria vector Anopheles gambiae. J Exp Biol. 2002;205:2429–2451. doi: 10.1242/jeb.205.16.2429. [DOI] [PubMed] [Google Scholar]
- He Y, Cao X, Zhang S, Rogers J, Hartson S, Jiang H. Changes in the plasma proteome of Manduca sexta larvae in relation to the transcriptome variations after an immune challenge: evidence for high molecular weight immune complex formation. Mol Cell Proteomics. 2016;15:1176–1187. doi: 10.1074/mcp.M115.054296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RA, Subramanian GM, Halpern A, Sutton GG, Charlab R, et al. The genome sequence of the malaria mosquito Anopheles gambiae. Science. 2002;298:129–149. doi: 10.1126/science.1076181. [DOI] [PubMed] [Google Scholar]
- Jiang H, Vilcinskas A, Kanost MR. Immunity in lepidopteran insects. Adv Exp Med Biol. 2010;708:181–204. doi: 10.1007/978-1-4419-8059-5_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalume DE, Okulate M, Zhong J, Reddy R, Suresh S, Deshpande N, Kumar N, Pandey A. A proteomic analysis of salivary glands of female Anopheles gambiae mosquito. Proteomics. 2005;5:3765–3777. doi: 10.1002/pmic.200401210. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Gorman MJ. Phenoloxidases in insect immunity. In: Beckage NE, editor. Insect Immunology. Elsevier; San Diego: 2008. pp. 69–96. [Google Scholar]
- Kanost MR, Jiang H. Clip-domain serine proteases as immune factors in insect hemolymph. Curr Opin Insect Sci. 2015;11:47–55. doi: 10.1016/j.cois.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C, Korayem AM, Scherfer C, Loseva O, Dushay MS, Theopold U. Proteomic analysis of the Drosophila larval hemolymph clot. J Biol Chem. 2004;279:52033–52041. doi: 10.1074/jbc.M408220200. [DOI] [PubMed] [Google Scholar]
- Larade K, Storey KB. Accumulation and translation of ferritin heavy chain transcripts following anoxia exposure in a marine invertebrate. J Exp Biol. 2004;207:1353–1360. doi: 10.1242/jeb.00872. [DOI] [PubMed] [Google Scholar]
- Lefevre T, Thomas F, Schwartz A, Levashina E, Blandin S, Brizard JP, Le Bourligu L, Demettre E, Renaud F, Biron DG. Malaria Plasmodium agent induces alteration in the head proteome of their Anopheles mosquito host. Proteomics. 2007;7:1908–1915. doi: 10.1002/pmic.200601021. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Moita LF, Blandin S, Vriend G, Lagueux M, Kafatos FC. Conserved role of a complement-like protein in phagocytosis revealed by dsRNA knockout in cultured cells of the mosquito, Anopheles gambiae. Cell. 2001;104:709–718. doi: 10.1016/s0092-8674(01)00267-7. [DOI] [PubMed] [Google Scholar]
- Li J, Wang X, Zhang G, Githure JI, Yan G, James AA. Genome-block expression-assisted association studies discover malaria resistance genes in Anopheles gambiae. Proc Natl Acad Sci USA. 2013;110:20675–20680. doi: 10.1073/pnas.1321024110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Xia X, Yu L, Vasseur L, Gurr GM, Yao F, Yang G, You M. Genome-wide identification and expression profiling of serine proteases and homologs in the diamondback moth, Plutella xylostella (L.) BMC Genomics. 2015;16:1054. doi: 10.1186/s12864-015-2243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K, Suwanchaichinda C, Morlais I, Lambrechts L, Cohuet A, Awono-Ambene PH, Simard F, Fontenille D, Kanost MR, Kafatos FC. Increased melanizing activity in Anopheles gambiae does not affect development of Plasmodium falciparum. Proc Natl Acad Sci USA. 2006;103:6858–6863. doi: 10.1073/pnas.0608033103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel W. Pathways of unconventional protein secretion. Curr Opin Biotechnol. 2010;21:621–626. doi: 10.1016/j.copbio.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Ong DS, Wang L, Zhu Y, Ho B, Ding JL. The response of ferritin to LPS and acute phase of Pseudomonas infection. J Endotoxin Res. 2005;11:267–280. doi: 10.1179/096805105X58698. [DOI] [PubMed] [Google Scholar]
- Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- Park JW, Kim CH, Rui J, Park KH, Ryu KH, Chai JH, Hwang HO, Kurokawa K, Ha NC, Söderhäll I, Söderhäll K, Lee BL. Beetle immunity. Adv Exp Med Biol. 2010;708:163–180. doi: 10.1007/978-1-4419-8059-5_9. [DOI] [PubMed] [Google Scholar]
- Paskewitz SM, Shi L. The hemolymph proteome of Anopheles gambiae. Insect Biochem Mol Biol. 2005;35:815–824. doi: 10.1016/j.ibmb.2005.03.002. [DOI] [PubMed] [Google Scholar]
- Pesch YY, Riedel D, Patil KR, Loch G, Behr M. Chitinases and imaginal disc growth factors organize the extracellular matrix formation at barrier tissues in insects. Sci Rep. 2016;6:18340. doi: 10.1038/srep18340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. SignalP 4.0: discriminating signal peptides from transmembrane regions. Nat Methods. 2011;8:785–786. doi: 10.1038/nmeth.1701. [DOI] [PubMed] [Google Scholar]
- Povelones M, Bhagavatula L, Yassine H, Tan LA, Upton LM, Osta MA, Christophides GK. The CLIP-domain serine protease homolog SPCLIP1 regulates complement recruitment to microbial surfaces in the malaria mosquito Anopheles gambiae. PLoS Pathog. 2013;9:e1003623. doi: 10.1371/journal.ppat.1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc Natl Acad Sci USA. 2015;112:1273–1280. doi: 10.1073/pnas.1423586112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Powers SJ. Real-time quantitative RT-PCR: design, calculations, and statistics. Plant Cell. 2009;21:1031–1033. doi: 10.1105/tpc.109.066001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HB, Chou KC. Signal-3L: A 3-layer approach for predicting signal peptides. Biochem Biophys Res Commun. 2007;363:297–303. doi: 10.1016/j.bbrc.2007.08.140. [DOI] [PubMed] [Google Scholar]
- Suwanchaichinda C, Kanost MR. The serpin gene family in Anopheles gambiae. Gene. 2009;442:47–54. doi: 10.1016/j.gene.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volz J, Müller HM, Zdanowicz A, Kafatos FC, Osta MA. A genetic module regulates the melanization response of Anopheles to Plasmodium. Cell Microbiol. 2006;8:1392–1405. doi: 10.1111/j.1462-5822.2006.00718.x. [DOI] [PubMed] [Google Scholar]
- Volz J, Osta MA, Kafatos FC, Müller HM. The roles of two clip domain serine proteases in innate immune responses of the malaria vector Anopheles gambiae. J Biol Chem. 2005;280:40161–40168. doi: 10.1074/jbc.M506191200. [DOI] [PubMed] [Google Scholar]
- WHO. World Malaria Report 2015. 2015. [Google Scholar]
- Yang F, Wang Y, He Y, Jiang H. In search of a function of Manduca sexta hemolymph protease–1 in the innate immune system. Insect Biochem Mol Biol. 2016;76:1–10. doi: 10.1016/j.ibmb.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassine H, Osta MA. Anopheles gambiae innate immunity. Cell Microbiol. 2010;12:1–9. doi: 10.1111/j.1462-5822.2009.01388.x. [DOI] [PubMed] [Google Scholar]
- Yu XQ, Jiang H, Wang Y, Kanost MR. Nonproteolytic serine proteinase homologs are involved in prophenoloxidase activation in the tobacco hornworm, Manduca sexta. Insect Biochem Mol Biol. 2003;33:197–208. doi: 10.1016/s0965-1748(02)00191-1. [DOI] [PubMed] [Google Scholar]
- Zhao P, Wang GH, Dong ZM, Duan J, Xu PZ, Cheng TC, Xiang ZH, Xia QY. Genome-wide identification and expression analysis of serine proteases and homologs in the silkworm Bombyx mori. BMC Genomics. 2010;11:405. doi: 10.1186/1471-2164-11-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Z, Jiang H. Manduca sexta serpin-6 regulates immune serine proteinases PAP-3 and HP8: cDNA cloning, protein expression, inhibition kinetics, and function elucidation. J Biol Chem. 2005;280:14341–14348. doi: 10.1074/jbc.M500570200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The 1,756 proteins identified in the larval hemolymph samples of A. gambiae