Abstract
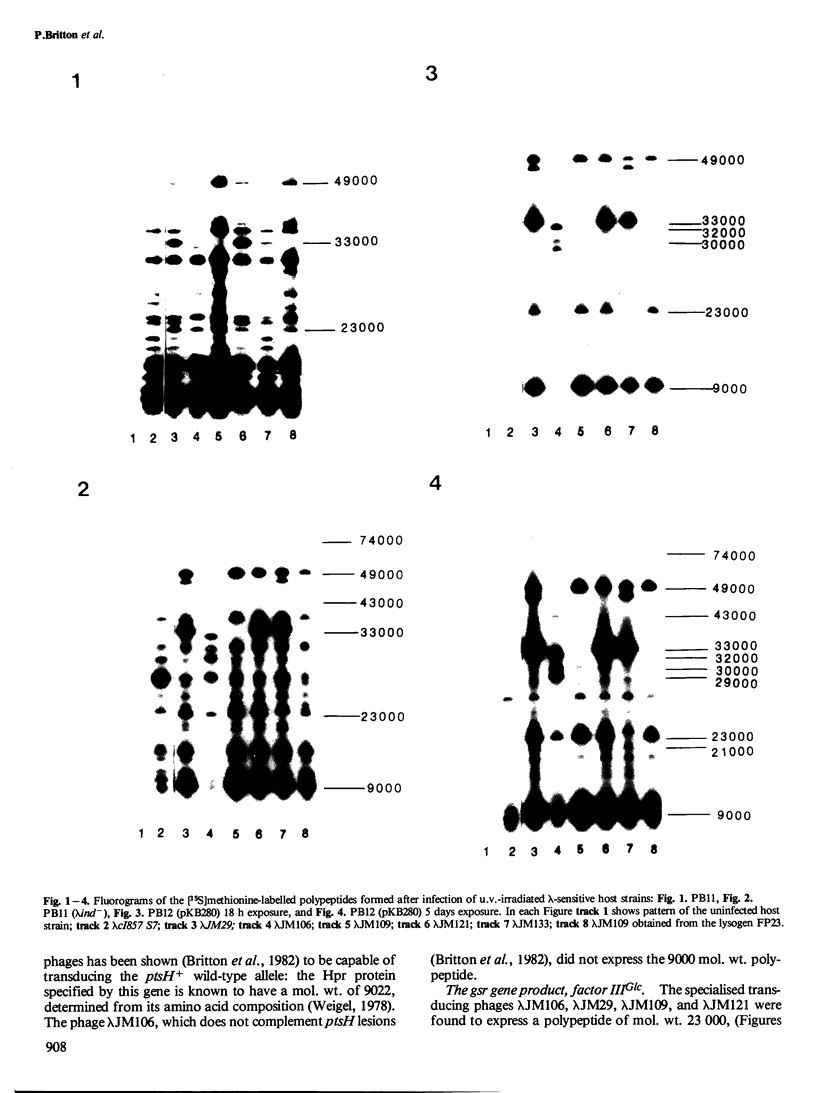
The expression of genes adjacent to ptsI was investigated using a series of specialised transducing phages carrying different, overlapping, segments of the cysA-gsr-ptsI-ptsH- iex - cysZ -lig region of the genome of Escherichia coli. The polypeptides were synthesised following the infection of u.v.-irradiated lysogenic and non-lysogenic uvrA recA hosts or a uvrA recA host carrying the lambda cI+ plasmid pKB280 . The polypeptides were identified by SDS-polyacrylamide gel electrophoresis and fluorography. The gsr gene product had a mol. wt. of 23 000. The product of the iex gene was tentatively identified as a protein of mol. wt. of either 33 000 or 21 000. Hpr, the product of the gene ptsH, had a mol. wt. of 9000. The gsr gene appeared to be expressed at a higher level in a non-immune host, which suggests that it was transcribed from lambda promoters. A new lambda host strain, suitable for the detection of small polypeptides (mol. wt. less than 30 000) is described.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Backman K., Ptashne M. Maximizing gene expression on a plasmid using recombination in vitro. Cell. 1978 Jan;13(1):65–71. doi: 10.1016/0092-8674(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Becker M. A., Kredich N. M., Tomkins G. M. The purification and characterization of O-acetylserine sulfhydrylase-A from Salmonella typhimurium. J Biol Chem. 1969 May 10;244(9):2418–2427. [PubMed] [Google Scholar]
- Buxton R. S., Hammer-Jespersen K., Valentin-Hansen P. A second purine nucleoside phosphorylase in Escherichia coli K-12. I. Xanthosine phosphorylase regulatory mutants isolated as secondary-site revertants of a deoD mutant. Mol Gen Genet. 1980;179(2):331–340. doi: 10.1007/BF00425461. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Douglas M. G., Butow R. A. Variant forms of mitochondrial translation products in yeast: evidence for location of determinants on mitochondrial DNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1083–1086. doi: 10.1073/pnas.73.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fimmel A. L., Loughlin R. E. Isolation and characterization of cysK mutants of Escherichia coli K12. J Gen Microbiol. 1977 Nov;103(1):37–43. doi: 10.1099/00221287-103-1-37. [DOI] [PubMed] [Google Scholar]
- Gershanovitch V. N., Ilyina T. S., Rusina O. Y., Yourovitskaya N. V., Bolshakova T. N. Repression of inducible enzyme synthesis in a mutant of Escherichia coli K 12 deleted for the ptsH gene. Mol Gen Genet. 1977 Jun 8;153(2):185–190. doi: 10.1007/BF00264734. [DOI] [PubMed] [Google Scholar]
- Hammer-Jespersen K., Buxton R. S., Hansen T. D. A second purine nucleoside phosphorylase in Escherichia coli K-12. II. Properties of xanthosine phosphorylase and its induction by xanthosine. Mol Gen Genet. 1980;179(2):341–348. doi: 10.1007/BF00425462. [DOI] [PubMed] [Google Scholar]
- Hulanicka D., Klopotowski T. Mutants of Salmonella typhimurium resistant to triazole. Acta Biochim Pol. 1972;19(3):251–260. [PubMed] [Google Scholar]
- Hulanicka M. D., Kredich N. M., Treiman D. M. The structural gene for O-acetylserine sulfhydrylase A in Salmonella typhimurium. Identity with the trzA locus. J Biol Chem. 1974 Feb 10;249(3):867–872. [PubMed] [Google Scholar]
- Jones-Mortimer M. C., Kornberg H. L. Genetic control of inducer exclusion by Escherichia coli. FEBS Lett. 1974 Nov 1;48(1):93–95. doi: 10.1016/0014-5793(74)81070-7. [DOI] [PubMed] [Google Scholar]
- KUNDIG W., GHOSH S., ROSEMAN S. PHOSPHATE BOUND TO HISTIDINE IN A PROTEIN AS AN INTERMEDIATE IN A NOVEL PHOSPHO-TRANSFERASE SYSTEM. Proc Natl Acad Sci U S A. 1964 Oct;52:1067–1074. doi: 10.1073/pnas.52.4.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Mizobuchi K, Demerec M, Gillespie D H. Cysteine Mutants of Salmonella Typhimurium. Genetics. 1962 Nov;47(11):1617–1627. doi: 10.1093/genetics/47.11.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modrich P., Anraku Y., Lehman I. R. Deoxyribonucleic acid ligase. Isolation and physical characterization of the homogeneous enzyme from Escherichia coli. J Biol Chem. 1973 Nov 10;248(21):7495–7501. [PubMed] [Google Scholar]
- Neville D. M., Jr Molecular weight determination of protein-dodecyl sulfate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971 Oct 25;246(20):6328–6334. [PubMed] [Google Scholar]
- Reeve J. N. Selective expression of transduced or cloned DNA in minicells containing plasmid pKB280. Nature. 1978 Dec 14;276(5689):728–729. doi: 10.1038/276728a0. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- Saier M. H., Roseman S. Inducer exclusion and repression of enzyme synthesis in mutants of Salmonella typhimurium defective in enzyme I of the phosphoenolpyruvate: sugar phosphotransferase system. J Biol Chem. 1972 Feb 10;247(3):972–975. [PubMed] [Google Scholar]
- Scholte B. J., Schuitema A. R., Postma P. W. Isolation of IIIGlc of the phosphoenolpyruvate-dependent glucose phosphotransferase system of Salmonella typhimurium. J Bacteriol. 1981 Oct;148(1):257–264. doi: 10.1128/jb.148.1.257-264.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate Accumulation and Metabolism in Escherichia coli: Characteristics of the Reversions of ctr Mutations. J Bacteriol. 1970 Dec;104(3):1318–1324. doi: 10.1128/jb.104.3.1318-1324.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiater A., Hulanicka D. Properties of cysK mutants of Escherichia coli K12. Acta Biochim Pol. 1979;26(1-2):21–28. [PubMed] [Google Scholar]
- Worcel A., Burgi E. Properties of a membrane-attached form of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):91–105. doi: 10.1016/0022-2836(74)90576-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]