Abstract
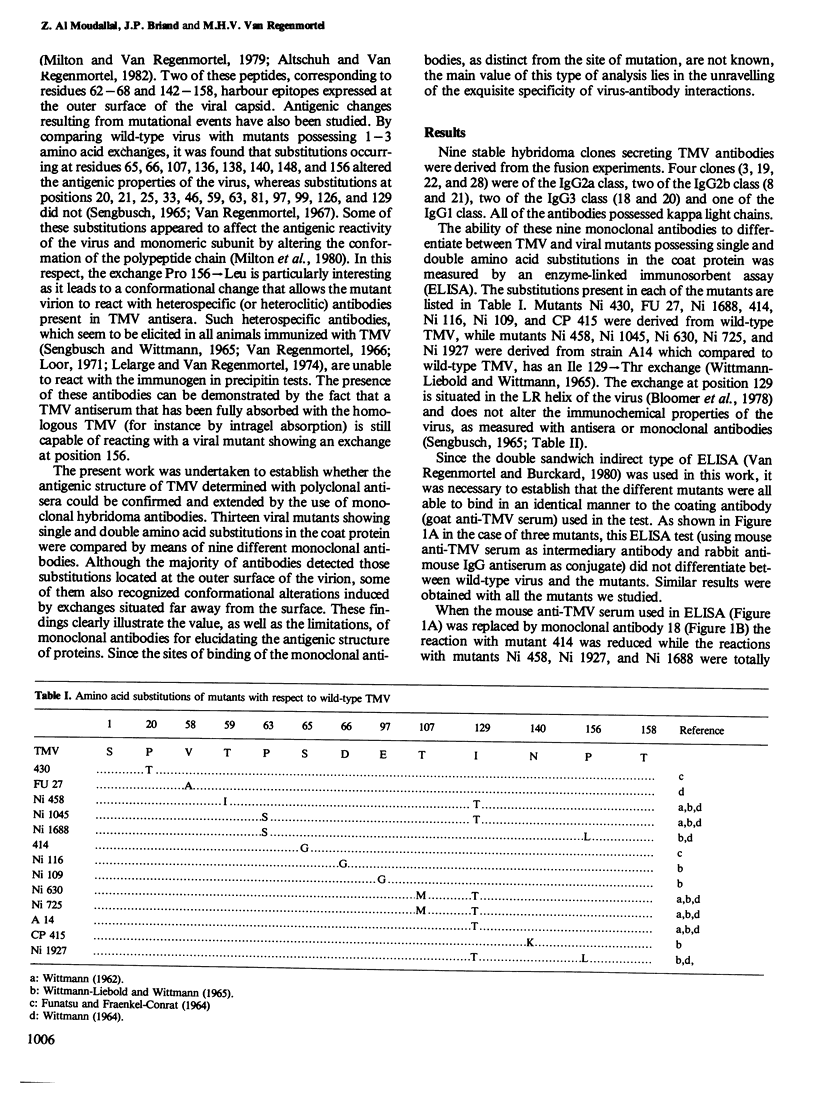
The antigenic structure of tobacco mosaic virus has been analysed by measuring the ability of nine monoclonal antibodies to distinguish between wild-type virus and 13 mutants showing single and double amino acid substitutions in the coat protein. Although the majority of antibodies detected those substitutions that were located at the outer surface of the virion, some of them also recognized conformation alterations induced by exchanges occurring deep inside the subunit. In the case of five mutants, the antibody reactivity was reduced compared with wild-type virus, while in the case of three others, it was significantly higher. Each monoclonal antibody possessed a unique discrimination pattern with respect to the different substitutions. The simultaneous presence of two exchanges led to the complete disappearance of any binding with six of the nine antibodies and to reduced binding with three others. The superior discriminatory capacity of monoclonal antibodies compared with polyclonal antisera was demonstrated by the fact that three exchanges not detected with antisera were found to alter the antigenicity when tested with monoclonal antibodies.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERER F. A., SCHLUMBERGER H. D. PROPERTIES OF DIFFERENT ARTIFICIAL ANTIGENS IMMUNOLOGICALLY RELATED TO TOBACCO MOSAIC VIRUS. Biochim Biophys Acta. 1965 Mar 8;97:503–509. doi: 10.1016/0304-4165(65)90163-7. [DOI] [PubMed] [Google Scholar]
- Altschuh D., Van Regenmortel M. H. Localization of antigenic determinants of a viral protein by inhibition of enzyme-linked immunosorbent assay (ELISA) with tryptic peptides. J Immunol Methods. 1982;50(1):99–108. doi: 10.1016/0022-1759(82)90307-6. [DOI] [PubMed] [Google Scholar]
- Anderer F. A., Ströbel G. Recognition of conjugated and native peptide determinants. I. Conformational and sequential specificities of rabbit antibodies versus tobacco mosaic virus. Eur J Immunol. 1972 Jun;2(3):274–277. doi: 10.1002/eji.1830020316. [DOI] [PubMed] [Google Scholar]
- Atassi M. Z. Determination of the entire antigenic structure of native lysozyme by surface-simulation synthesis, a novel concept in molecular recognition. CRC Crit Rev Biochem. 1979;6(4):371–400. doi: 10.3109/10409237909105426. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Buckenmeyer G. K., Hicks G., Gurd F. R., Feldmann R. J., Minna J. Topographic antigenic determinants recognized by monoclonal antibodies to sperm whale myoglobin. J Biol Chem. 1982 Mar 25;257(6):3189–3198. [PubMed] [Google Scholar]
- Berzofsky J. A., Hicks G., Fedorko J., Minna J. Properties of monoclonal antibodies specific for determinants of a protein antigen, myoglobin. J Biol Chem. 1980 Dec 10;255(23):11188–11191. [PubMed] [Google Scholar]
- Cameron D. J., Erlanger B. F. Evidence for multispecificity of antibody molecules. Nature. 1977 Aug 25;268(5622):763–765. doi: 10.1038/268763a0. [DOI] [PubMed] [Google Scholar]
- De L Milton R. C., van Regenmortel M. H. Immunochemical studies of tobacco mosaic virus--III. Demonstration of five antigenic regions in the protein sub-unit. Mol Immunol. 1979 Mar;16(3):179–184. doi: 10.1016/0161-5890(79)90143-3. [DOI] [PubMed] [Google Scholar]
- East I. J., Hurrell J. G., Todd P. E., Leach S. J. Antigenic specificity of monoclonal antibodies to human myoglobin. J Biol Chem. 1982 Mar 25;257(6):3199–3202. [PubMed] [Google Scholar]
- East I. J., Todd P. E., Leach S. J. On topographic antigenic determinants in myoglobins. Mol Immunol. 1980 Apr;17(4):519–525. doi: 10.1016/0161-5890(80)90091-7. [DOI] [PubMed] [Google Scholar]
- FUNATSU G., FRAENKEL-CONRAT H. LOCATION OF AMINO ACID EXCHANGES IN CHEMICALLY EVOKED MUTANTS OF TOBACCO MOSAIC VIRUS. Biochemistry. 1964 Sep;3:1356–1362. doi: 10.1021/bi00897a028. [DOI] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Gerhard W., Yewdell J., Frankel M. E., Webster R. Antigenic structure of influenza virus haemagglutinin defined by hybridoma antibodies. Nature. 1981 Apr 23;290(5808):713–717. doi: 10.1038/290713a0. [DOI] [PubMed] [Google Scholar]
- Hurrell J. G., Smith J. A., Todd P. E., Leach S. J. Cross-reactivity between mammalian myoglobins: linear vs spatial antigenic determinants. Immunochemistry. 1977 Apr;14(4):283–288. doi: 10.1016/0019-2791(77)90251-8. [DOI] [PubMed] [Google Scholar]
- Ibrahimi I. M., Eder J., Prager E. M., Wilson A. C., Arnon R. The effect of a single amino acid substitution on the antigenic specificity of the loop region of lysozyme. Mol Immunol. 1980 Jan;17(1):37–46. doi: 10.1016/0161-5890(80)90122-4. [DOI] [PubMed] [Google Scholar]
- Ibrahimi I. M., Prager E. M., White T. J., Wilson A. C. Amino acid sequence of California quail lysozyme. Effect of evolutionary substitutions on the antigenic structure of lysozyme. Biochemistry. 1979 Jun 26;18(13):2736–2744. doi: 10.1021/bi00580a008. [DOI] [PubMed] [Google Scholar]
- JERNE N. K. Immunological speculations. Annu Rev Microbiol. 1960;14:341–358. doi: 10.1146/annurev.mi.14.100160.002013. [DOI] [PubMed] [Google Scholar]
- Kabat E. A. Basic principles of Antigen-Antibody Reactions. Methods Enzymol. 1980;70(A):3–49. doi: 10.1016/s0076-6879(80)70040-x. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Fujio H., Kondo K., Dohi Y., Hirayama A., Takagaki Y., Kosaki G., Amano T. A monoclonal antibody specific for a distinct region of hen egg-white lysozyme. Mol Immunol. 1982 Apr;19(4):619–630. doi: 10.1016/0161-5890(82)90231-0. [DOI] [PubMed] [Google Scholar]
- Lane D., Koprowski H. Molecular recognition and the future of monoclonal antibodies. Nature. 1982 Mar 18;296(5854):200–202. doi: 10.1038/296200a0. [DOI] [PubMed] [Google Scholar]
- Loor F. On the existence of heterospecific antibodies in sera from rabbits immunized against tobacco mosaic virus determinants. Immunology. 1971 Oct;21(4):557–564. [PMC free article] [PubMed] [Google Scholar]
- Lubeck M., Gerhard W. Conformational changes at topologically distinct antigenic sites on the influenza A/PR/8/34 virus HA molecule are induced by the binding of monoclonal antibodies. Virology. 1982 Apr 15;118(1):1–7. doi: 10.1016/0042-6822(82)90313-0. [DOI] [PubMed] [Google Scholar]
- Metzger D. W., Miller A., Sercarz E. E. Sharing of an idiotypic marker by monoclonal antibodies specific for distinct regions of hen lysozyme. Nature. 1980 Oct 9;287(5782):540–542. doi: 10.1038/287540a0. [DOI] [PubMed] [Google Scholar]
- Milton R. C., Milton S. C., von Wechmar M. B., van Regenmortel M. H. Immunochemical studies of tobacco mosaic virus--IV. Influence of single amino acid exchanges on the antigenic activity of mutant coat proteins and peptides. Mol Immunol. 1980 Oct;17(10):1205–1212. doi: 10.1016/0161-5890(80)90016-4. [DOI] [PubMed] [Google Scholar]
- Richards F. F., Konigsberg W. H., Rosenstein R. W., Varga J. M. On the specificity of antibodies. Science. 1975 Jan 17;187(4172):130–137. doi: 10.1126/science.46122. [DOI] [PubMed] [Google Scholar]
- Richards F. F., Konigsberg W. H. Speculations. How specific are antibodies? Immunochemistry. 1973 Aug;10(8):545–553. doi: 10.1016/0019-2791(73)90227-9. [DOI] [PubMed] [Google Scholar]
- Smith-Gill S. J., Wilson A. C., Potter M., Prager E. M., Feldmann R. J., Mainhart C. R. Mapping the antigenic epitope for a monoclonal antibody against lysozyme. J Immunol. 1982 Jan;128(1):314–322. [PubMed] [Google Scholar]
- TALMAGE D. W. Immunological specificity, unique combinations of selected natural globulins provide an alternative to the classical concept. Science. 1959 Jun 19;129(3364):1643–1648. doi: 10.1126/science.129.3364.1643. [DOI] [PubMed] [Google Scholar]
- Van Regenmortel M. H. Plant virus serology. Adv Virus Res. 1966;12:207–271. doi: 10.1016/s0065-3527(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Van Regenmortel M. H. Serological studies on naturally occurring strains and chemically induced mutants of tobacco mosaic virus. Virology. 1967 Mar;31(3):467–480. doi: 10.1016/0042-6822(67)90228-0. [DOI] [PubMed] [Google Scholar]
- WITTMANN H. G. PROTEINANALYSEN VON CHEMISCH INDUZIERTEN MUTANTEN DES TABAKMOSAIKVIRUS. Z Vererbungsl. 1964 Dec 30;95:333–344. [PubMed] [Google Scholar]
- White T. J., Ibrahimi I. M., Wilson A. C. Evolutionary substitutions and the antigenic structure of globular proteins. Nature. 1978 Jul 6;274(5666):92–94. doi: 10.1038/274092a0. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Wilson I. A., Skehel J. J. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981 Jan 29;289(5796):373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- Wittmann-Liebold B., Wittmann H. G. Lokalisierung von Aminosäureaustauschen bei Nitritmutanten des Tabakmosaikvirus. Z Vererbungsl. 1965;97(3):305–326. [PubMed] [Google Scholar]
- von Sengbusch P. Aminosäureaustausche und Tertiärstruktur eines Proteins. Vergleich von Mutanten des Tabakmosaikvirus mit serologischen und physikochemischen Methoden. Z Vererbungsl. 1965;96(4):364–386. [PubMed] [Google Scholar]


