Abstract
Calcium plays an important role in regulating hundreds of biological processes due to its primary role as one of the most ubiquitous second messengers. As a result, the levels of calcium are tightly regulated as are the peak and trough calcium concentrations during a calcium signal. Calcium levels are controlled via a variety of feedback mechanisms and exchangers/transporters. Here the role of calcium in the feedback regulation of ion channel function is reviewed, with an emphasis on the molecular mechanisms governing calcium-dependent function. In particular, the role of calcium in the regulation of voltage-gated sodium, calcium, and potassium channels are reviewed as well as its effects on the ryanodine receptor.
Keywords: Calcium, calmodulin, EF-hand, ion channels, regulation
INTRODUCTION
Calcium takes part in hundreds of biological processes in its role as one of the most ubiquitous second messengers.1 Intracellular calcium concentrations are tightly regulated to a very low level of approximately ~100 nM;2 the general mechanism by which calcium alters cellular biology involves a transient spike in calcium concentration, reaching over 10 μM and possibly much higher in certain local environments.3 This represents a 100-fold change in calcium concentration. As a rough approximation, this means that a calcium binding protein at 1 μM concentation with an affinity (Kd) of 1 μM will go from less than 10% saturated to over 85% saturated due to this change in calcium concentration. Note that these numbers are highly dependent on the concentration of the calcium-binding protein in question as well as the concentration of other cellular targets and proteins competing for calcium.
The EF-hand is the most common signal transduction element for translating this abrupt rise in calcium concentration into a biochemical signal. Over 1000 have been identified in animal genomes from their unique sequence signatures.4 Structurally, the EF-hand is a helix-loop-helix motif first identified in parvalbumin.5 The “EF” refers to the two helices in the motif. It is called a hand because, if thumb and index finger are used to form an L shape and the other three fingers curled inward so the fingertips touch the palm, the two extended fingers approximately represent the helices of the motif and the curled fingers together with the palm approximately represent the calcium binding loop. The angle between thumb and index finger, representing the interhelical angle, can be variable; calcium binding can cause changes in this interhelical angle, resulting in widespread conformational changes which can then transduce the calcium signal into a biochemical event by altering the binding surface presented by the protein.
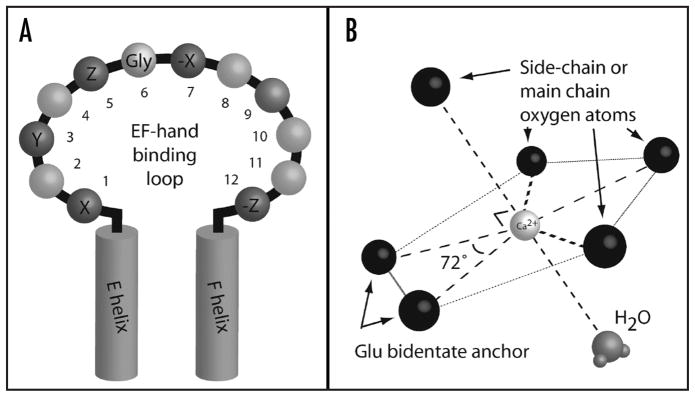
The consensus loop of the EF-hand is comprised of twelve residues (Fig. 1A) that surround the bound calcium (II) ion, coordinating the ion with oxygen atoms arranged in a pentagonal bipyramidal geometry,6,7 as shown in Figure 1B. The side chains of residues at positions 1, 3, and 5 each provide one oxygen ligand, thus Asn, Asp, Gln, or Glu are most frequently found at these positions. The main chain oxygen of residue 7 provides a fourth ligand. In order to attain appropriate positioning of residues 5 and 7, the residue in position 6 must occupy an unusual backbone conformation favorable only for glycine, resulting in strong conservation of Gly in this position. The Glu residue at position 12 is strictly conserved because it provides two coordination sites for a critical bidentate ligand. Residue 9 is also involved in coordinating the calcium ion by hydrogen bonding the water molecule that chelates the ion, completing the coordination sphere.
Figure 1.
EF-hand calcium binding loop coordinates the calcium (II) ion with pentagonal bipyramidal geometry. (A) Consensus EF-hand calcium binding loop sequence. Residues 1, 3, 5, 7 and 12, contribute oxygens for calcium coordination; see text for details. Position 6 Gly is strongly conserved for steric/conformational reasons. Position 9, participates in coordination by hydrogen bonding with a water molecule needed to complete the coordination sphere. (B) Pentagonal bipyramidal geometry of calcium coordination in an EF-hand. All of the peripheral large spheres represent oxygen atoms participating in coordination.
It is important to understand that calcium affinity is tuned not only by the calcium binding loop but also by many other elements of the EF-hand structural domain.8,9 This is observed, for example, in the difference between calbindin D9k and the ubiquitous calcium sensor calmodulin (CaM). Both contain EF-hands (one pair in calbindin, two pairs in calmodulin) with reasonable sequence homology, but the former has an affinity in the nM range, while the latter has calcium affinity in the μM range.
One part of the equation, then, is the transduction of a calcium signal into a biochemical signal. The other part of the equation, obviously, is the generation of a calcium signal. The mechanisms for regulating intracellular calcium concentrations in excitable cells involve, in part, feedback mechanisms where calcium acts directly or indirectly on ion channels. The purpose of this review is to briefly survey these ion channel feedback mechanisms in order to gain some appreciation of the variations and commonalities in calcium-dependent regulation of these channels. Other mechanisms by which calcium levels (both cellularly and systemically) are regulated, such as those involving ion exchangers or G-protein coupled receptors, are important but beyond the scope of this paper and have been reviewed elsewhere.10–17
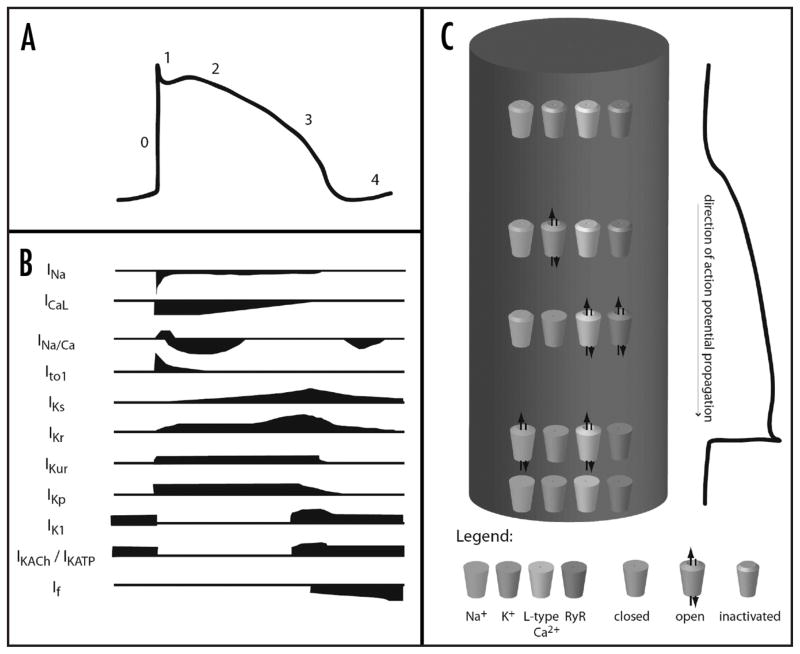
Calcium appears to regulate the function of nearly every channel involved in excitation. In brief, during an action potential, voltage-gated sodium channels open following an initial depolarization event, leading to rapid depolarization. These sodium channels are regulated in two ways by calcium. In cardiac and skeletal myocytes and in neural cells, dihydropyridine sensitive L-type calcium channels (LTCC) on the cell surface open concurrently, resulting in an inward rush of calcium ions. In mammalian ventricular myocytes, T-tubules transmit the depolarization to the interior of the cell, where more LTCC’s also open. This results in further calcium release into the cytoplasm.18 The LTCC’s interact with the ryanodine-sensitive calcium release channel (ryanodine receptors, RyR), which are found on the sarcoplasmic reticulum (SR). Activation of LTCC’s leads to activation of RyR’s, releasing calcium stored in the SR into the cytoplasm. Both of these calcium-conducting membrane proteins are tightly regulated by calcium, resulting variably in positive or negative feedback regulation of calcium release, depending on calcium concentration. In other cell types, the action potential activates P/Q-type calcium channels, resulting in other downstream events such as neurotransmitter release or enzyme secretion. These channels are also regulated by calcium. Throughout the action potential, but especially towards the middle and late stages, potassium channels open, resulting in repolarization of the cell membrane. Certain members of the potassium channel family are regulated by calcium. The contributions of various currents to the overall cardiac action potential are shown in Figure 2.19
Figure 2.
The contributions of various ionic currents to an action potential. (A) demonstrates the changes in transmembrane potential that occur during an action potential. (B) indicates the currents of sodium (INa), calcium (ICaL), and potassium (IK…) that occur during the course of the action potential. (C) diagrams in a very general way the channel openings, closings, and inactivations that occur during an action potential, where the cylinder represents a single myocyte. (A and B) adapted with permission from Tamargo19.
VOLTAGE-GATED SODIUM CHANNELS
Voltage-gated sodium channels are the essential element in action potential initiation and propagation in excitable tissue (e.g., muscle, nervous tissue). These channels consist of a large α subunit, constituting the pore of conduction, associated with smaller β subunits. These channels are mainly gated by alterations in membrane voltage, and it has only recently come to the fore that calcium is able to regulate voltage-gated sodium channels. In humans, there are nine α subunit isoforms, named NaV1.1 to NaV1.9. All possess the same structural organization: four homologous domains of six transmembrane segments each formed from a single polypeptide chain. This is the prototypic primary sequence of voltage-gated sodium and calcium channels.
A common calmodulin binding motif, known as the IQ motif, so named for the typical isoleucine-glutamine pair found in such motifs, is strongly conserved in the human sodium channel family,20 and a number of groups have published data on the role of calmodulin in the regulation of sodium channel function. The initial report related to the IQ motif was published by Tan and colleagues in 2002.21 The main data presented were electrophysiological results showing the effect of exogenous Ca2+-calmodulin added to the pipette solution on human cardiac voltage-gated sodium channel, enhancing its entry into a slow inactivated state (in the literature, this channel is referred to as NaV1.5, hH1 and SCN5A.). The study also presented data indicating that an IQ motif peptide is able to shift the migration of CaM produced by polyacrylamide gel electrophoresis in the presence of calcium, but not in the absence of calcium.
It is likely that calmodulin is actually able to bind the IQ motif in the absence of calcium even though this was not detected in the initial report. The IQ motif is commonly understood to bind calmodulin in a calcium-independent manner, and a number of follow up reports have established that the IQ motif does indeed bind to both apo and calcium-loaded calmodulin.22–24 Mori et al. reported lobe specific binding of calmodulin to the IQ motif, in both the presence and absence of calcium. Specifically, the group found that an IQ peptide alters the circular dichroism (CD) spectrum of only the C-lobe of calmodulin in the absence of calcium, but alters the CD spectrum of both lobes in the presence of calcium. Kim et al. found that a bacterially expressed fragment of the hH1 C-terminus, which included the IQ motif, eluted from a gel filtration column as a complex with calmodulin in a calcium-independent manner.23
However, it is important to note that other groups have been unable to recapitulate some of these results. Deschenes et al.25 were unable to detect a calmodulin-dependent effect on hH1, although it did have a significant effect on hSkM1, the skeletal muscle isoform of the channel. This group also reported that inhibition of CaM-dependent kinase II (CaMK) affected hH1 channel function slowing the current decay, the rate of entry into inactivation and shifting its voltage dependence. Interestingly, close examination of the data uncovers an effect not noticed by the authors—namely that calcium has a direct effect on channel function. A second group detected interactions between calmodulin and some but not all human sodium channel isoforms (NaV1.1, .2, .3, .4, .6 and .7).20 It has been speculated that the interaction with hH1 (NaV1.5) was missed due to lower affinity.26
It has also recently been discovered that an EF-hand calcium binding motif is present in hH1, the cardiac isoform of the voltage-gated sodium channel NaV1.5.27 This EF-hand is strongly conserved in the human sodium channel family. While this finding has been disputed,23 newer studies indicate that calcium does indeed bind to the hH1 C-terminus and plays an important role in concert with calmodulin to regulate channel function.24
This study extended an initial report, based on electrophysiological and spectroscopic data, that calcium regulates the cardiac voltage-gated sodium channel hH1 (NaV1.5). Combining all available evidence, the following model for the mechanism of calcium-dependent regulation of hH1 was proposed (Fig. 3). NMR and fluorescence titration data suggest that calmodulin is bound to the hH1 IQ motif via its C-lobe at basal calcium levels with nM affinity. Upon an increase in calcium levels, the binding of calcium to CaM causes the conformation of CaM to change, weakening and altering the CaM/IQ interaction, also demonstrated by NMR and fluorescence titration data. One or both lobes of calcium-loaded calmodulin are then more readily able to dissociate. The partial or complete dissociation of CaM frees the IQ motif to bind to the EF-hand domain, as NMR data shows will happen in the absence of calmodulin. This causes a concomitant calcium-dependent change in the conformation of the EF-hand domain, helping drive the equilibrium toward IQ interaction with the EF-hand domain and freeing up CaM. The EF-hand domain interacting with the IQ has μM calcium affinity and binds calcium.
Figure 3.
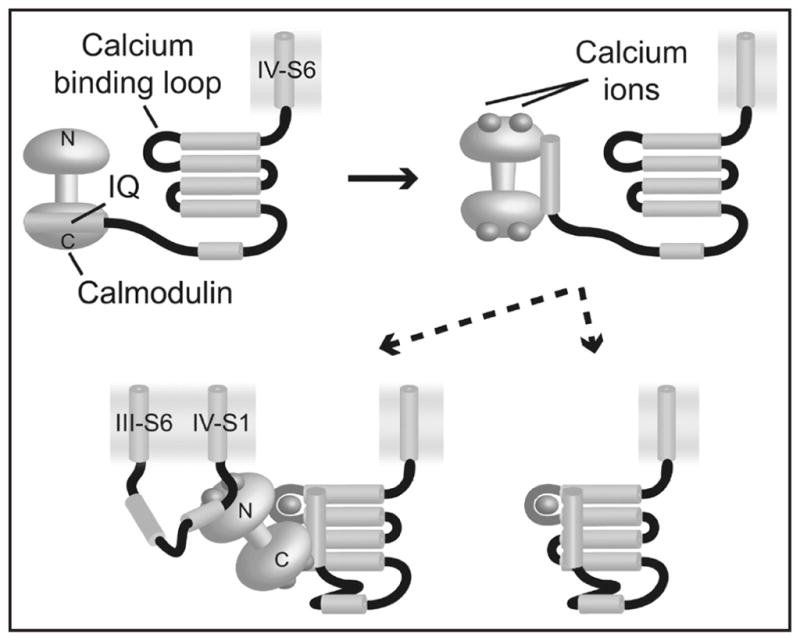
Model for the action of calcium in the hH1 C-terminus. At basal levels of calcium in the cell, calmodulin (yellow dumbbell) is localized to the IQ motif (light blue cylinder), bound via the C-lobe (top left). As calcium levels rise, calmodulin binds calcium (top right), which weakens and alters the CaM/IQ interaction. The consequent release of one or both CaM domains enables the IQ motif to interact with the EF-hand core (bottom panels), raising its calcium affinity by three orders of magnitude which in turn leads to calcium binding in that site.
It is likely that the debate will continue over the role of direct calcium binding and regulation versus the role of calmodulin mediated calcium-dependent regulation of voltage-gated sodium channel function. There appears to be a strong consensus, however, that calcium does indeed regulate sodium channel function through at least one of these mechanisms, and that new insights into these processes may lead to more fundamental breakthroughs in the overall mechanisms governing channel availability and inactivation.
CALCIUM CHANNELS
Voltage-gated calcium channels
The voltage-gated calcium channel family consists of three groups named CaV1, CaV2 and CaV3. In a manner analogous to sodium channel topology, functional channels consist of a large α subunit, which forms the pore, associated with different regulatory subunits (α2/δ, β, γ). Most of the studies looking at calcium-dependent regulation of voltage-gated calcium channels have been performed on CaV1.2 (L-type) and CaV2.1 (P/Q-type) channels.
CaV1.2 (L type)
CaV1 channels generate the so-called L-type calcium current and are sensitive to inhibition by the drug dihydropyridine. CaV1.2 (also known as LTCC), is an interesting case study in the regulation of ion channels by calcium because it exhibits strong calcium-dependent behavior. The channel undergoes inactivation with a fairly slow (~1000 ms) rate constant in the presence of barium, a divalent ion commonly used to mimic calcium, but inactivation speeds up five-fold (~200 ms) in the presence of calcium.28
It has been long recognized that the channel contains an EF-hand in the proximal part of the C-terminus.29 This region was initially proposed30 to mediate calcium-dependent inactivation of this channel, but subsequent studies have ruled this out.31,32 Sequence analysis suggests that this EF-hand may not even bind calcium with any significant affinity; there is a proline at position 2, and position 5 is a lysine instead of an acidic residue.32
In light of these and other revelations, the calcium-dependent behavior of the channel is now attributed to a calmodulin-mediated mechanism. It has been discovered that a number of noncontiguous sites in the C-terminus provide binding sites for calmodulin, including a canonical IQ-type motif.33–35 These sites appear to form a ternary complex with calmodulin at basal calcium levels, followed by rearrangement of the complex during a calcium signal.36 Recently, two research teams succeeded in elucidating the structure of the complex between the calmodulin and the IQ motif by X-ray crystallography. These structures revealed that the binding between the calmodulin and the IQ motif is in a parallel orientation and mainly mediated by hydrophobic interactions. (In both structures, the four Ca2+ binding sites of the CaM are saturated).37,38 As discussed in the section on voltage-gated sodium channels, IQ motifs are known to bind apo (calcium-free) calmodulin, and it is commonly held that a major role for IQ motifs is to recruit calmodulin at basal calcium levels, which then serves as the calcium sensor for local calcium-dependent events.
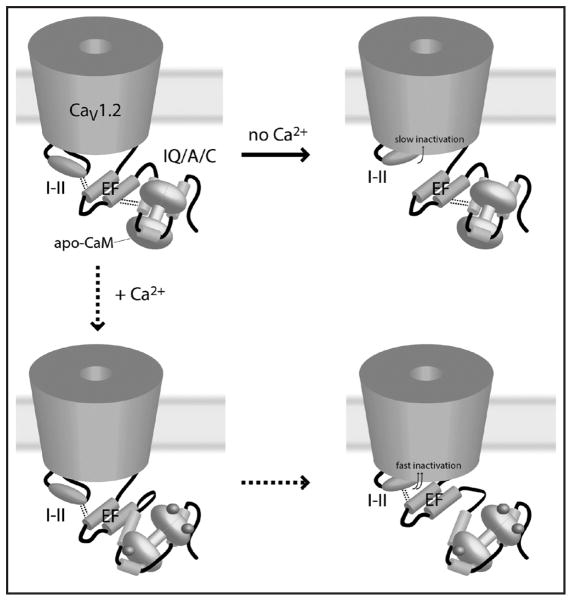
A number of proposals have been made for the mechanism of calcium-dependent behavior in this channel and the role of the IQ motif in this mechanism. The most recent, proposed by Pitt and colleagues,28 integrates data related to calcium-dependent binding of calmodulin sites in the LTCC C-terminus with new mutational data and newly uncovered interactions between the C-terminus and the I-II linker “inactivation particle”. The authors propose that the EF-hand region serves a structural role in calcium-dependent channel behavior. According to their proposal, the complex of calmodulin and the calmodulin-binding-region in the C-terminus interacts constitutively with the EF-hand. The EF-hand, in turn, interacts with the I-II linker in a way that inhibits I-II mediated channel inactivation. Under basal calcium conditions, this results in slowing of I-II mediated inactivation. During conditions of elevated calcium, however, conformational changes in calmodulin release the EF-hand and allow the I-II linker to inactivate the channel more quickly. The proposed model elegantly ties voltage-dependent inactivation and calcium-dependent inactivation into a single mechanism, providing a mechanistic model for calcium-dependent effects on both of these supposedly independent types of inactivation. The proposed mechanism of action is summarized in Figure 4.
Figure 4.
The proposed mechanism of calcium-dependent inactivation in CaV1.2. Top left figure shows the channel in the open state. Dotted lines from the EF-domain to the I-II region and to the IQ/A/C region indicate binding interactions. In the absence of calcium, these interactions must be interrupted for I-II mediated inactivation. In the presence of calcium, conformational changes allow the I-II to inactivate while preserving the I-II/EF interaction, speeding the process fivefold. Figure based on reference 28.
This model bears some resemblance to the one proposed by Shah et al.24 for the cardiac voltage-gated sodium channel NaV1.5. Both pair an IQ motif with an intrinsic EF-hand in the C-terminus. However, the overall mechanism of calcium-dependent regulation is quite different. The major difference is that the proposed mechanism for CaV1.2 relies on the EF-hand domain to transduce a calcium-binding signal that occurs at CaM, whereas the proposal for NaV1.5 suggests that the IQ-motif is the site of interaction between the CTD (C-terminal domain) and the cytoplasmic loop. Additionally, it appears that the interaction of CaM with the Ca2+-channel is mediated by a number of noncontiguous stretches of residues in the C-terminus, whereas the IQ motif is necessary and sufficient for the interaction of CaM with the Na+-channel C-terminus. Another key difference is that the intrinsic calcium binding site does not play a role in calcium-dependent properties of CaV1.2, but is critical in mediating calcium-dependent changes in the voltage-dependent steady state availability of hH1.24,27 Finally, the evidence in hH1 favors a model where CaM dissociates or binds elsewhere on the channel, whereas the proposed mechanism in CaV1.2 requires CaM to remain associated with the C-terminus.
The findings related to and the model proposed for the mechanism of calcium-dependent regulation of CaV1.2 are important not just for understanding this particular system, but shed light onto the inactivation process in general. Further characterization, including the intramolecular targets of the I-II linker and the site of its interaction with the EF-hand may provide important clues toward the development of new drugs targeting calcium channels.
Ca2+-CaM-dependent protein kinase II (CaMK) is able to modify the activity of voltage-gated calcium channels as well as voltage-gated sodium channels. The activation of CaMK induces a modal gating shift that favors prolonged LTCC openings.39 Based on findings that CaMK can bind to the CaV1.2 C-terminal domain in a region that involves IQ and calmodulin-binding (CB) domains, Dzhura et al. hypothesize that activated CaMKII prevents Ca2+-CaM binding to CB and IQ, reducing inactivation, and enhances the interaction of these domains with a ‘facilitation site’. This hypothetical site is speculated to be formed by the β subunit.40 A recent study proposes that following intracellular entry of Ca2+, Ca2+-CaM binds to CaMK. This interaction targets CaMK to certain intracellular domains of CaV1.2. After autophosphorylation of CaMK due to cell’s depolarization, CaMK exposes a CaV1.2 binding site in the C-terminal domain. Then, CaMK phosphorylates the N- and/or C-teminal domain of CaV1.2 to regulate the channel activity.41
CaV2.1 (P/Q type)
CaV2.1 also provides a good case study in the regulation of ion channels by calcium. This channel is mainly located in the brain; its activity is modulated in two ways by calmodulin. CaM has two different effects on the channel, as it promotes both Ca2+-dependent facilitation (CDF) and Ca2+-dependent inhibition (CDI) of this channel.42,43 These two effects are mediated by the interaction of CaM with two CaM-binding sites in the C terminal domain of the channel, an IQ motif and a CaM-binding domain. CaV2.1 possesses lobe-specific regulation by CaM-Ca2+ binding to one or the other lobe of CaM differentially regulates the channel. Ca2+ binding to the C-lobe of CaV2.1-associated CaM results in CDF, whereas Ca2+ binding to the N-lobe of CaM results in CDI. DeMaria et al42 proposed that the ability of CaM to serve a bifunctional role in voltage-gated calcium channels regulation reflects the different Ca2+ affinities of the two lobes.
An EF-hand like domain has also been found in the C terminal domain of CaV2.1 and, in contrast to CaV1.2, seems to be involved in CDF. Alternative splicing of the EF-hand and the CaV2.1 C-terminal region controls the form of Ca2+-dependent facilitation in the channel, and individual cells are thought to express only one of these alternative mRNA transcripts.44
Ryanodine-sensitive calcium release channels
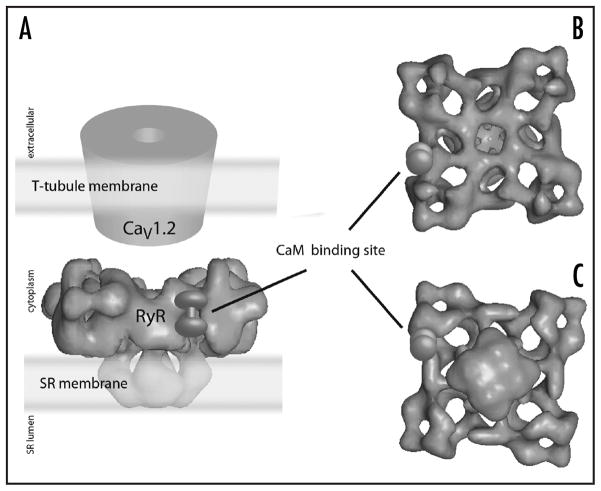
The ryanodine-sensitive calcium release channels (ryanodine receptors, RyR) are found on the SR membrane at locations physically proximate to the T-tubule.45,46 Ryanodine receptors are massive (~5000 amino acid) proteins with four to twelve transmembrane helices47,48 that form tetrameric structures that are able to conduct calcium (stored in the SR lumen under resting conditions) when activated by calcium flowing through LTCC45 or by interaction with LTCC. Its opening increases Ca2+ concentration in the cytoplasm, which leads to the well-characterized process called excitation-contraction coupling. Most of the bulk of the protein is on the cytoplasmic face of the SR membrane, forming 27 nm × 27 nm × 14 nm “feet,” which can be visualized by electron microscopy49–52 in close association with LTCC (more so in skeletal than in cardiac muscle) as shown in Figure 5.
Figure 5.
Three-dimensional reconstruction of the structure of the ryanodine receptor (RyR) tetramer by high-resolution electron microscopy. (A) shows a side view and emphasizes the proximity of RyR to L-type calcium channels in the T-tubule system. (B) shows a view from the cytoplasmic perspective, and (C) shows a view from the SR lumen perspective. The location of calmodulin binding on a single subunit of the tetramer is indicated. Figure adapted with permission from reference 45.
L-type calcium channels found in the transverse (T) tubule system of mammalian ventricular myocytes stimulate calcium release from the sarcoplasmic reticulum (SR) of muscle cells.53 The T-tubule system, formed by invaginations of the cell membrane, conducts depolarization occuring on the surface of the cell deep into the interior. Depolarization activates the voltage-gated L-type calcium channels, resulting in calcium flow from the T-tubule system into the cytoplasm. In the heart, this calcium flow in turn activates ryanodine receptors54 whereas in the skeletal muscle, the RyR channels are activated by a direct interaction with the LTCC channels.55
Three isoforms of RyR are found in mammals.45 RyR1 is found predominantly in skeletal muscle and RyR2 in cardiac muscle. RyR3 has more widespread distribution but is also found in muscle. These channels are activated by ATP and low μM cytoplasmic calcium (calcium-induced calcium release), but inhibited by calmodulin and mM range cytoplasmic calcium.3 At low Ca2+ concentration (<1 μM), calmodulin activates RyR1 and RyR3 channels and inhibits RyR2 channels but at high Ca2+ concentration (>1 μM), calmodulin inhibits all three RyR isoforms.56 Another aspect of the channel’s overall response to calcium is that measurement of the response to calcium but itself results in a bell-shaped curve, with maximal response to calcium occurring in the range of 1 to 10 μm which decreases as calcium concentration increases or decreases.57
These receptors contain two low-calcium-affinity EF-hands, only one of which is thought to bind calcium.57 Because the affinity is low, it is assumed that this EF-hand is involved somehow in channel inactivation, but little is known about the mechanisms by which calcium-dependent activation and inactivation occur.
Not much is known about the calmodulin dependent processes regulating the protein either. Calmodulin binding sites have been characterized by high resolution electron microscopy, which have elucidated a pocket on the massive cytoplasmic “foot” where calmodulin binds49 (Fig. 5). Apo-calmodulin is able to bind to the RyR, and the conformational changes accompanying the calcium-loading of calmodulin are thought to cause the calmodulin binding site to shift by a few amino acids; the change in position of calmodulin is observable by high resolution EM. Calmodulin is known to have different effects on the different isoforms of the RyR, but in all cases inhibits calcium release at higher calcium concentrations. A significant amount of work remains to be done to understand the calcium-dependent mechanisms that regulate these channels, a task that is especially important given their role in the generation of muscular calcium spikes.
Ca2+-CaM-dependent protein kinase II (CaMK) has also been shown to play a role in RyR2 channel activation. A phosphorylation site in rabbit RyR2 (serine-2809) was discovered, and it was assumed that phosphorylation at this site principally mediated CaMK dependent effects. However, another site (serine-2815) has now been identified and the evidence suggests that this site is responsible for CaMK dependent RyR2 activation and Ca2+ release.45,58,59
The concentration of free calcium in SR has also been suggested to modulate channel activity,61 resulting in so called luminal regulation, but the mechanisms by which this occur remain unclear. Another channel with similar localization is the inositol 1,4,5-triphosphate receptor, are located in the endoplasmic reticulum and Golgi apparatus. With RyR, they are the other channel responsible for the release of intracellular stored calcium and so are involved in diverse physiological functions. In mammal, three isoforms of inositol 1,4,5-triphosphate receptors exist. The activation of the receptor needs both inositol 1,4,5-triphosphate and calcium but it may be modulated by calmodulin, adenosine triphosphate and other compounds. The characteristics of this receptor have been reviewed elsewhere (see Bultynck et al.62). As Ryr, inositol 1,4,5-triphosphate receptor responds to calcium in a bell-shaped manner.57
POTASSIUM CHANNELS
A wide variety of potassium channels exist, acting to repolarize the cell membrane by conducting potassium ions, which are maintained at a concentration gradient with a very negative Nernst potential. Some of these channels act during various phases of the action potential to modulate membrane potential changes caused by the flux of other ions. Mechanistically, these channels open slowly compared to sodium channels, with the result that they begin to act just as sodium channels inactivate.
KV channels
The voltage-gated potassium channel family forms the largest group of potassium channels with twelve families named KV1 to KV12. In constrast to sodium and calcium channels, voltage-gated potassium channels are tetramers of four α subunits, each α subunit containing six transmembranes helices. The functional diversity of these families increases through homo- or hetero-associations of α subunits or association with auxiliary cytoplasmic β subunits. The fourth transmembrane helix is involved in voltage-gating, and the fifth and sixth from each subunit form the ion conduction pore.
The ether a go-go channels are members of the KV10 family and are mainly involved in cell cycle control. They are activated by membrane depolarization but were found to be inhibited by low intracellular Ca2+.60,61 The Ca2+ sensitivity of the channels is due to calmodulin; a calmodulin-binding site has been localized in the C-terminal domain (CTD) of the channel.61 Though the channels are tetrameric, only one molecule of calmodulin is required for inhibition of channel function. Two other CaM binding sites have been recently detected. One is located in the C terminus of the channel in the vicinity of the first site discovered. The other is located in the N terminus.62 Calmodulin binds all sites in a Ca2+-dependent manner, and experiments with mutant CaM have demonstrated that binding at all sites is primarily dependent on the C-lobe of CaM. The molecular mechanism by which CaM acts is unclear at this point, though structural information about the complex between CaM and the channel would likely be useful in elucidating the series of events.62
It has been also reported that CaM was able to bind several channels in the KV7 family in both the absence and presence of Ca2+. Calmodulin can binds to the C terminus of these channels at two distinct sites, termed IQ1 and IQ2. Only the binding on IQ2 seems to be Ca2+-dependent.63–66 Functionally, CaM appears to inhibit channel function at elevated Ca2+ concentrations. For example, in heteromeric KV7.2/KV7.3 channels, increases in Ca2+ led to a decline in the channel current, which was recovered when Ca2+ was lowered.64 CaM may also play a structural role in KV7 function. A recent study shows that CaM participates in the assembly of KV7.1, an isoform involved in cardiac action potential repolarization.66 This is in addition to its functional effects on the channel; the chelation of Ca2+ below physiological concentrations or the incorporation of a mutant CaM unable to bind Ca2+ induces prominent channel inactivation during the depolarizing pulses and reduces current amplitude of Kv7.1.66
Calcium-dependent regulation of voltage-gated potassium channels is mediated by a number of proteins aside from CaM. KV4 channels, also involved in cardiac action potential repolarization, are modulated by a family of Ca2+ sensor proteins termed Kv channel-interacting proteins (KChIP’s). The coexpression of KChIP with KV4 leads to an increase in the current intensity.67 These proteins interact with KV4 channels via the N and C terminal regions of the channel,68 resulting in an increase in current density, a reduction in the inactivation rates and an acceleration of recovery from inactivation. Studies have shown that the effects of KChIP on current density and inactivation kinetics occur independently of Ca2+, whereas the effects on the rate of recovery from inactivation are Ca2+-dependent.69 Also regulating KV4 is CaMK; the effects on channel inactivation are similar to those produced by KchIP.70 Sergeant et al found that mutation of serine-550 in the C terminal domain of KV4.3 eliminated the effects of CaMK, suggesting that the channel is a direct target of CaMK. They proposed that elevation of cytosolic Ca2+ activates CaMK, causing the inactivation rate of KV4.3 to slow.70
The KV1.4 channel is also regulated by CaMK. The inactivation gating of this Shaker-related fast-inactivating KV channel is controlled by CaMK and protein phosphatase B2. The balance between phosphorylated and dephosphorylated KV1.4 channels is regulated by changes in intracellular Ca2+ concentration, rendering KV1.4 inactivation gating Ca 2+-sensitive.71 Phosphorylation of the N terminal domain reduces its rate of inactivation, increases its recovery from inactivation, and shifts the potential of voltage dependant steady-state inactivation to more positive potentials.
Jow et al. uncovered Ca2+-dependent regulation of the inactivation of voltage-gated KV1.1 associated with the β subunit KVβ1.1. Ca2+ switchs the kinetics of KV1.1/KVβ1.1 inactivation from fast to slow. Alteration of the N terminal domain of KVβ1.1 abolishes Ca2+-dependent modulation, although no Ca2+-binding site have been detected in this region. No role for CaM has been suggested. The authors hypothesize that the slowed inactivation of voltage-gated KV1.1/KVβ1.1 channels by Ca2+ could serve as a negative feedback mechanism to increase potassium conductance and may contribute to the regulation of repolarization and afterhyperpolarization during the action potentials in neurons.72
Additional potassium channels
One class of potassium channels act late in the cardiac action potential to repolarize the cell membrane to end the current action potential and prepare the cell for the next one. Among these channels are the inward rectifiers, which activate and then inactivate extremely rapidly during initial depolarization, resulting in little conduction of potassium ion. During the course of the action potential, however, these channels recover from inactivation and are able to activate again if the membrane potential is more positive than −30 mV.19 During this second activation, the channels will remain open longer if the membrane potential is between 0 mV and −30 mV.19 This results in increased potassium current and acceleration of repolarization.
The calcium-activated potassium channels (KCa) form a second group. In contrast to KV channels, these channels open in response to calcium, acting late in the action potential to contribute potassium currents towards repolarization of the cell in response to elevated intracellular calcium. Three different subtypes of KCa were initially identified on the basis of conductance: big conductance (BKCa), intermediate conductance (IKCa), and small conductance (SKCa).73 IKCa and SKCa channels have since been recognized to be closely related to each other.
BKCa channels
These channels are expressed in smooth muscle74 and nerve cells.75,76 Recently, they have also been implicated in functions as diverse as innate immunity in neutrophil leukocytes77 and protection against ischemia in cardiac myocyes.78 The channels are tetramers of α subunits that are associated with a regulatory β subunit. The α subunit appears to have a canonical six-transmembrane topology, with a number of positively charged residues in the S4 segment. Interestingly, hydropathy plots indicate that there may be another transmembrane helix preceding the S1 segment of the channel (termed S0), as well as four hydrophobic segments distal to S6 (termed S7–S10) involved in forming a large intracellular domain.79 This intracellular region contains several different important functional domains: a tetramerization domain, several leucine zipper domains, multiple phosphorylation sites, two “regulator of conductance for potassium” domains (referred to as RCK domains) located around the S7 and S8 segments80,81 and a “calcium-bowl” located between the S9 and S10 segments.82,83
BKCa channels are able to respond to both voltage signals and calcium signals.84 The S4 helix likely mediates voltage-gating, given what is known generally about six-transmembrane voltage-gated channels. It is currently believed that calcium sensitivity is mediated by the C-terminal domain, based on cross-species domain swapping experiments, which demonstrated that transfer of the distal hydrophobic segments can transfer calcium dependent properties across species.85
At present there remains quite a bit of controversy over the number, affinity, and location of calcium binding sites in BKCa. What is clear is that there are no canonical calcium binding sites anywhere in the protein. Several putative ion-binding sites have been identified in the channel, including two Ca2+ binding sites proposed to be located in the RCK domains.86,87 It has also been demonstrated by alanine-scanning mutagenesis that an acid-rich region in the S9–S10 loop is important for calcium binding.88 As mentioned above, this region has been termed the “calcium-bowl” and is thought by some to serve as one of the calcium binding sites in the protein. Standing in contrast to all of this data is the finding by another group that most of the voltage- and calcium-dependent properties of the channel are retained even when the last four hydrophobic helices S7–S10 are truncated.89 With regards to calcium-dependent regulation by an extrinsic calcium sensor, studies examining the role of calmodulin in Ca2+ dependent activation of BKCa channels have concluded that it is not involved.90,91 However, sequence analysis using the CaM Target Database92 suggests a calmodulin binding site may exist in the C-terminal region of some isoforms.
SKCa and IKCa channels
SKCa channels are expressed throughout the body in muscle and nerve cells.93,94 In humans, this family is comprised of four members encoded by the genes SK1, SK2, SK3 and IK1.94 Note that the intermediate conductance channel is included in this gene family; although it has a larger single-channel conductance and different drug sensitivity than the other members of the family, sequence homology indicates that this channel functions in a similar manner and is closely related to SKCa channels. The initial separation of these channels into specific groups stemmed from the fact that SK isoforms were sensitive to the bee venom toxin apamin, whereas the IK isoforms were not. SKCa channels are the only known target of apamin.94
Each of the SKCa channels has the canonical transmembrane topology of potassium channels, namely, six transmembrane helices and tetramerization to form a functional channel. In contrast to the S4 segments in voltage-gated potassium channels (KV), which carry positively charged residues important for voltage-dependent gating, the S4 segments of SKCa channels are populated with primarily neutral resides at comparable positions. Presumably, this explains the voltage-independent behavior of these channels.73 The focus of research on the three SK isoforms of this channel has been mainly restricted to their function in neural contexts,95–101 but it has been demonstrated that the SK2 isoform is expressed in the atria and ventricles of both human and mouse heart.102 Apamin blockade resulted in substantially longer lasting plateau and repolarization phases of the cardiac action potential. This and other observations revealed both the presence of SK2 in cardiac myocytes and that the role of SK2 in this context is to accelerate repolarization of the cell following calcium-mediated excitation-contraction coupling.
Unlike BKCa channels, quite a bit is known about the structural mechanism of calcium-dependent sensitivity and gating in SKCa channels. The proximal C-terminal domain of the channel binds calmodulin via a novel motif that does not conform to known Ca2+-independent CaM binding motifs (such as the IQ motif) or the traditional Ca2+-dependent motifs (reviewed in refs. 92 and 103). This region binds calmodulin in such a way that the C-terminal lobe of calmodulin appears to mediate a Ca2+-independent interaction with the channel, while the N-terminal lobe mediates Ca2+-dependent changes to these interactions.104 Mechanistic insights have been obtained from a combination of biochemical data104–106 and crystal structures of the SKCa Ca2+-binding domain in complex with both apo107 and Ca2+-loaded calmodulin.108 Only the N-lobe EF-hand calcium-binding sites of calmodulin are occupied by calcium in the “Ca2+-loaded” structure.
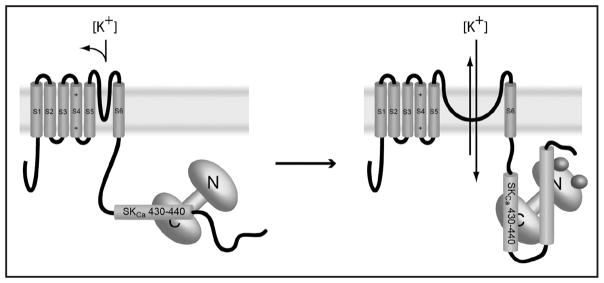
The consensus view is that binding of calcium to the N-lobe of calmodulin orders large parts of the SKCa C-terminus. At the same time, the conformation of the binding surface between the calmodulin C-lobe and the SKCa C-terminus alters significantly, reorienting the helical residues in the interaction region (residues 430–440) by greater than 90°.107 The comparison of the apo-structure with the Ca2+-loaded structure suggests that this rotation may directly result in gating of the channel. Since these changes occur in the proximal C-terminus, they directly affect the covalently attached inner helix gate of the channel. The authors conclude a rotation of this magnitude will likely cause significant conformational change in the gate, resulting in channel opening. This proposed mechanism is summarized in Figure 6.
Figure 6.
Proposed mechanism for calcium dependent activation of SKCa channels. Closed channels, at left, constitutively bind the C-lobe of calmodulin. Calcium binding causes a >90° rotation in the 430–440 segment of the SKCa C-terminus, altering the conformation of S6 and gating the channel to the open position. Calcium binding also causes a significant ordering of other parts of the C-terminus as well; the crystal structure of Ca-CaM with the SKCa-CTD is a domain swapped dimer with both lobes of CaM binding different parts of the SKCa-CTD.
DISCUSSION
As is clear from the examples described here, evolution has strongly selected for systems which self-limit calcium signals. The existence of calcium-dependent feedback mechanisms in sodium, potassium, and calcium signal generation underlines the importance of tightly regulated intracellular calcium levels. In aggregate, the calcium-dependent behavior of these channels is likely to tune the peak and trough calcium concentrations during an action potential. This effect is both direct, by modulation of calcium current, and indirect, by providing calcium-dependent repolarization mechanisms. These processes may also provide some protection against pathological processes such as ischemia in which both extracellular109,110 and intracellular111 calcium concentrations rise significantly.
Not explored here, but also likely to play a role in the regulation of ion channels, is Ca2+- dependent changes in the expression of channels. For example, Varga et al have demonstrated that CaMK does not affect KV4.2 channel biophysics but does directly modulate neuronal excitability by increasing cell-surface expression of A-type K+ channels.112
It is clear that calmodulin plays a significant role in many of these regulatory processes. One commonality that has emerged from this review is the importance of recruiting calmodulin at basal calcium concentrations via the IQ motif (Na+ channels and CaV1.2) or other motifs (SKCa channels and ryanodine-sensitive calcium release channel). This localizes a molecule of calmodulin to the area where it will take action, providing for faster kinetics in the response to calcium as well as stabilizing CaM in the “off” position. Additionally, it has been shown that the IQ motif interaction alters the Ca2+ on and off-rates from calmodulin,113,114 further ensuring a more rapid kinetic on-response during a calcium signal and off-response to the basal state once a calcium signal is complete.
Intrinsic calcium binding sites regulate many of these channels as well, serving structural (CaV1.2) or functional (NaV1.5) roles. Further study of these systems should lead to new understanding of how these regulatory systems evolved and why these sites were conserved. Important discoveries may also come from better understanding of these channels. For example, it is likely that BKCa channels are regulated by a novel calcium binding protein and/or contain a novel intrinsic calcium binding motif. Characterization of this motif may uncover similar motifs in other proteins not currently recognized as calcium binding factors. A better molecular understanding of the mechanisms behind calcium-dependent control of these channels may also lead to new strategies for anti-arrhythmic and anti-epileptic therapy.
Acknowledgments
The authors wish to thank Tammy L. Wingo and Jeffrey R. Balser for their ongoing support, encouragement and helpful discussions. Research on calcium signaling in our laboratory is supported by operating grants from the National Institutes of Health (RO1 GM 40120 and RO1 GM62112 to WJC; RO1 GM 56307 to J.R.B.). V.N.S. was supported in part by the NIH Medical Scientist training program (T32 GM07347).
Footnotes
NOTE
Color figures are available in the online pdf at http://landesbioscience.com/journals/cbproteins/article/3496.
References
- 1.Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braveny P. Heart, calcium and time. Experimental and Clinical Cardiology. 2002;7:111–78. [PMC free article] [PubMed] [Google Scholar]
- 3.Martonosi AN, Pikula S. The network of calcium regulation in muscle. Acta Biochim Pol. 2003;50:1–30. [PubMed] [Google Scholar]
- 4.Henikoff S, Greene EA, Pietrokovski S, Bork P, Attwood TK, Hood L. Gene families: The taxonomy of protein paralogs and chimeras. Science. 1997;278:609–14. doi: 10.1126/science.278.5338.609. [DOI] [PubMed] [Google Scholar]
- 5.Kretsinger RH, Nockolds CE. Carp muscle calcium-binding protein. II. Structure determination and general description. J Biol Chem. 1973;248:3313–26. [PubMed] [Google Scholar]
- 6.Drake SK, Zimmer MA, Kundrot C, Falke JJ. Molecular tuning of an EF-hand-like calcium binding loop. Contributions of the coordinating side chain at loop position 3. J Gen Physiol. 1997;110:173–84. doi: 10.1085/jgp.110.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang W, Lee HW, Hellinga H, Yang JJ. Structural analysis, identification, and design of calcium-binding sites in proteins. Proteins. 2002;47:344–56. doi: 10.1002/prot.10093. [DOI] [PubMed] [Google Scholar]
- 8.Bunick CG, Nelson MR, Mangahas S, Hunter MJ, Sheehan JH, Mizoue LS, Bunick GJ, Chazin WJ. Designing sequence to control protein function in an EF-hand protein. J Am Chem Soc. 2004;126:5990–8. doi: 10.1021/ja0397456. [DOI] [PubMed] [Google Scholar]
- 9.Nelson MR, Thulin E, Fagan PA, Forsen S, Chazin WJ. The EF-hand domain: A globally cooperative structural unit. Protein Sci. 2002;11:198–205. doi: 10.1110/ps.33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parekh AB, Putney JW., Jr Store-operated calcium channels. Physiol Rev. 2005;85:757–810. doi: 10.1152/physrev.00057.2003. [DOI] [PubMed] [Google Scholar]
- 11.Tfelt-Hansen J, Brown EM. The calcium-sensing receptor in normal physiology and pathophysiology: A review. Crit Rev Clin Lab Sci. 2005;42:35–70. doi: 10.1080/10408360590886606. [DOI] [PubMed] [Google Scholar]
- 12.van der Heyden MA, Wijnhoven TJ, Opthof T. Molecular aspects of adrenergic modulation of cardiac L-type Ca2+ channels. Cardiovasc Res. 2005;65:28–39. doi: 10.1016/j.cardiores.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 13.Iwamoto T. Forefront of Na+/Ca2+ exchanger studies: Molecular pharmacology of Na+/Ca2+ exchange inhibitors. J Pharmacol Sci. 2004;96:27–32. doi: 10.1254/jphs.fmj04002x6. [DOI] [PubMed] [Google Scholar]
- 14.Komuro I, Ohtsuka M. Forefront of Na+/Ca2+ exchanger studies: Role of Na+/Ca2+ exchanger-lessons from knockout mice. J Pharmacol Sci. 2004;96:23–6. doi: 10.1254/jphs.fmj04002x5. [DOI] [PubMed] [Google Scholar]
- 15.Uehara A, Iwamoto T, Nakamura Y, Imanaga I. Forefront of Na+/Ca2+ exchanger studies: Physiology and molecular biology of monovalent cation sensitivities in Na+/Ca2+ exchangers. J Pharmacol Sci. 2004;96:19–22. doi: 10.1254/jphs.fmj04002x4. [DOI] [PubMed] [Google Scholar]
- 16.Hinata M, Kimura J. Forefront of Na+/Ca2+ exchanger studies: Stoichiometry of cardiac Na+/Ca2+ exchanger; 3:1 or 4:1? J Pharmacol Sci. 2004;96:15–8. doi: 10.1254/jphs.fmj04002x3. [DOI] [PubMed] [Google Scholar]
- 17.Matsuoka S. Forefront of Na+/Ca2+ exchanger studies: Regulation kinetics of Na+/Ca2+ exchangers. J Pharmacol Sci. 2004;96:12–4. doi: 10.1254/jphs.fmj04002x2. [DOI] [PubMed] [Google Scholar]
- 18.Sperelakis N. Cell physiology sourcebook: A molecular approach. San Diego: Academic Press; 2001. [Google Scholar]
- 19.Tamargo J, Caballero R, Gomez R, Valenzuela C, Delpon E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Herzog RI, Liu C, Waxman SG, Cummins TR. Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1. 6 and differentially modulates their functional properties. J Neurosci. 2003;23:8261–70. doi: 10.1523/JNEUROSCI.23-23-08261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AA, Anderson ME, Balser JR. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415:442–7. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- 22.Mori M, Konno T, Morii T, Nagayama K, Imoto K. Regulatory interaction of sodium channel IQ-motif with calmodulin C-terminal lobe. Biochem Biophys Res Commun. 2003;307:290–6. doi: 10.1016/s0006-291x(03)01183-5. [DOI] [PubMed] [Google Scholar]
- 23.Kim J, Ghosh S, Liu H, Tateyama M, Kass RS, Pitt GS. Calmodulin mediates Ca2+ sensitivity of sodium channels. J Biol Chem. 2004;279:45004–12. doi: 10.1074/jbc.M407286200. [DOI] [PubMed] [Google Scholar]
- 24.Shah VN, Wingo TL, Weiss KL, Williams CK, Balser JR, Chazin WJ. Calcium-dependent regulation of the voltage-gated sodium channel hH1: Intrinsic and extrinsic sensors use a common molecular switch. Proc Natl Acad Sci USA. 2006 doi: 10.1073/pnas.0507397103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deschenes I, Neyroud N, DiSilvestre D, Marban E, Yue DT, Tomaselli GF. Isoform-specific modulation of voltage-gated Na(+) channels by calmodulin. Circ Res. 2002;90:E49–57. doi: 10.1161/01.res.0000012502.92751.e6. [DOI] [PubMed] [Google Scholar]
- 26.Abriel H, Kass RS. Regulation of the voltage-gated cardiac sodium channel Nav1. 5 by interacting proteins. Trends Cardiovasc Med. 2005;15:35–40. doi: 10.1016/j.tcm.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Wingo TL, Shah VN, Anderson ME, Lybrand TP, Chazin WJ, Balser JR. An EF-hand in the sodium channel couples intracellular calcium to cardiac excitability. Nat Struct Mol Biol. 2004;11:219–25. doi: 10.1038/nsmb737. [DOI] [PubMed] [Google Scholar]
- 28.Kim J, Ghosh S, Nunziato DA, Pitt GS. Identification of the components controlling inactivation of voltage-gated Ca2+ channels. Neuron. 2004;41:745–54. doi: 10.1016/s0896-6273(04)00081-9. [DOI] [PubMed] [Google Scholar]
- 29.Babitch J. Channel hands. Nature. 1990;346:321–2. doi: 10.1038/346321b0. [DOI] [PubMed] [Google Scholar]
- 30.de Leon M, Wang Y, Jones L, Perez-Reyes E, Wei X, Soong TW, Snutch TP, Yue DT. Essential Ca(2+)-binding motif for Ca(2+)-sensitive inactivation of L-type Ca2+ channels. Science. 1995;270:1502–6. doi: 10.1126/science.270.5241.1502. [DOI] [PubMed] [Google Scholar]
- 31.Peterson BZ, Lee JS, Mulle JG, Wang Y, de Leon M, Yue DT. Critical determinants of Ca(2+)-dependent inactivation within an EF-hand motif of L-type Ca(2+) channels. Biophys J. 2000;78:1906–20. doi: 10.1016/S0006-3495(00)76739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Olcese R, Qin N, Noceti F, Birnbaumer L, Stefani E. Feedback inhibition of Ca2+ channels by Ca2+ depends on a short sequence of the C terminus that does not include the Ca2+-binding function of a motif with similarity to Ca2+-binding domains. Proc Natl Acad Sci USA. 1997;94:2301–5. doi: 10.1073/pnas.94.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erickson MG, Liang H, Mori MX, Yue DT. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron. 2003;39:97–107. doi: 10.1016/s0896-6273(03)00395-7. [DOI] [PubMed] [Google Scholar]
- 34.Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW. Molecular basis of calmodulin tethering and Ca2+-dependent inactivation of L-type Ca2+ channels. J Biol Chem. 2001;276:30794–802. doi: 10.1074/jbc.M104959200. [DOI] [PubMed] [Google Scholar]
- 35.Xiong L, Kleerekoper QK, He R, Putkey JA, Hamilton SL. Sites on calmodulin that interact with the C-terminal tail of Cav1. 2 channel. J Biol Chem. 2005;280:7070–9. doi: 10.1074/jbc.M410558200. [DOI] [PubMed] [Google Scholar]
- 36.Mouton J, Feltz A, Maulet Y. Interactions of calmodulin with two peptides derived from the c-terminal cytoplasmic domain of the Ca(v)1. 2 Ca2+ channel provide evidence for a molecular switch involved in Ca2+-induced inactivation. J Biol Chem. 2001;276:22359–67. doi: 10.1074/jbc.M100755200. [DOI] [PubMed] [Google Scholar]
- 37.Van Petegem F, Chatelain FC, Minor DL., Jr Insights into voltage-gated calcium channel regulation from the structure of the CaV1. 2 IQ domain-Ca2+/calmodulin complex. Nat Struct Mol Biol. 2005;12:1108–15. doi: 10.1038/nsmb1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fallon JL, Halling DB, Hamilton SL, Quiocho FA. Structure of calmodulin bound to the hydrophobic IQ domain of the cardiac Ca(v)1. 2 calcium channel. Structure. 2005;13:1881–6. doi: 10.1016/j.str.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 39.Dzhura I, Wu Y, Colbran RJ, Balser JR, Anderson ME. Calmodulin kinase determines calcium-dependent facilitation of L-type calcium channels. Nat Cell Biol. 2000;2:173–7. doi: 10.1038/35004052. [DOI] [PubMed] [Google Scholar]
- 40.Dzhura I, Wu Y, Zhang R, Colbran RJ, Hamilton SL, Anderson ME. C terminus L-type Ca2+ channel calmodulin-binding domains are ‘auto-agonist’ ligands in rabbit ventricular myocytes. J Physiol. 2003;550:731–8. doi: 10.1113/jphysiol.2003.043778. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Hudmon A, Schulman H, Kim J, Maltez JM, Tsien RW, Pitt GS. CaMKII tethers to L-type Ca2+ channels, establishing a local and dedicated integrator of Ca2+ signals for facilitation. J Cell Biol. 2005;171:537–47. doi: 10.1083/jcb.200505155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–9. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 43.Halling DB, Aracena-Parks P, Hamilton SL. Regulation of voltage-gated Ca2+ channels by calmodulin. Sci STKE. 2005;2005:re15. doi: 10.1126/stke.3152005re15. [DOI] [PubMed] [Google Scholar]
- 44.Chaudhuri D, Chang SY, DeMaria CD, Alvania RS, Soong TW, Yue DT. Alternative splicing as a molecular switch for Ca2+/calmodulin-dependent facilitation of P/Q-type Ca2+ channels. J Neurosci. 2004;24:6334–42. doi: 10.1523/JNEUROSCI.1712-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bers DM. Macromolecular complexes regulating cardiac ryanodine receptor function. J Mol Cell Cardiol. 2004;37:417–29. doi: 10.1016/j.yjmcc.2004.05.026. [DOI] [PubMed] [Google Scholar]
- 46.Franzini-Armstrong C, Protasi F, Ramesh V. Shape, size, and distribution of Ca(2+) release units and couplons in skeletal and cardiac muscles. Biophys J. 1999;77:1528–39. doi: 10.1016/S0006-3495(99)77000-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, et al. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989;339:439–45. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- 48.Zorzato F, Fujii J, Otsu K, Phillips M, Green NM, Lai FA, Meissner G, MacLennan DH. Molecular cloning of cDNA encoding human and rabbit forms of the Ca2+ release channel (ryanodine receptor) of skeletal muscle sarcoplasmic reticulum. J Biol Chem. 1990;265:2244–56. [PubMed] [Google Scholar]
- 49.Samso M, Wagenknecht T. Apocalmodulin and Ca2+-calmodulin bind to neighboring locations on the ryanodine receptor. J Biol Chem. 2002;277:1349–53. doi: 10.1074/jbc.M109196200. [DOI] [PubMed] [Google Scholar]
- 50.Sharma MR, Penczek P, Grassucci R, Xin HB, Fleischer S, Wagenknecht T. Cryoelectron microscopy and image analysis of the cardiac ryanodine receptor. J Biol Chem. 1998;273:18429–34. doi: 10.1074/jbc.273.29.18429. [DOI] [PubMed] [Google Scholar]
- 51.Wagenknecht T, Samso M. Three-dimensional reconstruction of ryanodine receptors. Front Biosci. 2002;7:d1464–74. doi: 10.2741/A853. [DOI] [PubMed] [Google Scholar]
- 52.Samso M, Wagenknecht T, Allen PD. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo-EM. Nat Struct Mol Biol. 2005;12:539–44. doi: 10.1038/nsmb938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ Res. 2001;88:1151–8. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- 54.Meissner G. Molecular regulation of cardiac ryanodine receptor ion channel. Cell Calcium. 2004;35:621–8. doi: 10.1016/j.ceca.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 55.Cheng W, Altafaj X, Ronjat M, Coronado R. Interaction between the dihydropyridine receptor Ca2+ channel beta-subunit and ryanodine receptor type 1 strengthens excitation-contraction coupling. Proc Natl Acad Sci USA. 2005;102:19225–30. doi: 10.1073/pnas.0504334102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaguchi N, Xu L, Pasek DA, Evans KE, Chen SR, Meissner G. Calmodulin regulation and identification of calmodulin binding region of type-3 ryanodine receptor calcium release channel. Biochemistry. 2005;44:15074–81. doi: 10.1021/bi051251t. [DOI] [PubMed] [Google Scholar]
- 57.Bezprozvanny I, Watras J, Ehrlich BE. Bell-Shaped Calcium-Response Curves of Ins(1,4,5)P3-Gated and Calcium-Gated Channels from Endoplasmic-Reticulum of Cerebellum. Nature. 1991;351:751–4. doi: 10.1038/351751a0. [DOI] [PubMed] [Google Scholar]
- 58.Hamada T, Sakube Y, Ahnn J, Kim do H, Kagawa H. Molecular dissection, tissue localization and Ca2+ binding of the ryanodine receptor of Caenorhabditis elegans. J Mol Biol. 2002;324:123–35. doi: 10.1016/s0022-2836(02)01032-x. [DOI] [PubMed] [Google Scholar]
- 59.Witcher DR, Kovacs RJ, Schulman H, Cefali DC, Jones LR. Unique phosphorylation site on the cardiac ryanodine receptor regulates calcium channel activity. J Biol Chem. 1991;266:11144–52. [PubMed] [Google Scholar]
- 60.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 61.Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Gyorke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circulation Research. 2002;91:414–20. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- 62.Bultynck G, Sienaert I, Parys JB, Callewaert G, De Smedt H, Boens N, Dehaen W, Missiaen L. Pharmacology of inositol trisphosphate receptors. Pflugers Archiv-European Journal of Physiology. 2003;445:629–42. doi: 10.1007/s00424-002-0971-1. [DOI] [PubMed] [Google Scholar]
- 63.Stansfeld CE, Roper J, Ludwig J, Weseloh RM, Marsh SJ, Brown DA, Pongs O. Elevation of intracellular calcium by muscarinic receptor activation induces a block of voltage-activated rat ether-a-go-go channels in a stably transfected cell line. Proc Natl Acad Sci U S A. 1996;93:9910–4. doi: 10.1073/pnas.93.18.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schonherr R, Lober K, Heinemann SH. Inhibition of human ether a go-go potassium channels by Ca(2+)/calmodulin. Embo J. 2000;19:3263–71. doi: 10.1093/emboj/19.13.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ziechner U, Schonherr R, Born AK, Gavrilova-Ruch O, Glaser RW, Malesevic M, Kullertz G, Heinemann SH. Inhibition of human ether a go-go potassium channels by Ca2+/calmodulin binding to the cytosolic N- and C-termini. Febs J. 2006;273:1074–86. doi: 10.1111/j.1742-4658.2006.05134.x. [DOI] [PubMed] [Google Scholar]
- 66.Wen H, Levitan IB. Calmodulin is an auxiliary subunit of KCNQ2/3 potassium channels. J Neurosci. 2002;22:7991–8001. doi: 10.1523/JNEUROSCI.22-18-07991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gamper N, Shapiro MS. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. Journal of General Physiology. 2003;122:17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yus-Najera E, Santana-Castro I, Villarroel A. The identification and characterization of a noncontinuous calmodulin-binding site in noninactivating voltage-dependent KCNQ potassium channels. J Biol Chem. 2002;277:28545–53. doi: 10.1074/jbc.M204130200. [DOI] [PubMed] [Google Scholar]
- 69.Ghosh S, Nunziato DA, Pitt GS. KCNQ1 Assembly and Function Is Blocked by Long-QT Syndrome Mutations That Disrupt Interaction With Calmodulin. Circ Res. 2006 doi: 10.1161/01.RES.0000218863.44140.f2. [DOI] [PubMed] [Google Scholar]
- 70.An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–6. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- 71.Callsen B, Isbrandt D, Sauter K, Hartmann LS, Pongs O, Bahring R. Contribution of N- and C-terminal channel domains to Kv4. 2 domains to KChIP interaction [corrected] J Physiol. 2005;568:397–412. doi: 10.1113/jphysiol.2005.094359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Patel SP, Campbell DL, Strauss HC. Elucidating KChIP effects on Kv4. 3 inactivation and recovery kinetics with a minimal KChIP2 isoform. J Physiol. 2002;545:5–11. doi: 10.1113/jphysiol.2002.031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sergeant GP, Ohya S, Reihill JA, Perrino BA, Amberg GC, Imaizumi Y, Horowitz B, Sanders KM, Koh SD. Regulation of Kv4. 3 currents by Ca2+/calmodulin-dependent protein kinase II. Am J Physiol Cell Physiol. 2005;288:C304–13. doi: 10.1152/ajpcell.00293.2004. [DOI] [PubMed] [Google Scholar]
- 74.Roeper J, Lorra C, Pongs O. Frequency-dependent inactivation of mammalian A-type K+ channel KV1. 4 regulated by Ca2+/calmodulin-dependent protein kinase. J Neurosci. 1997;17:3379–91. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jow F, Zhang ZH, Kopsco DC, Carroll KC, Wang K. Functional coupling of intracellular calcium and inactivation of voltage-gated Kv1.1/Kvbeta1. 1 A-type K+ channels. Proc Natl Acad Sci U S A. 2004;101:15535–40. doi: 10.1073/pnas.0402081101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–70. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 77.Brayden JE, Nelson MT. Regulation of arterial tone by activation of calcium-dependent potassium channels. Science. 1992;256:532–5. doi: 10.1126/science.1373909. [DOI] [PubMed] [Google Scholar]
- 78.Moczydlowski EG. BK channel news: full coverage on the calcium bowl. J Gen Physiol. 2004;123:471–3. doi: 10.1085/jgp.200409069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robitaille R, Garcia ML, Kaczorowski GJ, Charlton MP. Functional colocalization of calcium and calcium-gated potassium channels in control of transmitter release. Neuron. 1993;11:645–55. doi: 10.1016/0896-6273(93)90076-4. [DOI] [PubMed] [Google Scholar]
- 80.Ahluwalia J, Tinker A, Clapp LH, Duchen MR, Abramov AY, Pope S, Nobles M, Segal AW. The large-conductance Ca2+-activated K+ channel is essential for innate immunity. Nature. 2004;427:853–8. doi: 10.1038/nature02356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–33. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 82.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (SO-S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14066–71. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang YX, Pico A, Cadene M, Chait BT, MacKinnon R. Structure of the RCK domain from the E. coli K+ channel and demonstration of its presence in the human BK channel. Neuron. 2001;29:593–601. doi: 10.1016/s0896-6273(01)00236-7. [DOI] [PubMed] [Google Scholar]
- 84.Roosild TP, Le KT, Choe S. Cytoplasmic gatekeepers of K+-channel flux: a structural perspective. Trends in Biochemical Sciences. 2004;29:39–45. doi: 10.1016/j.tibs.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 85.Schreiber M, Salkoff L. A novel calcium-sensing domain in the BK channel. Biophysical Journal. 1997;73:1355–63. doi: 10.1016/S0006-3495(97)78168-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wei A, Jegla T, Salkoff L. Eight potassium channel families revealed by the C-elegans genome project. Neuropharmacology. 1996;35:805–29. doi: 10.1016/0028-3908(96)00126-8. [DOI] [PubMed] [Google Scholar]
- 87.Cox DH. The BKCa channel’s Ca2+-binding sites, multiple sites, multiple ions. J Gen Physiol. 2005;125:253–5. doi: 10.1085/jgp.200509270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wei A, Solaro C, Lingle C, Salkoff L. Calcium sensitivity of BK-type KCa channels determined by a separable domain. Neuron. 1994;13:671–81. doi: 10.1016/0896-6273(94)90034-5. [DOI] [PubMed] [Google Scholar]
- 89.Bao L, Rapin AM, Holmstrand EC, Cox DH. Elimination of the BKCa channel’s high-affinity Ca2+ sensitivity. Journal of General Physiology. 2002;120:173–89. doi: 10.1085/jgp.20028627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xia XM, Zeng XH, Lingle CJ. Multiple regulatory sites in large-conductance calcium-activated potassium channels. Nature. 2002;418:880–4. doi: 10.1038/nature00956. [DOI] [PubMed] [Google Scholar]
- 91.Bao L, Kaldany C, Holmstrand EC, Cox DH. Mapping the BKCa channel’s “Ca2+ bowl”: side-chains essential for Ca2+ sensing. J Gen Physiol. 2004;123:475–89. doi: 10.1085/jgp.200409052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piskorowski R, Aldrich RW. Calcium activation of BK(Ca) potassium channels lacking the calcium bowl and RCK domains. Nature. 2002;420:499–502. doi: 10.1038/nature01199. [DOI] [PubMed] [Google Scholar]
- 93.Ikemoto Y, Ono K, Yoshida A, Akaike N. Delayed activation of large-conductance Ca2+-activated K channels in hippocampal neurons of the rat. Biophys J. 1989;56:207–12. doi: 10.1016/S0006-3495(89)82665-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kihira M, Matsuzawa K, Tokuno H, Tomita T. Effects of calmodulin antagonists on calcium-activated potassium channels in pregnant rat myometrium. Br J Pharmacol. 1990;100:353–9. doi: 10.1111/j.1476-5381.1990.tb15808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yap KL, Kim J, Truong K, Sherman M, Yuan T, Ikura M. Calmodulin target database. J Struct Funct Genomics. 2000;1:8–14. doi: 10.1023/a:1011320027914. [DOI] [PubMed] [Google Scholar]
- 96.Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurobiol. 1998;8:321–9. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- 97.Bond CT, Maylie J, Adelman JP. SK channels in excitability, pacemaking and synaptic integration. Curr Opin Neurobiol. 2005;15:305–11. doi: 10.1016/j.conb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 98.Dunn PM, Benton DC, Campos Rosa J, Ganellin CR, Jenkinson DH. Discrimination between subtypes of apamin-sensitive Ca(2+)-activated K+ channels by gallamine and a novel bis-quaternary quinolinium cyclophane, UCL 1530. Br J Pharmacol. 1996;117:35–42. doi: 10.1111/j.1476-5381.1996.tb15151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Galanakis D, Davis CA, Del Rey Herrero B, Ganellin CR, Dunn PM, Jenkinson DH. Synthesis and structure-activity relationships of dequalinium analogues as K+ channel blockers. Investigations on the role of the charged heterocycle. J Med Chem. 1995;38:595–606. doi: 10.1021/jm00004a005. [DOI] [PubMed] [Google Scholar]
- 100.Galanakis D, Ganellin CR, Dunn PM, Jenkinson DH. On the concept of a bivalent pharmacophore for SKCa channel blockers: synthesis, pharmacological testing, and radioligand binding studies on mono-, bis-, and tris-quinolinium compounds. Arch Pharm (Weinheim) 1996;329:524–8. doi: 10.1002/ardp.19963291203. [DOI] [PubMed] [Google Scholar]
- 101.Sah P. Ca(2+)-activated K+ currents in neurones: types, physiological roles and modulation. Trends Neurosci. 1996;19:150–4. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- 102.Selyanko AA, Sim JA, Brown DA. Small (SKCa) Ca2+-activated K+ channels in cultured rat hippocampal pyramidal neurones. Pflugers Arch. 1998;437:161–3. doi: 10.1007/s004240050762. [DOI] [PubMed] [Google Scholar]
- 103.Villalobos C, Shakkottai VG, Chandy KG, Michelhaugh SK, Andrade R. SKCa channels mediate the medium but not the slow calcium-activated afterhyperpolarization in cortical neurons. J Neurosci. 2004;24:3537–42. doi: 10.1523/JNEUROSCI.0380-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang GY, Robinson DW, Chalupa LM. Calcium-activated potassium conductances in retinal ganglion cells of the ferret. J Neurophysiol. 1998;79:151–8. doi: 10.1152/jn.1998.79.1.151. [DOI] [PubMed] [Google Scholar]
- 105.Xu Y, Tuteja D, Zhang Z, Xu D, Zhang Y, Rodriguez J, Nie L, Tuxson HR, Young JN, Glatter KA, Vazquez AE, Yamoah EN, Chiamvimonvat N. Molecular identification and functional roles of a Ca(2+)-activated K+ channel in human and mouse hearts. J Biol Chem. 2003;278:49085–94. doi: 10.1074/jbc.M307508200. [DOI] [PubMed] [Google Scholar]
- 106.Bhattacharya S, Bunick CG, Chazin WJ. Target selectivity in EF-hand calcium binding proteins. Biochim Biophys Acta. 2004;1742:69–79. doi: 10.1016/j.bbamcr.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 107.Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, Janowsky A, Fakler B, Adelman JP, Maylie J. Domains responsible for constitutive and Ca(2+)-dependent interactions between calmodulin and small conductance Ca(2+)-activated potassium channels. J Neurosci. 1999;19:8830–8. doi: 10.1523/JNEUROSCI.19-20-08830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Maylie J, Bond CT, Herson PS, Lee WS, Adelman JP. Small conductance Ca2+-activated K+ channels and calmodulin. J Physiol. 2004;554:255–61. doi: 10.1113/jphysiol.2003.049072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, Maylie J, Adelman JP. Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature. 1998;395:503–7. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]
- 110.Schumacher MA, Crum M, Miller MC. Crystal structures of apocalmodulin and an apocalmodulin/SK potassium channel gating domain complex. Structure (Camb) 2004;12:849–60. doi: 10.1016/j.str.2004.03.017. [DOI] [PubMed] [Google Scholar]
- 111.Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–4. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- 112.Kristian T, Siesjo BK. Calcium in ischemic cell death. Stroke. 1998;29:705–18. doi: 10.1161/01.str.29.3.705. [DOI] [PubMed] [Google Scholar]
- 113.Kitakaze M, Weisfeldt ML, Marban E. Acidosis during early reperfusion prevents myocardial stunning in perfused ferret hearts. J Clin Invest. 1988;82:920–7. doi: 10.1172/JCI113699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lee JA, Allen DG. Changes in intracellular free calcium concentration during long exposures to simulated ischemia in isolated mammalian ventricular muscle. Circ Res. 1992;71:58–69. doi: 10.1161/01.res.71.1.58. [DOI] [PubMed] [Google Scholar]
- 115.Varga AW, Yuan LL, Anderson AE, Schrader LA, Wu GY, Gatchel JR, Johnston D, Sweatt JD. Calcium-calmodulin-dependent kinase II modulates Kv4. 2 channel expression and upregulates neuronal A-type potassium currents. J Neurosci. 2004;24:3643–54. doi: 10.1523/JNEUROSCI.0154-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Putkey JA, Kleerekoper Q, Gaertner TR, Waxham MN. A new role for IQ motif proteins in regulating calmodulin function. J Biol Chem. 2003;278:49667–70. doi: 10.1074/jbc.C300372200. [DOI] [PubMed] [Google Scholar]