Abstract
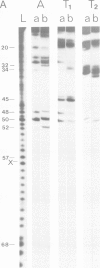
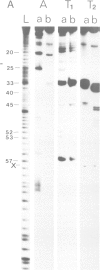
We have used RNases T1, T2 and A to digest two aminoacyl-tRNAs, Escherichia coli Phe-tRNAPhe and E. coli Met- tRNAMetm both in the naked forms and in ternary complexes with E. coli elongation factor Tu (EF-Tu) and GTP. An analysis of the 'footprinting' results has led to an interpretation that has localized the part of the three-dimensional structure of aminoacyl-tRNA covered by the protein in the ternary complex. In terms of the three-dimensional structure of tRNA established for yeast tRNAPhe, EF-Tu covers the aa-end, aa-stem, T-stem, and extra loop on the side of the L-shaped tRNA that exposes the extra loop.
Full text
PDF

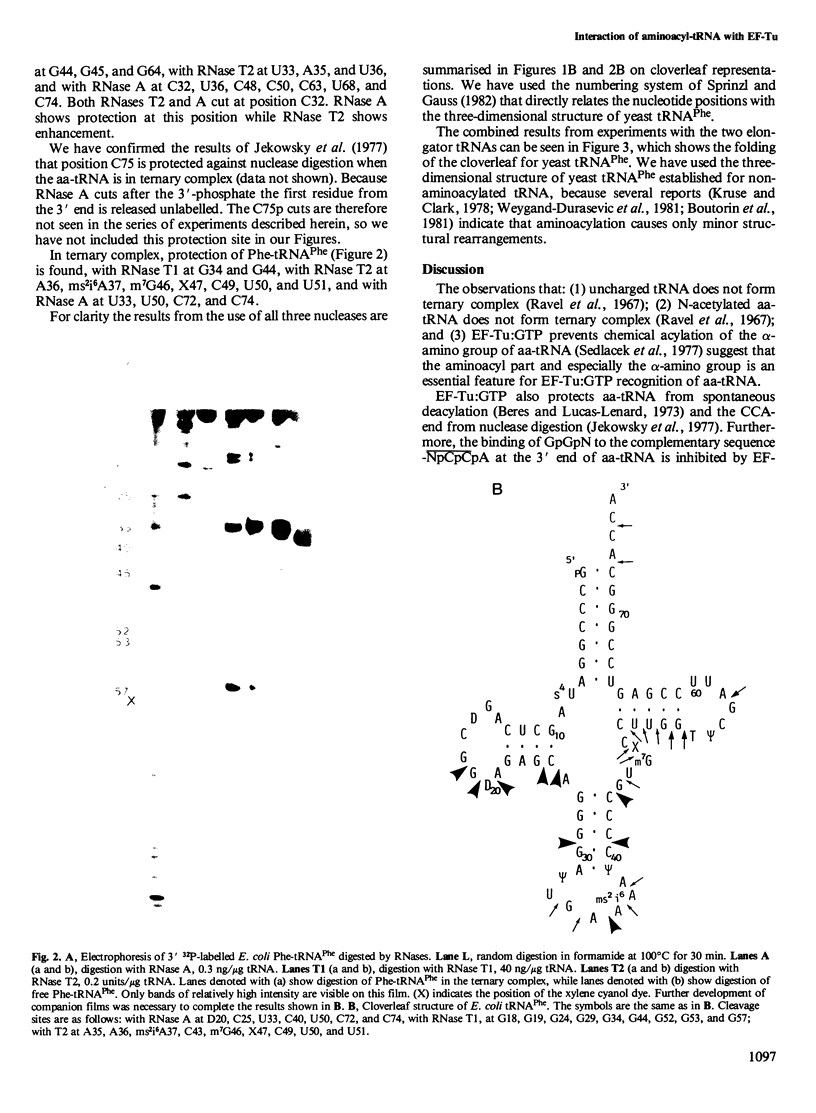
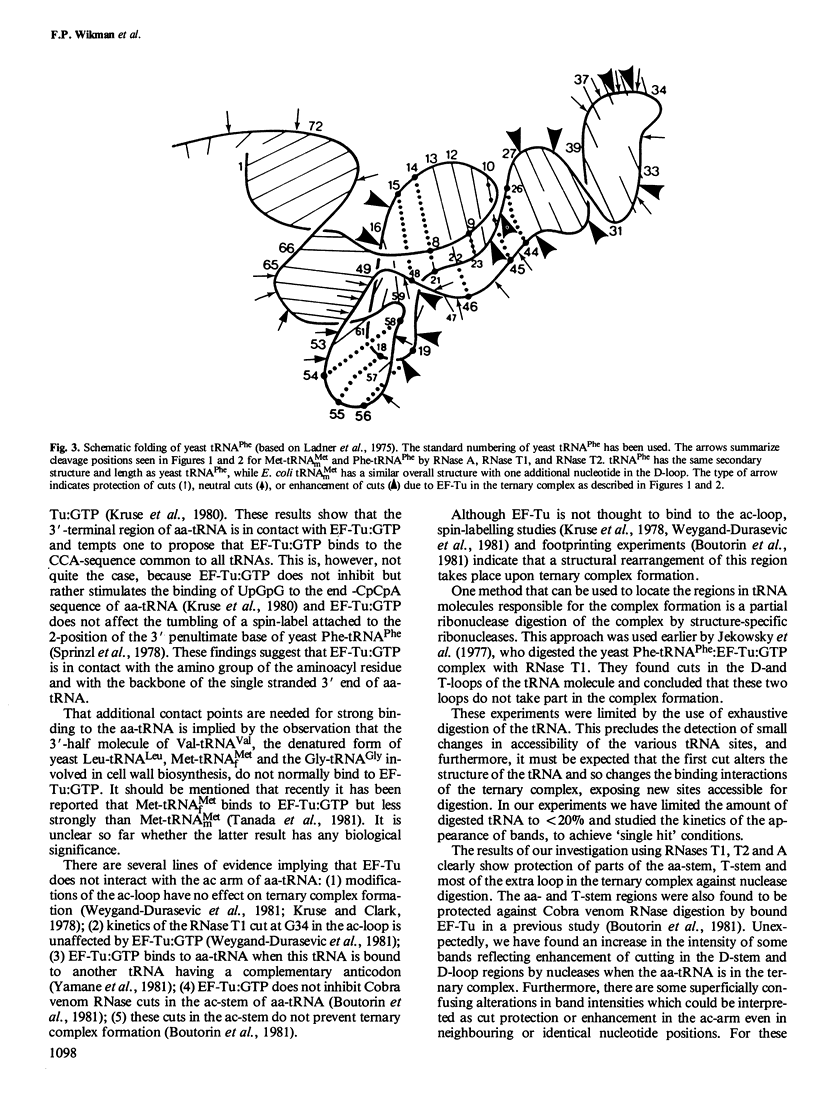


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beres L., Lucas-Lenard J. Studies on the fluorescence of the Y base of yeast phenylalanine transfer ribonucleic acid. Effect of pH, aminoacylation, and interaction with elongation factor Tu. Biochemistry. 1973 Sep 25;12(20):3998–4002. doi: 10.1021/bi00744a033. [DOI] [PubMed] [Google Scholar]
- Boutorin A. S., Clark B. F., Ebel J. P., Kruse T. A., Petersen H. U., Remy P., Vassilenko S. A study of the interaction of Escherichia coli elongation factor-Tu with aminoacyl-tRNAs by partial digestion with cobra venom ribonuclease. J Mol Biol. 1981 Nov 5;152(3):593–608. doi: 10.1016/0022-2836(81)90271-0. [DOI] [PubMed] [Google Scholar]
- Cory S., Marcker K. A., Dube S. K., Clark B. F. Primary structure of a methionine transfer RNA from Escherichia coli. Nature. 1968 Dec 7;220(5171):1039–1040. doi: 10.1038/2201039a0. [DOI] [PubMed] [Google Scholar]
- Deutscher M. P. Synthesis and functions of the -C-C-A terminus of transfer RNA. Prog Nucleic Acid Res Mol Biol. 1973;13:51–92. doi: 10.1016/s0079-6603(08)60100-2. [DOI] [PubMed] [Google Scholar]
- Jekowsky E., Schimmel P. R., Miller D. L. Isolation, characterization and structural implications of a nuclease-digested complex of aminoacyl transfer RNA and Escherichia coli elongation factor Tu. J Mol Biol. 1977 Aug 15;114(3):451–458. doi: 10.1016/0022-2836(77)90262-5. [DOI] [PubMed] [Google Scholar]
- Kruse T. A., Clark B. F., Appel B., Erdmann V. A. The structure of the CCA end of tRNA, aminoacyl-tRNA and aminoacyl-tRNA in the ternary complex. FEBS Lett. 1980 Aug 11;117(1):315–318. doi: 10.1016/0014-5793(80)80970-7. [DOI] [PubMed] [Google Scholar]
- Kruse T. A., Clark B. F. The effect of specific structural modification on the biological activity of E. coli arginine tRNA. Nucleic Acids Res. 1978 Mar;5(3):879–892. doi: 10.1093/nar/5.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner J. E., Jack A., Robertus J. D., Brown R. S., Rhodes D., Clark B. F., Klug A. Structure of yeast phenylalanine transfer RNA at 2.5 A resolution. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4414–4418. doi: 10.1073/pnas.72.11.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Elongation factor Tu and the aminoacyl-tRNA-EFTu-GTP complex. Methods Enzymol. 1974;30:219–232. doi: 10.1016/0076-6879(74)30024-9. [DOI] [PubMed] [Google Scholar]
- Pingoud A., Urbanke C., Krauss G., Peters F., Maass G. Ternary complex formation between elongation factor Tu, GTP and aminoacyl-tRNA: an equilibrium study. Eur J Biochem. 1977 Sep;78(2):403–409. doi: 10.1111/j.1432-1033.1977.tb11752.x. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Seeman N. C., Suddath F. L., Rich A., Sussman J. L., Kim S. H. Hydrogen bonding in yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4866–4870. doi: 10.1073/pnas.72.12.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel J. M., Shorey R. L., Shive W. Evidence for a guanine nucleotide-aminoacyl-RNA complex as an intermediate in the enzymatic transfer of aminoacyl-RNA to ribosomes. Biochem Biophys Res Commun. 1967 Oct 11;29(1):68–73. doi: 10.1016/0006-291x(67)90542-6. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Gauss D. H. Compilation of tRNA sequences. Nucleic Acids Res. 1982 Jan 22;10(2):r1–55. [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Siboska G. E., Pedersen J. A. Properies of tRNAPhe from yeast carrying a spin label on the 3'-terminal. Interaction with yeast phenylalanyl-tRNA Synthetase and elongation factor Tu from Escherichia coli. Nucleic Acids Res. 1978 Mar;5(3):861–877. doi: 10.1093/nar/5.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanada S., Kawakami M., Yoneda T., Takemura S. Interaction of initiator Met-tRNArMet (Escherichia coli) and Gly-tRNAIGly (Staphylococcus epidermidis) with bacterial elongation factor Tu:GTP complex. J Biochem. 1981 May;89(5):1565–1572. doi: 10.1093/oxfordjournals.jbchem.a133350. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., Clayton D. A. Altered mobility of polydeoxyribonucleotides in high resolution polyacrylamide gels due to removal of terminal phosphates. Nucleic Acids Res. 1981 Dec 21;9(24):6787–6794. doi: 10.1093/nar/9.24.6787. [DOI] [PMC free article] [PubMed] [Google Scholar]