Abstract
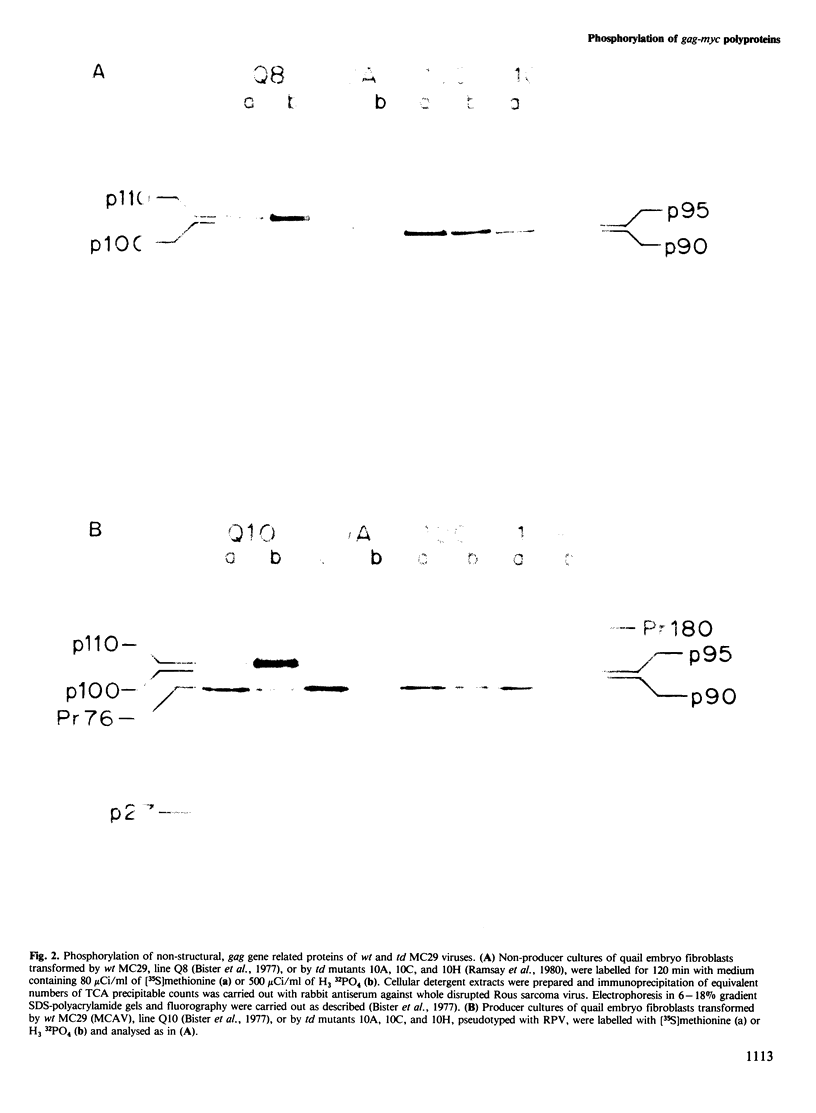
The putative transforming proteins of the four acute leukaemia viruses belonging to the MC29 subgroup were shown to be phosphorylated in vivo. Comparison of the MC29 and CM11 encoded phosphoproteins revealed identical tryptic phosphopeptide maps, with both the gag and myc domains being phosphorylated. In contrast, the MH2 phosphoprotein was only phosphorylated on the gag domain. Analysis of partial transformation-defective MC29 deletion mutants revealed that the deletions had removed the v-myc specific phosphopeptides. Phosphoamino acid analysis showed that these deleted phosphopeptides were phosphorylated on threonine. Moreover, a back mutant that had regained transforming ability had regained these phosphopeptides. These studies correlate the phosphorylation of the gag-myc protein with the transformation capability of the virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Beemon K. Transforming proteins of some feline and avian sarcoma viruses are related structurally and functionally. Cell. 1981 Apr;24(1):145–153. doi: 10.1016/0092-8674(81)90510-9. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Courtneidge S. A., Levinson A. D., Oppermann H., Quintrell N., Sheiness D. K., Weiss S. R., Varmus H. E. Origin and function of avian retrovirus transforming genes. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):919–930. doi: 10.1101/sqb.1980.044.01.099. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Genetic structure of avian acute leukemia viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):801–822. doi: 10.1101/sqb.1980.044.01.086. [DOI] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Lee W. H., Duesberg P. H. Phosphorylation of the nonstructural proteins encoded by three avian acute leukemia viruses and by avian fujinami sarcoma virus. J Virol. 1980 Nov;36(2):617–621. doi: 10.1128/jvi.36.2.617-621.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Löliger H. C., Duesberg P. H. Oligoribonucleotide map and protein of CMII: detection of conserved and nonconserved genetic elements in avian acute leukemia viruses CMII, MC29, and MH2. J Virol. 1979 Oct;32(1):208–219. doi: 10.1128/jvi.32.1.208-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Ramsay G. M., Hayman M. J. Deletions within the transformation-specific RNA sequences of acute leukemia virus MC29 give rise to partially transformation-defective mutants. J Virol. 1982 Mar;41(3):754–766. doi: 10.1128/jvi.41.3.754-766.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Ramsay G., Hayman M. J., Duesberg P. H. OK10, an avian acute leukemia virus of the MC 29 subgroup with a unique genetic structure. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7142–7146. doi: 10.1073/pnas.77.12.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiswell D. J., Ramsay G., Hayman M. J. Two virus-specific rna species are present in cells transformed by defective leukemia virus OK10. J Virol. 1981 Oct;40(1):301–304. doi: 10.1128/jvi.40.1.301-304.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Erikson E., Brugge J. S., Erikson R. L. Phosphorylated and nonphosphorylated forms of avian sarcoma virus polypeptide p19. Virology. 1977 Jul 1;80(1):177–185. doi: 10.1016/0042-6822(77)90390-7. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H., von Kirchbach A., Hayman M. J. Three new types of viral oncogenes in defective avian leukemia viruses. II. Biological, genetic, and immunochemical evidence. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1225–1234. doi: 10.1101/sqb.1980.044.01.133. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Kitchener G., Graf T. Cells transformed by avian myelocytomatosis virus strain CMII contain a 90K gag-related protein. Virology. 1979 Oct 15;98(1):191–199. doi: 10.1016/0042-6822(79)90537-3. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Moscovici C., Vogt P. K. The defectiveness of Mill Hill 2, a carcinoma-inducing avian oncovirus. Virology. 1978 Aug;89(1):162–178. doi: 10.1016/0042-6822(78)90049-1. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitchener G., Hayman M. J. Comparative tryptic peptide mapping studies suggest a role in cell transformation for the gag-related protein of avian erythroblastosis virus and avian myelocytomatosis virus strains CMII and MC29. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1637–1641. doi: 10.1073/pnas.77.3.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G. M., Hayman M. J. Isolation and biochemical characterization of partially transformation-defective mutants of avian myelocytomatosis virus strain MC29: localization of the mutation to the myc domain of the 110,000-dalton gag-myc polyprotein. J Virol. 1982 Mar;41(3):745–753. doi: 10.1128/jvi.41.3.745-753.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Graf T., Hayman M. J. Mutants of avian myelocytomatosis virus with smaller gag gene-related proteins have an altered transforming ability. Nature. 1980 Nov 13;288(5787):170–172. doi: 10.1038/288170a0. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Hayman M. J. Analysis of cells transformed by defective leukemia virus OK10: production of noninfectious particles and synthesis of Pr76gag and an additional 200,000-dalton protein. Virology. 1980 Oct 15;106(1):71–81. doi: 10.1016/0042-6822(80)90222-6. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Rubin C. S., Rosen O. M. Protein phosphorylation. Annu Rev Biochem. 1975;44:831–887. doi: 10.1146/annurev.bi.44.070175.004151. [DOI] [PubMed] [Google Scholar]
- Stéhelin D., Saule S., Roussel M., Sergeant A., Lagrou C., Rommens C., Raes M. B. Three new types of viral oncogenes in defective avian leukemia viruses. I. Specific nucleotide sequences of cellular origin correlate with specific transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1215–1223. doi: 10.1101/sqb.1980.044.01.132. [DOI] [PubMed] [Google Scholar]