Abstract
Medicinal chemistry has produced small-molecule agents with drug-like character that potently and safely modulate the activity of discrete endocannabinoid system components as potential treatments for medical disorders, including various psychiatric conditions. Two cannabinoid (CB) receptors (CB1 and CB2) currently represent prime endocannabinoid-system therapeutic targets for ligands that either mimic endocannabinoid signalling processes and/or potentiate endocannabinoid-system activity (agonists) or attenuate pathologically heightened endocannabinoid-system transmission (antagonists). Two endocannabinoid deactivating enzymes, fatty acid amide hydrolase (FAAH) and soluble monoacylglycerol lipase (MGL), are increasingly prominent targets for inhibitors that indirectly potentiate endocannabinoid-system signalling. Continued profiling of drug candidates in relevant disease models, identification of additional cannabinoid-related therapeutic targets, and validation of new pharmacological modes of endocannabinoid system modulation will undoubtedly invite further translational efforts in the cannabinoid field for treating psychiatric disorders and other medical conditions.
Introduction
Pharmacotherapeutic tuning of endocannabinoid signalling as a means to treat psychiatric disorders is supported by several lines of evidence. Some plant cannabinoids (CBs) (phytocannabinoids) isolated from Cannabis sp. exert neurobiological, behavioural, and/or psychological (e.g. anxiolytic, antipsychotic) actions in experimental animals and man (Leweke & Koethe, 2008; Pertwee, 2008). Such biological effects mainly reflect the phytocannabinoid agonist (activating ligand) property at two major CB G protein-coupled receptor (GPCR) subtypes, designated CB1 and CB2 (Woelkart, Salo-Ahen, & Bauer, 2008). Endogenous CB-receptor agonists (endocannabinoids) and synthetic CB-receptor ligands influence diverse neurological, psychological, and behavioural processes in health and disease (Drago, 2007; Janero & Makriyannis, 2007; Moriera & Lutz, 2008). Endocannabinoid signalling is also intrinsically neuroprotective (Karanian et al., 2007).
This article highlights exemplary natural and synthetic compounds that modulate prime druggable targets within the endocannabinoid system. Emphasis is placed on agents having application to psychiatric, neurobiological, and/or behavioural indications.
Phytocannabinoids
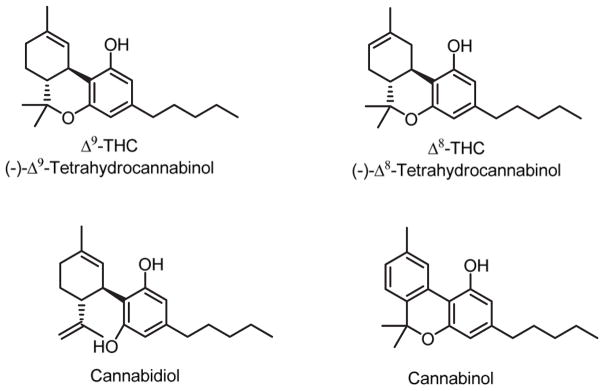
Cannabis contains some 70 unique phytocannabinoids, most prominently the ‘classical cannabinoids’ (−)-Δ -9-tetrahydrocannabinol (Δ-9-THC), (−)- Δ-8-tetrahydrocannabinol (Δ-8-THC), cannabidiol, and cannabinol (Thakur, Duclos, & Makriyannis, 2005; Woelkart et al., 2008) (Figure 1). The archtypical phytocannabinoid and the main psychotrophic constituent of cannabis, Δ-9-THC is a fused-ring tricyclic terpenoid derivative incorporating a polar benzopyran ring with a terminal, hydrophobic alkyl (n-pentyl) side-chain, a characteristic lipophilic domain, and hydrogen-bonding substituents (Thakur et al., 2005). Δ-9-THC can activate the CB1 receptor as a partial agonist, binding to both CB1 and CB2 receptors with low nanomolar affinity (Pertwee, 2008). Among its wide-ranging effects, Δ-9-THC can influence brain function and neuropsychological performance and may induce some behavioural and cognitive changes reminiscent of psychiatric disorders including depression and anxiety (Gonzalez, 2007). The psychotrophic effects of cannabis are believed mainly to reflect Δ-9-THC activation of CNS presynaptic CB1 receptors (Pertwee, 2008). Δ-8- and Δ-9-THC are virtually equivalent as to CB-receptor affinity and pharmacological activity, although Δ-8-THC is the more chemically stable isomer (Charalambous et al., 1991; Thakur et al., 2005).
Figure 1.
Structures of the principal phytocannabinoids.
In contrast to THC, the phytocannabinoids cannabinol and cannabidiol display significantly lower affinity for CB receptors, modest CB2-receptor selectivity, and little or no psychotropic activity (Pertwee, 2008; Woelkart et al., 2008). Aside from its anti-inflammatory effects, cannabidiol is neuro-protective, antipsychotic, and anxiolytic, and preliminary studies show that it reduces schizophrenic symptoms in patients (Mechoulam, Peters, Murillo-Rodriguez, & Hanuš, 2007; Pertwee, 2008).
Over the past 40 years, some two dozen randomized controlled clinical trials (exclusive of studies on recreational cannabis use in humans) have been conducted with medical cannabinoid-based preparations, often containing Δ-9-THC with or without cannabidiol. Although the principal indications addressed were non-psychiatric (e.g. pain, multiple sclerosis), the most frequently reported adverse events in medicinal cannabinoid trials were ‘nervous system’ and/or ‘altered mood’ disorders (Wang, Collet, Shapiro, & Ware, 2008). The few cannabinoid-related marketed pharmaceuticals have a direct relationship to cannabis and, hence, act as non-selective agonists at both CB1 and CB2 receptors to treat nausea and emesis (Di Marzo, Bifulco, & De Petrocellis, 2004). Regarding discrete psychiatric applications, clinical studies have recently been completed to evaluate cannabidiol as an antipsychotic agent (University of Cologne, 2006). A clinical trial is ongoing for a mixture of Δ-9-THC and cannabidiol in bipolar affective disorder (University of British Columbia, 2006). Human trials are planned testing cannabidiol in schizophrenic cognitive dysfunction (Yale University, 2008).
Synthetic CB1/CB2-receptor agonists
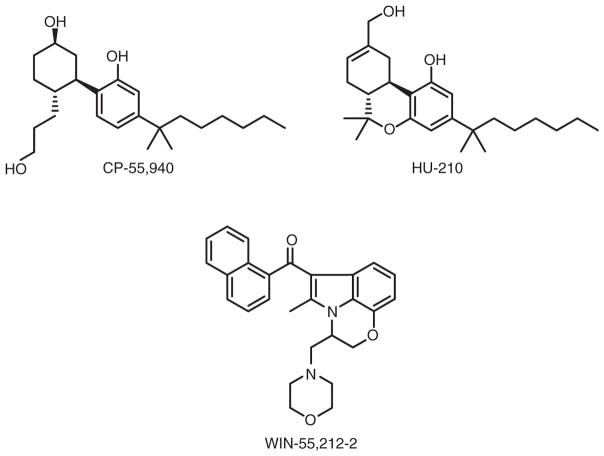
In an approach towards structural simplicity and improved agonist activity as compared to Δ-9-THC, bicyclic ‘nonclassical cannabinoids’ lacking the hallmark CB dihydropyran ring were first synthesized by Pfizer (Makriyannis & Rapaka, 1990). The most thoroughly studied bicyclic compound, (−)-CP-55,940, shows high affinity and efficacy at both CB1 and CB2 receptors and increased potency with reduced lipophilicity relative to Δ-9-THC (Herkenham et al., 1990) (Figure 2). Similar to Δ-9-THC, the C-3 alkyl side-chain and phenolic hydroxyl are pharmacophoric elements crucial to the biological activity of CP-55,940 and related open pyran ring non-classical cannabinoids (Ashton, Wright, McPartland, & Tyndall, 2008). As exemplified by CP-55,940 and HU-210 (Figure 2), a hydroxyl group at the C-9 or C-11 enhances cannabinoid affinity and potency for both CB receptors (Mechoulam et al., 1988).
Figure 2.
Structures of synthetic CB1/CB2-receptor agonists.
Although bearing no structural relationship to cannabinoids, the aminoalkylindole WIN-55,212-2 (Figure 2) engages CB receptors (Bell et al., 1991). The aminoalklyindoles are less lipophilic than classical and non-classical CB-receptor ligands (Ashton et al., 2008). The (~) enantiomer of WIN-55,212-2 is a potent CB1 and CB2 agonist with high (nanomolar) CB-receptor affinity and moderate (~5–10-fold) preference for the human CB2 versus CB1 receptor (Ashton et al., 2008; Reggio et al., 1998). The 3-aroyl moiety and the 1-chain of WIN-55,212-2 are important for its cannabinergic activity (Xie, Eissenstat, & Makriyannis, 1995).
These synthetic CB1/CB2-receptor agonists have been used experimentally to investigate how CB signalling affects learning/memory and behaviour (Bambico, Katz, Debonnel, & Gobbi, 2007) and substance abuse (Vinod et al., 2008).
Synthetic selective CB2-receptor agonists
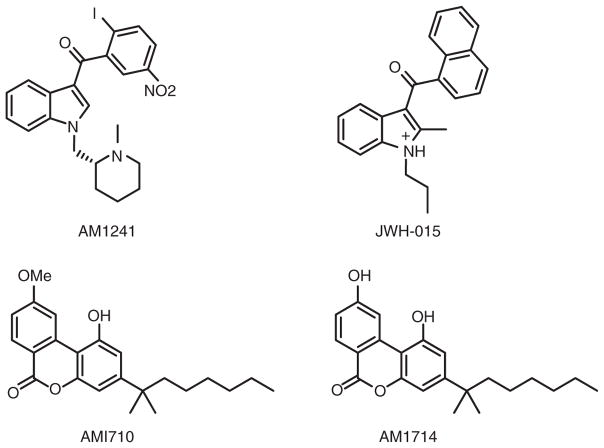
The moderate CB2-receptor selectivity of WIN-55,212-2 and the very limited CB2 receptor expression in brain, which would be expected to render CB2-receptor agonists largely devoid of the central psychotrophic effects, to encouraged the rational design of highly selective CB2-receptor agonists (Malan et al., 2003). This effort first met success with AM1241 (Ibrahim et al., 2003) (Figure 3), a racemic aminoalkylindole with an N-methylpiperidinyl-2-methyl substituent at the N-1 position and a 2-iodo-5-nitrobenzoyl group as the C-3 indole substituent. AM1241 can be crystallized and exhibits high binding potencies (i.e. low nanomolar Ki values) and selectivities of 110-fold and 34-fold for the human and rat CB2 over the CB1 receptor, respectively (Ibrahim et al., 2003; Malan et al., 2001). Preclinical data show that AM1241 exerts peripheral analgesia and potent full agonist activity in vivo (Malan et al., 2001).
Figure 3.
Structures of synthetic selective CB2-receptor agonists.
As summarized (Marriott and Huffman, 2008), efforts to develop structure-activity relationships in the indole CB2-receptor agonist class have met with limited success. JWH-015 is an indole in which the WIN-55,212-2 morpholine ring has been replaced by a short alkyl tail (Figure 3). JWH-015 exhibits low nanomolar affinity for the CB2 receptor, 3- to 10-fold selectivity at the human CB2 versus CB1 receptor, and ~3-fold greater affinity for the human versus rat CB2 receptor (Mukherjee et al., 2004). In laboratory animals, JWH-015 exerts anti-inflammatory, immunosuppressive, and analgesic effects without psychotropic liability or tolerance (Lombard, Nagarkatti, & Nagarkatti, 2007; Romero-Sandoval, Nutile-McMenemy, & DeLeo, 2008) and shows efficacy in neurodegeneration models (Ehrhart et al., 2005).
Two novel planar ring cannabilactones, AM1710 and AM1714, have emerged as selective CB2-receptor agonists (Khanolkar et al., 2007) (Figure 3), AM1714 displaying the greater (490-fold) selectivity and subnanomolar CB2-receptor affinity. Pronounced species-dependent affinity and selectivity favouring the rat versus the human cannabinoid CB2 receptor have been observed with AM1710 and AM1714 (Khanolkar et al., 2007; Mukherjee et al., 2004). Devoid of CB1 receptor-mediated side effects, both AM1710 and AM1714 exert peripheral analgesic activity in animal models of neuropathic pain (Khanolkar et al., 2007).
Synthetic selective CB1-receptor agonists
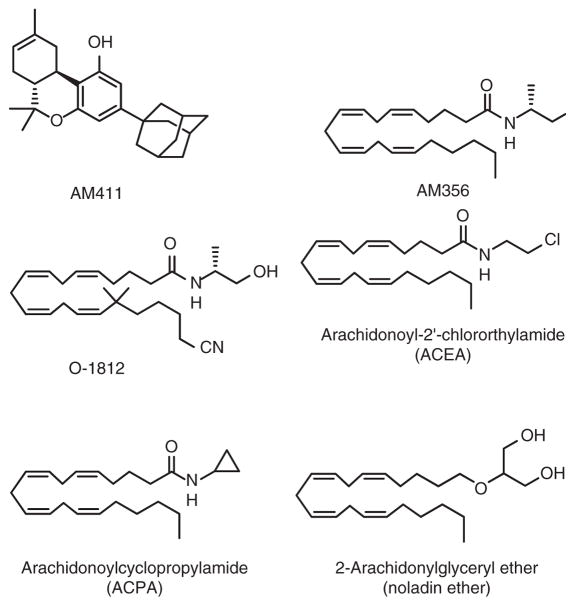
Cyclic variations in the C-3 alkyl side chain of classical cannabinoids led to prototypic compounds with some CB1-receptor selectivity. Of these, the synthetic adamantyl analog AM411 (Figure 4) was the first pharmacologically active classical cannabinoid to be crystallized (Lu et al., 2005). AM411 demonstrates low nanomolar affinity and ~8-fold selectivity as a CB1-receptor agonist without eliciting rapid receptor desensitization (Lu et al., 2005; Luk et al., 2004).
Figure 4.
Structures of synthetic selective CB1-receptor agonists.
Another approach to CB1 receptor-selective agonists enhanced the marginal selectivity of the endocannabinoid ananadmide (AEA). This approach yielded the metabolically more stable chiral AEA analog, AM356 [R-(+)-methanandamide] (Figure 4), which is ~50-fold selective for the CB1 versus CB2 receptor with ~4-fold greater CB1-receptor affinity than AEA itself (Abadji et al., 1994; Lin et al., 1998). A cyano analog of AM356 (O-1812), along with the readily hydrolizable arachidonoyl-2′-chloroethylamide (ACEA) and arachidonoylcyclopropylamide (ACPA) and the putative endocannabinoid 2-arachidonoylglyceryl ether (noladin ether) (Figure 4), are other AEA analogs with reasonably high (~40- to 1500-fold) CB1-receptor agonist selectivities relative to the CB2 receptor and low nM CB1-receptor affinities. In general, these CB1-receptor agonists exert cannabimimetic effects in vivo (Di Marzo et al., 2001; Hanuš et al., 2001; Hillard et al., 1999).
Synthetic selective CB1-receptor antagonists/inverse agonists and neutral antagonists
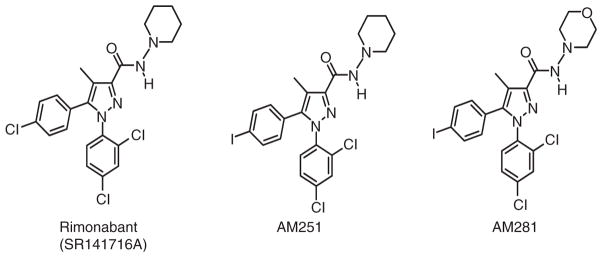
CB1-receptor antagonists have the potential to treat a number of persistent global healthcare problems (e.g. substance abuse disorders, overweight/obesity, metabolic syndrome) often accompanied by co-morbid psychological conditions ( Jagerovic, Fernandez-Fernandez, & Goya, 2008; Janero & Makriyannis, 2007; Lange & Kruse, 2008; Vemuri, Janero, & Makriyannis, 2008). Among the first selective CB1-receptor antagonists, the diarylpyrazole analogue rimonabant (SR141716A) (Figure 5) engages the CB1 receptor with low nanomolar affinity and ~150-fold selectivity versus the CB2 receptor (Rinaldi-Carmona et al., 1994). The C-3 piperidinylamide group, the N-1 dichlorophenyl substituent, and the C-5 phenyl ring contribute to rimonabant’s high CB1-receptor affinity and selectivity (Jagerovic et al., 2008). Rimonabant’s suppression of appetite leading to weight loss in adult, non-obese rats (Colombo et al., 1998) incited an intense search for other novel CB1-receptor antagonists and set rimonabant itself on the path to eventual approval outside the USA as a weight control drug. However, rimonabant has been withdrawn from some major markets due to prominent side effects including nausea and psychiatric/mood disorders, and all rimonabant clinical studies have been halted (Rumsfeld & Nallamothu, 2008; Sanofi-Aventis, 2008a, 2008b). No data have yet appeared in the referenced clinical literature as to the safety or efficacy of most other CB1-receptor antagonists (i.e. surinabant, ibipinabant, AVE1625, otenabant, rosonabant). Adverse psychological effects similar to rimonabant’s have been associated with the CB1-receptor antagonist taranabant, whose development programme has been discontinued (Fulmer, 2008; Merck, 2008). A similar corporate decision was rendered for otenabant (Pfizer, 2008).
Figure 5.
Structures of synthetic selective CB1-receptor antagonists/inverse agonists.
Replacement of rimonabant’s 4-chloro group with a 4-iodo substituent generated AM251 (Figure 5), which exhibits improved (low nanomolar) CB1-receptor affinity and selectivity (~300-fold) with respect to the CB2 receptor (Gatley et al., 1997). A related CB1-receptor antagonist, AM281 (Figure 5), was derived by substituting the N-(piperidin-1-yl) moiety of rimonabant with an N-(morpholin-4-yl) group. AM281 has low nano-molar affinity for the CB1-receptor comparable to that of AM251, but with greater CB1-receptor (~350-fold) selectivity, oral bioavailability, and brain penetration (Gatley et al., 1998).
Rimonabant, AM251, and AM281 have served as tool compounds for investigations that have enhanced our appreciation of overactive CB1-receptor transmission in the etiology of disorders (overweight/obesity, drug addiction, substance abuse) having a reward-supported appetitive component (Muccioli, 2007).
Traditional pharmacology functionally classifies receptor ligands as either agonists, which activate receptors, or neutral antagonists, which block agonist receptor activation but do not elicit a biological response themselves. Accumulating evidence indicates that GPCRs, including the CB1 receptor, display agonist-independent constitutive activity (Canals & Milligan, 2008). In certain test systems in vitro, many ligands previously classified as neutral antagonists demonstrate ‘negative efficacy’ on constitutive GPCR activity – i.e. an ‘inverse agonist’ property. Biochemical profiling of rimonabant and most other CB1-receptor antagonists indicates that they act as antagonists/inverse agonists, not as truly neutral antagonists, and as such may affect constitutive endocannabinoid signalling in the absence of CB1-receptor stimulation, promoting signal transduction responses opposing those of agonists (Hodge et al., 2008; Hurst et al., 2002). Translational interest rests with the proposition that at least some untoward side effects of CB1-receptor antagonists/inverse agonists noted in clinical trials (e.g. nausea, adverse psychological responses) may reflect their functional suppression of basal CB1-receptor signalling (i.e. their inverse-agonist property) (Bergman et al., 2008). AM4113, a pyrazole analogue related to rimonabant (structure undisclosed), has emerged as the first well-characterized CB1-receptor neutral antagonist and has shown impressive preclinical effects in suppressing food intake and food-reinforced behaviour without inducing signs of nausea or emesis (Salamone, McLaughlin, Sink, Makriyannis, & Parker, 2007; Sink et al., 2008). If CB1-receptor neutral antagonists were to suppress food intake without producing nausea and undesirable psychological side effects in humans, they would offer concrete therapeutic advantage over CB1-receptor antagonists/inverse agonists such as rimonabant and taranabant.
Restricting blood-brain barrier penetration of CB1-receptor antagonists so as to favour their peripheral action might improve their safety by reducing the opportunity for adverse, centrally mediated side effects (Kunos, Osei-Hyiaman, Bátaki, Sharkey, & Makriyannis, 2008). This approach appears from initial laboratory data potentially attractive for treating metabolic syndrome, insulin resistance/glucose intolerance, and steatosis (Osei-Hyiaman et al., 2008).
Synthetic selective CB2-receptor antagonists/inverse agonists
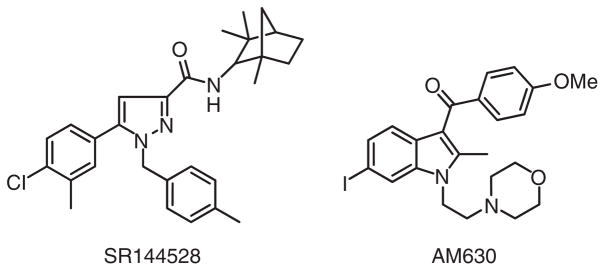
The best known selective CB2-receptor antagonist/inverse agonist, SR144528, was developed by Sanofi around a pyrazole moiety and displays ~700-fold selectivity and sub-nanomolar affinity for the CB2 versus CB1 receptor (Bouaboula, Dussossoy, & Casellas, 1999; Rinaldi-Carmona et al., 1998) (Figure 6). The CB2-receptor selectivity of SR144528 is partly conferred by its 4-methylbenzyl group (Rinaldi-Carmona et al., 1998). Another high-profile CB2-receptor antagonist/inverse agonist, AM630, contains a 6-iodoindole moiety and exhibts a 170-fold CB2- versus CB1-receptor selectivity (Pertwee et al., 1995) (Figure 6). Preclinical data suggest the potential therapeutic utility of CB2-receptor antagonists in inflammatory and allergic syndromes (Ashton & Glass, 2007).
Figure 6.
Structures of synthetic selective CB2-receptor antagonists/inverse agonists.
Endocannabinoids
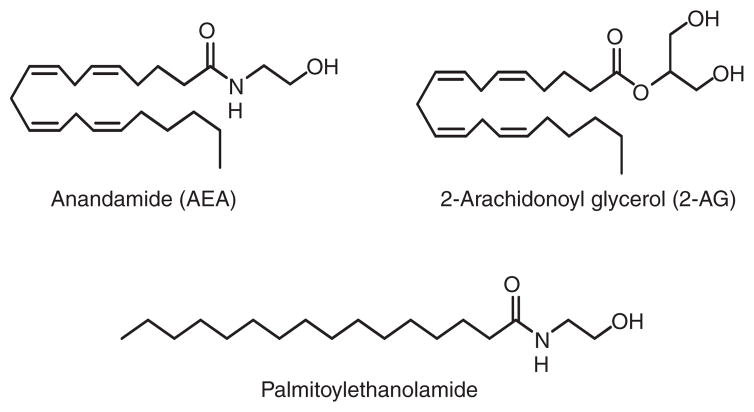
AEA and 2-arachidonoyl glycerol (2-AG) are the most extensively studied endocannabinoids (Figure 7). AEA is a partial CB1-receptor agonist with modest affinity (Ki = 61 nM (rat) and 240 nM (human)) and a relatively weak CB2-receptor ligand (Ki = 440–1930 nM for rodent and human CB2 receptors) with low overall efficacy (Lin et al., 1998; McPartland, Glass, & Pertwee, 2007). Produced in much greater amounts than AEA, 2-AG is a full agonist that binds to both CB1 and CB2 receptors with lower affinity (Ki = 472 and 1400 nM, respectively), but with greater efficacy, relative to AEA (Mechoulam et al., 1995; Thakur et al., 2005). Common structural features of both the phytocannabinoid Δ-9-THC and the endocannabinoids AEA and 2-AG, including a polar head group and a hydrophobic chain with a terminal n-pentyl moiety, allow both classes of compounds to share similar CB1-receptor binding motifs (Thakur et al., 2005). Among their myriad roles, AEA and 2-AG participate in neuromodulation as retrograde synaptic messengers and immune-cell function (Di Marzo & Petrosino, 2007).
Figure 7.
Structures of the principal endocannabinoids.
First identified decades ago (Bachur, Masek, Melmon, & Udenfriend, 1965), N-palmitoylethanolamide (PEA) (Figure 7) is a saturated N-acylamide AEA congener more abundant than AEA in most tissues that shares some actions with Δ-9-THC (Mackie & Stella, 2006). PEA synthesis and inactivation involve metabolic routes distinct from those of 2-AG and AEA (Tsuboi et al., 2005). At physiologically relevant concentrations, PEA does not bind to rat or human CB1 and CB2 receptors (Lambert et al., 1999), whereas it activates the GPR55 receptor in the nanomolar range (Pertwee, 2007). Nonetheless, PEA exhibits many pharmacological properties reminiscent of a CB2-receptor agonist (Lambert & Di Marzo, 1999). Synthesized in cells during tissue damage and inflammation, PEA exerts tissue-protective anti-inflammatory actions and relieves neurogenic and neuropathic pain (Lambert, Vandevoorde, Jonsson, & Fowler, 2002; Re, Barbero, Miolo, & Di Marzo, 2007). Although its mode of action is as yet undefined, PEA has been cited as the subject of clinical trials for the treatment of chronic lumbrosiatalgia and multiple sclerosis (Lambert et al., 2002). PEA may also find therapeutic use in stroke and chronic inflammatory central nervous system disorders such as Alzheimer’s, Huntington’s, and Parkinson’s diseases (Carbonare et al., 2008; Schomacher, Müller, Sommer, Schwab, & Schäbitz, 2008).
Fatty acid amide hydrolase (FAAH) inhibitors
Given AEA’s greater affinity for the CB1 versus CB2 receptor and the role of FAAH in catalytic AEA inactivation (Ahn, McKinney, & Cravatt, 2008), a FAAH inhibitor should potentiate signalling through the CB1 receptor. A major attraction of such an indirect approach for enhancing endocannabinoid activity rests with its potential to offer ‘site- and event-specific’ therapeutic relief in those tissues where endocannabinoids are being released as part of a physiological protective mechanism. In contrast, direct agonist activation of all accessible CB receptors indiscriminately may invite unwanted CB receptor-mediated psychotrophic effects (Gonzalez, 2007; Moriera & Lutz, 2008; Pertwee, 2008).
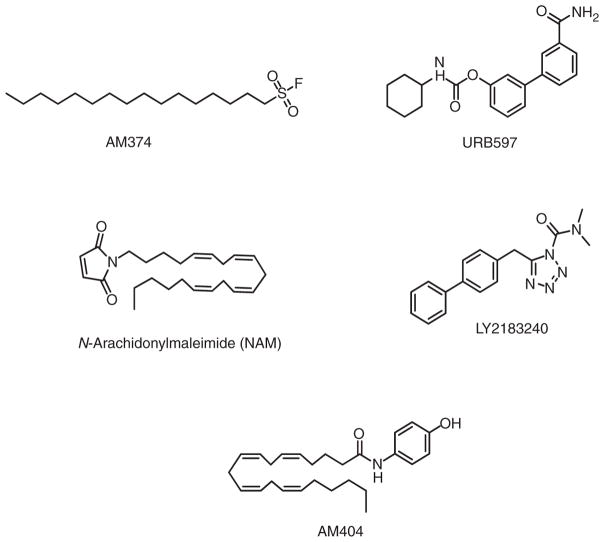
Palmitylsulfonyl fluoride (AM374) inhibits FAAH irreversibly at low nanomolar concentrations (Figure 8). However, AM374 also acts on the CB1 receptor at micromolar concentrations as well as E. coli outer-membrane phospholipase A (Deutsch et al., 1997). AM374 effectively potentiates anandamide concentrations and signalling in vitro and in vivo, thereby providing neuroprotection and functional protection against excitotoxic brain injury in rats (Gifford et al., 1999; Karanian et al., 2007) and reducing hyperkinesia in a model of Huntington’s disease (Lastres-Becker et al., 2003).
Figure 8.
Structures of FAAH (AM374, URB597, LY2183240), MGL (LY2183240, NAM) and endocannabinoid transport (AM404) inhibitors.
The carbamate ester URB597 (Figure 8) inhibits FAAH at low nanomolar concentrations (Fegley et al., 2005) as well as multiple mouse and human serine hydrolases (Ahn et al., 2008). URB597 potency for FAAH is modulated by the shape of its rigid aromatic biphenyl moiety. Carbamates such as URB597 irreversibly inactivate FAAH through carbamylation of the enzyme’s active-site serine 241 residue (Alexander & Cravatt, 2005). URB597 effectively enhances endogenous brain levels of AEA (and congeners) and elicits anxiolytic, analgesic, and antinociceptive effects without inducing cardinal cannabimimetic responses (e.g., catalepsy, hypothermia, hypomotility, hyperphagia) characteristic of CB1-receptor agonists (Jayamanne et al., 2006). FAAH inhibition may thus represent an innovative approach to managing pain and mood disorders/depression. URB597 is devoid of reinforcing effects in primates, which distinguishes it from direct-acting CB agonists such as Δ-9-THC and suggests that FAAH inhibitors might be used therapeutically without abuse liability or triggering a drug relapse in patients with a substance abuse disorder (Gaetani, Cuomo, & Piomelli, 2003). Phase-I clinical testing of URB597 has been noted (Labar & Michaux, 2007).
The heterocyclic urea compound LY2183240 (Figure 8) targets covalently mouse-brain FAAH (IC50 ~ 13 nM) and other brain serine hydrolases (Alexander & Cravatt, 2006). Analogous to the mechanism of action of URB597, LY2183240 inactivates FAAH by carbamylating a critical serine (Alexander & Cravatt, 2005). In laboratory animals, LY2183240 increases brain AEA concentrations and exerts analgesic and antinociceptive effects (Dickason-Chesterfield et al., 2006).
Monoacylglycerol lipase (MGL) inhibitors
N-Arachidonoylmaleimide (NAM) was modelled after the 2-AG substrate MGL to target MGL sulfhydryl group(s) at (or near) the enzyme’s catalytic site (Saario et al., 2005) (Figure 8). NAM inhibits rat-membrane MGL with an IC50 of 0.14 μM, as compared to an IC50 of 3.3 μM for FAAH inhibition. NAM inhibits human MGL by alkylating cysteine residues 215 and/or 249, cysteine 249 being of paramount importance for catalysis (Zvonok et al., 2008).
LY2183240 (as a mixture of its two isomers) (Figure 8) inhibits recombinant mouse MGL in a time-dependent manner (IC50 ~5.3 nM) some-what more potently than it inhibits FAAH (IC50 ~13 nM) (Alexander & Cravatt, 2006) (vide supra). The 2,5-isomer (AM6701) is a more potent inhibitor of human recombinant MGL than the 1,5-isomer (AM6702) (IC50 = 0.9 versus 9.1 nM, respectively) (Zvonok et al., 2008). MGL inhibition by AM6701 involves a covalent interaction resulting in serine carbamylation in the MGL catalytic triad (Zvonok et al., 2008). In vivo, LY2183240 exerts antinociceptive effects (Maione et al., 2008).
Inhibitors of endocannabinoid transport
As the first novel inhibitor of endocannabinoid transport into cells, AM404 (Figure 8) inhibits cellular AEA uptake at low micromolar concentrations and increases AEA concentrations in plasma and, in some cases, select brain areas when administered to experimental animals (Bortolato et al., 2006; Giuffrida, Rodriguez de Fonseca, Nava, Loubet-Lescoulié, Piomelli, 2000). AM404 may also inhibit FAAH, bind to CB1 receptors, and interact weakly with other targets (Pertwee 2005; Rawls, Ding, & Cowan, 2006). Structural analogues of AM404 show marginal, if any, improvement in selectivity or potency (Pertwee, 2005). AM404 potentiates and prolongs many of the physiological and behavioural effects of AEA (Solinas et al., 2007) and has anxiolytic properties in rodents (Bortolato et al., 2006). Since a discrete endocannabinoid transport protein has not been isolated or expressed, the molecular mechanism underlying these effects should be approached cautiously (Fowler et al., 2004). Paracetamol (acetaminophen) may exert its analgesic (and, perhaps, anti-pyretic) effects in vivo by its FAAH-dependent conversion to AM404 in the nervous system (Anderson, 2008).
Discussion
Translational mining of the endocannabinoid system is mandated by the increasing number of medical conditions considered amenable to treatment through pharmacotherapeutic modulation of endocannabinoid signalling.
In preclinical models, modulators of the endocannabinoid system exert therapeutically relevant effects on biological and behavioural endpoints related to neurobiological/psychiatric function.
Novel pharmacological modalities will be increasingly applied to the endocannabioid system for potential therapeutic benefit with improved safety: e.g. neutral (Bergman et al., 2008), peripherally directed (Kunos et al., 2008), and allosteric (Ross, 2007) CB1-receptor ligands.
Emerging targets, e.g. diacylglycerol lipase (Ortar et al., 2008), and metabolites, e.g. endocannabinoid-derived oxidation products including prostag-landin ethanolamides (Khanapure, Garvey, Janero, & Letts, 2007; Kozak & Marrnett, 2002), will be studied for potential therapeutic relevance.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abadji V, Lin S, Taha G, Griffin G, Stevenson LA, Pertwee RG, et al. (R)-methanandamide: A chiral novel anandamide possessing higher potency and metabolic stability. Journal of Medicinal Chemistry. 1994;37:1889–1893. doi: 10.1021/jm00038a020. [DOI] [PubMed] [Google Scholar]
- Ahn K, McKinney MK, Cravatt BF. Enzymatic pathways that regulate endocannabinoid signaling in the nervous system. Chemical Reviews. 2008;108:1687–1707. doi: 10.1021/cr0782067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JP, Cravatt BF. Mechanism of carbamate inactivation of FAAH: Implications for the design of covalent inhibitors and in vivo functional probes for enzymes. Chemistry & Biology. 2005;12:1179–1187. doi: 10.1016/j.chembiol.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander JP, Cravatt BF. The putative endocannabinoid transport blocker LY2183240 is a potent inhibitor of FAAH and several other brain serine hydrolases. Journal of the American Chemical Society. 2006;128:9699–9704. doi: 10.1021/ja062999h. [DOI] [PubMed] [Google Scholar]
- Anderson BJ. Paracetamol (Acetaminophen): Mechanisms of action. Paediatric Anesthesiology. 2008;18:915–921. doi: 10.1111/j.1460-9592.2008.02764.x. [DOI] [PubMed] [Google Scholar]
- Ashton JC, Glass M. The cannabinoid CB2 receptor as a target for inflammation-dependent neurodegeneration. Current Neuropharmacology. 2007;5:73–80. doi: 10.2174/157015907780866884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Wright JL, McPartland JM, Tyndall DA. Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists. Current Medicinal Chemistry. 2008;15:1428–1443. doi: 10.2174/092986708784567716. [DOI] [PubMed] [Google Scholar]
- Bachur NR, Masek K, Melmon KL, Udenfriend S. Fatty acid amides of ethanolamine in mammalian tissues. Journal of Biological Chemistry. 1965;240:1019–1024. [PubMed] [Google Scholar]
- Bambico FR, Katz N, Debonnel G, Gobbi G. Cannabinoids elicit antidepressant-like behavior and activate serotoneric neurons through the medial prefrontal cortex. Journal of Neuroscience. 2007;27:11700–11711. doi: 10.1523/JNEUROSCI.1636-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell MR, D’Ambra TE, Kumar V, Eissenstat MA, Herrmann JL, Jr, Wetzel JR, et al. Antinociceptive (aminoalkyl)indoles. Journal of Medicinal Chemistry. 1991;34:1099–1110. doi: 10.1021/jm00107a034. [DOI] [PubMed] [Google Scholar]
- Bergman J, Delatte MS, Paronis CA, Vemuri K, Thakur GA, Makriyannis A. Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiology & Behavior. 2008;93:666–670. doi: 10.1016/j.physbeh.2007.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolato M, Campolongo P, Mangieri RA, Scattoni ML, Frau R, Trezza V, et al. Anxiolytic-like properties of the anandamide transport inhibitor AM404. Neuropsychopharmacology. 2006;31:2652–2659. doi: 10.1038/sj.npp.1301061. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Dussossoy D, Casellas P. Regulation of peripheral cannabinoid receptor CB2 phosphorylation by the inverse agonist SR 144528. Implications for receptor biological responses. Journal of Biological Chemistry. 1999;274:29307–29405. doi: 10.1074/jbc.274.29.20397. [DOI] [PubMed] [Google Scholar]
- Canals M, Milligan G. Constitutive activity of the cannabinoid CB1 receptor regulates the function of co-expressed mu opioid receptors. Journal of Biological Chemistry. 2008;283:11424–11434. doi: 10.1074/jbc.M710300200. [DOI] [PubMed] [Google Scholar]
- Carbonare MD, Del Giudice E, Stecca A, Colavito D, Fabris M, D’Arrigo A, et al. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: A neglected story. Journal of Neuroendocrinology. 2008;20(Suppl 1):26–34. doi: 10.1111/j.1365-2826.2008.01689.x. [DOI] [PubMed] [Google Scholar]
- Charalambous A, Lin S, Marciniak G, Banijamali A, Friend FL, Compton DR, et al. Pharmacological evaluation of halogenated Δ8-THC analogs. Pharmacology, Biochemistry, and Behavior. 1991;40:509–512. doi: 10.1016/0091-3057(91)90355-6. [DOI] [PubMed] [Google Scholar]
- Colombo G, Agabio R, Diaz G, Lobina C, Reali R, Gessa GL. Appetite supression and weight loss after the cannabinoid antagonist SR 141716. Life Sciences. 1998;63:PL113–PL117. doi: 10.1016/s0024-3205(98)00322-1. [DOI] [PubMed] [Google Scholar]
- Deutsch DG, Lin S, Hill WA, Morse KL, Salehani D, Arreaza G, et al. Fatty acid sulfonyl fluorides inhibit anandamide metabolism and bind to the cannabinoid receptor. Biochemical and Biophysical Research Communications. 1997;231:217–221. doi: 10.1006/bbrc.1997.6072. [DOI] [PubMed] [Google Scholar]
- Dickason-Chesterfield AK, Kidd SR, Moore SA, Schaus JM, Liu B, Nomikos GG, et al. Pharmacological characterization of endocannabinoid transport and fatty acid amide hydrolase inhibitors. Cellular and Molecular Neurobiology. 2006;26:407–423. doi: 10.1007/s10571-006-9072-6. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bifulco M, De Petrocellis L. The endocannabinoid system and its therapeutic exploitation. Nature Reviews Drug Discovery. 2004;3:771–784. doi: 10.1038/nrd1495. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Bisogno T, De Petrocellis L, Brandi I, Jefferson RG, Winckler RL, et al. Highly selective CB1 cannabinoid receptor ligands and novel CB1/VR1 vanilloid receptor ‘hybrid’ ligands. Biochemmical and Biophysical Research Communications. 2001;281:444–451. doi: 10.1006/bbrc.2001.4354. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Petrosino S. Endocannabinoids and the regulation of their levels in health and disease. Current Opinion in Lipidology. 2007;18:129–140. doi: 10.1097/MOL.0b013e32803dbdec. [DOI] [PubMed] [Google Scholar]
- Drago F. Endocannabinoids and psycopathology: The therapy inside. Pharmacological Research. 2007;56:357–359. doi: 10.1016/j.phrs.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Ehrhart J, Obregon D, Mori T, Hou H, Sun N, Bai Y, et al. Stimulation of cannabinoid receptor 2 (CB2) suppresses microglial activation. Neuroinflammation. 2005;2:29. doi: 10.1186/1742-2094-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fegley D, Gaetani S, Duranti A, Tontini A, Mor M, Tarzia G, et al. Characterization of the fatty acid amide hydrolase inhibitor cyclohexyl carbamic acid 3′-carba-moyl-biphenyl-3-yl ester (URB597): Effects on anandamide and oleoylethanolamide deactivation. Journal of Pharmacology and Experimental Therapeutics. 2005;313:352–358. doi: 10.1124/jpet.104.078980. [DOI] [PubMed] [Google Scholar]
- Fowler CJ, Tiger G, Ligresti A, López-Rodríguez ML, Di Marzo V. Selective inhibition of anandamide cellular uptake versus enzymatic hydrolysis–a difficult issue to handle. European Journal of Pharmacology. 2004;492:1–11. doi: 10.1016/j.ejphar.2004.03.048. [DOI] [PubMed] [Google Scholar]
- Fulmer T. Not cutting the fat. BioCentury. 2008;16:A9–A10. [Google Scholar]
- Gaetani S, Cuomo V, Piomelli D. Anandamide hydrolysis: A new target for anti-anxiety drugs. Trends in Molecular Medicine. 2003;9:474–478. doi: 10.1016/j.molmed.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Pyatt B, Gifford AN, Volkow ND, Makriyannis A. Binding of the non-classical cannabinoid CP 55,940, and the diarylpyrazole AM251 to rodent brain cannabinoid receptors. Life Sciences. 1997;61:L191–L197. doi: 10.1016/s0024-3205(97)00690-5. [DOI] [PubMed] [Google Scholar]
- Gatley SJ, Lan R, Volkow ND, Pappas N, King P, Wong CT, et al. Imaging the brain marijuana receptor: Development of a radioligand that binds to cannabinoid CB1 receptors in vivo. Journal of Neurochemistry. 1998;70:417–423. doi: 10.1046/j.1471-4159.1998.70010417.x. [DOI] [PubMed] [Google Scholar]
- Gifford A, Bruneus M, Lin S, Goutopoulos A, Makriyannis A, Zvolkow ND, et al. Potentiation of the action of anandamide on hippocampal slices by the fatty acid amide hydrolase inhibitor, palmitylsulphonyl fluoride (AM 374) European Journal of Pharmacology. 1999;383:9–14. doi: 10.1016/s0014-2999(99)00609-3. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Rodriguez de Fonseca F, Nava F, Loubet-Lescoulié P, Piomelli D. Elevated circulating levels of anandamide after administration of the transport inhibitor, AM404. European Journal of Pharmacology. 2000;408:161–168. doi: 10.1016/s0014-2999(00)00786-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychology Reviews. 2007;17:347–361. doi: 10.1007/s11065-007-9036-8. [DOI] [PubMed] [Google Scholar]
- Hanuš L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, et al. 2-Arachidonoyl glyceryl ether, an endogeous agonist of the cannabinoid CB1 receptor. Proceedings of the National Academy of Sciences of the USA. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Coata BR, et al. Cannabinoid receptor localization in brain. Proceedings of the National Academy of Sciences of the USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard CJ, Manna S, Greenberg MJ, Dicamelli R, Ross RA, Stevenson LA, et al. Synthesis and characterization of potent and selective agonists of the neuronal cannabinoid receptor (CB1) Journal of Pharmacology and Experimental Therapeutics. 1999;289:1427–1433. [PubMed] [Google Scholar]
- Hodge J, Bow JP, Plyler KS, Vemuri VK, Wisniecki A, Salamone JD, et al. The cannabinoid CB1 receptor inverse agonist AM 251 and antagonist AM 4113 produce similar effects on the behavioral satiety sequence in rats. Behavioral Brain Research. 2008;193:298–305. doi: 10.1016/j.bbr.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst DP, Lynch DL, Barnett-Norris J, Hyatt SM, Seltzman HH, Zhong M, et al. N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2, 4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide (SR141716A) interaction with LYS 3.28(192) is crucial for its inverse agonism at the cannabinoid CB1 receptor. Molecular Pharmacology. 2002;62:1274–1287. doi: 10.1124/mol.62.6.1274. [DOI] [PubMed] [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: Pain inhibition by receptors not present in the CNS. Proceedings of the National Academy of Sciences of the USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagerovic N, Fernandez-Fernandez C, Goya P. CB1 cannabinoid antagonists: Structure-activity relationships and potential therapeutic applications. Current Topics in Medicinal Chemistry. 2008;8:205–230. doi: 10.2174/156802608783498050. [DOI] [PubMed] [Google Scholar]
- Janero DR, Makriyannis A. Targeted modulators of the endogenous cannabinoid system: future medications to treat addiction disorders and obesity. Current Psychiatry Reports. 2007;9:365–373. doi: 10.1007/s11920-007-0047-1. [DOI] [PubMed] [Google Scholar]
- Jayamanne A, Greenwood R, Mitchell VA, Asian S, Piomelli D, Vaughan CW. Actions of the FAAH inhibitor URB597 in neuropathic and inflammatory chronic pain models. British Journal of Pharmacology. 2006;147:281–288. doi: 10.1038/sj.bjp.0706510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karanian DA, Karim SL, Wood JT, Williams JS, Lin S, Makriyannis A, et al. Endocannnabinoid enhancement protects against kainic acid-induced seizures and associated brain damage. Journal of Pharmacology and Experimental Therapeutics. 2007;322:1059–1066. doi: 10.1124/jpet.107.120147. [DOI] [PubMed] [Google Scholar]
- Khanapure SP, Garvey DS, Janero DR, Letts LG. Eicosanoids in inflammation: Biosynthesis, pharmacology, and therapeutic frontiers. Current Topics in Medicinal Chemistry. 2007;7:311–340. doi: 10.2174/156802607779941314. [DOI] [PubMed] [Google Scholar]
- Khanolkar AD, Lu D, Ibrahim M, Duclos RI, Jr, Thakur GA, Malan TP, Jr, et al. Cannabilactones: A novel class of CB2 selective agonists with peripheral analgesic activity. Journal of Medicinal Chemistry. 2007;50:6493–6550. doi: 10.1021/jm070441u. [DOI] [PubMed] [Google Scholar]
- Kozak KR, Marrnett LJ. Oxidative metabolism of endocannabinoids. Prostaglandins Leukotrienes Essential Fatty Acids. 2002;66:211–220. doi: 10.1054/plef.2001.0359. [DOI] [PubMed] [Google Scholar]
- Kunos G, Osei-Hyiaman D, Bátaki S, Sharkey KA, Makriyannis A. Should peripheral CB1 cannabinoid receptors be selectively targeted for therapeurtic gain? Trends in Pharmacological Sciences. 2008;30:1–7. doi: 10.1016/j.tips.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labar G, Michaux C. Fatty acid amide hydrolase: From characterization to therapeutics. Chemistry & Biodiversity. 2007;4:1882–1902. doi: 10.1002/cbdv.200790157. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Di Marzo V. The palmitoylethanolamide and oleamide enigmas: Are these two fatty acid amides cannabimimetic? Current Medicinal Chemistry. 1999;6:757–773. [PubMed] [Google Scholar]
- Lambert DM, DiPaolo FG, Sonveaux P, Kanyonyo M, Govaerts SJ, Hermans E, et al. Analogs and homologs of n-palmitoylethanolamide, a putative endogenous CB(2) cannabinoid, as potential ligands for the cannabinoid receptors. Biochimica et Biophysica Acta. 1999;1440:266–274. doi: 10.1016/s1388-1981(99)00132-8. [DOI] [PubMed] [Google Scholar]
- Lambert DM, Vandevoorde S, Jonsson KO, Fowler CJ. The palmitoylethanolamide family: A new class of anti-inflammatory agents? Current Medicinal Chemistry. 2002;9:663–674. doi: 10.2174/0929867023370707. [DOI] [PubMed] [Google Scholar]
- Lange JHM, Kruse CG. Cannabinoid CB1 receptor antagonists in therapeutic and structural perspectives. Chemical Record. 2008;8:156–168. doi: 10.1002/tcr.20147. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, de Miguel R, De Petrocellis L, Makriyannis A, Di Marzo V, Fernández-Ruiz J. Compounds acting at the endocannabinoid and/or endovanil-loid systems reduce hyperkinesia in a rat model of Huntington’s disease. Journal of Neurochemistry. 2003;84:1097–1109. doi: 10.1046/j.1471-4159.2003.01595.x. [DOI] [PubMed] [Google Scholar]
- Leweke FM, Koethe D. Cannabis and psychiatric disorders: It is not only addiction. Addiction Biology. 2008;13:264–275. doi: 10.1111/j.1369-1600.2008.00106.x. [DOI] [PubMed] [Google Scholar]
- Lin S, Khanolkar AD, Fan P, Goutopoulos A, Qin C, Papahadjis D, et al. Novel analogues of arachidonoy-lethanolamide (anandamide): Affinities for the CB1 and CB2 cannabinoid receptors and metabolic stability. Journal of Medicinal Chemistry. 1998;41:5353–5361. doi: 10.1021/jm970257g. [DOI] [PubMed] [Google Scholar]
- Lombard C, Nagarkatti M, Nagarkatti P. CB2 cannabinoid receptor agonist, JWH-015, triggers apoptosis in immune cells: Potential role for CB2-selective ligands as immunosuppressive agents. Clinical Immunology. 2007;122:259–270. doi: 10.1016/j.clim.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Meng Z, Thakur GA, Fan P, Steed J, Tartal CL, et al. Adamantyl cannabinoids: A novel class of cannabinergic ligands. Journal of Medicinal Chemistry. 2005;48:4576–4585. doi: 10.1021/jm058175c. [DOI] [PubMed] [Google Scholar]
- Luk T, Jin W, Zvonok A, Lu D, Lin XZ, Chavkin C, et al. Identification of a potent and highly efficacious, yet slowly desensitizing CB1 cannabinoid receptor agonist. British Journal of Pharmacology. 2004;142:495–500. doi: 10.1038/sj.bjp.0705792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie K, Stella N. Cannabinoid receptors and endocannabinoids: Evidence for new players. American Association of Pharmaceutical Scientists Journal. 2006;8:E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maione S, Morera E, Marabese I, Ligresti A, Luongo L, Ortar G, et al. Antinociceptive effects of tetrazole inhibitors of endocannabinoid inactivation: Cannabinoid and non-cannabinoid receptor-mediated mechanisms. British Journal of Pharmacology. 2008;155:775–782. doi: 10.1038/bjp.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makriyannis A, Rapaka R. The molecular basis of cannabinoid activity. Life Sciences. 1990;47:2173–2184. doi: 10.1016/0024-3205(90)90147-j. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Deng H, Liu Q, Mata HP, Vanderah T, et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain. 2001;93:239–245. doi: 10.1016/S0304-3959(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Malan TP, Jr, Ibrahim MM, Lai J, Vanderah TW, Makriyannis A, Porreca F. CB2 cannabinoid receptor agonists: Pain relief without psychoactive effects? Current Opinion in Pharmacology. 2003;3:62–67. doi: 10.1016/s1471-4892(02)00004-8. [DOI] [PubMed] [Google Scholar]
- Marriott KSC, Huffman JW. Recent advances in the development of selective ligands for the cannabinoid CB2 receptor. Current Topics in Medicinal Chemistry. 2008;8:187–204. doi: 10.2174/156802608783498014. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: Interspecies differences. British Journal of Pharmacology. 2007;152:583–593. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochemical Pharmacology. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Feigenbaum JJ, Lander N, Segal M, Järbe TU, Hiltunen AJ, et al. Enantiomeric cannabinoids: Stereopsecificity of psychotropic activity. Experientia. 1988;44:762–774. doi: 10.1007/BF01959156. [DOI] [PubMed] [Google Scholar]
- Mechoulam R, Peters M, Murillo-Rodriguez E, Hanuš LO. Cannabidiol—recent advances. Chemistry & Biodiversity. 2007;4:1678–1692. doi: 10.1002/cbdv.200790147. [DOI] [PubMed] [Google Scholar]
- Merck Research and Development News. [accessed October 2008];Merck discontinues development of investigational medicine taranabant for obesity. 2008 Available at: http://www.merck.com/newsroom/press_releases/research_and_development/2008_1002.html.
- Moriera FA, Lutz B. The endocannabinoid system: Emotion, learning and addiction. Addiction Biology. 2008;13:196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- Muccioli GG. Blocking the cannabinoid receptors: Drug candidates and therapeutic promises. Chemistry & Biodiversity. 2007;4:1805–1827. doi: 10.1002/cbdv.200790153. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Adams M, Whiteaker K, Daza A, Kage K, Cassar S, et al. Species comparison and pharmacological characterization of rat and human CB2 cannabinoid receptors. European Journal of Pharmacology. 2004;505:1–9. doi: 10.1016/j.ejphar.2004.09.058. [DOI] [PubMed] [Google Scholar]
- Ortar G, Bisogno T, Ligresti A, Morera E, Nalli M, Di Marzo V. Tetrahydrolipstatin analogues as modulators of endocannabinoid 2-arachidonoylglycerol metabolism. Journal of Medicinal Chemistry. 2008;51:6970–6979. doi: 10.1021/jm800978m. [DOI] [PubMed] [Google Scholar]
- Osei-Hyiaman D, Liu J, Zhou L, Godlewski G, Harvey-White J, Jeong W, et al. Hepatic CB1 receptor is required for development of diet-induced steatosis, dyslipidemia, and insulin and leptin resistance in mice. Journal of Clinical Investigation. 2008;118:3160–3169. doi: 10.1172/JCI34827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. The therapeutic potential of drugs that target cannabinoid receptors or modulate tissue levels or actions of endocannabinoids. American Association of Pharmaceutical Scientists Journal. 2005;7:E625–E654. doi: 10.1208/aapsj070364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG. GPR55: A new member of the cannabinoid receptor clan? British Journal of Pharmacology. 2007;152:984–986. doi: 10.1038/sj.bjp.0707464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Δ9-tetrahydrocannabinol, cannabidiol and Δ9-tetrahydrocannabivarin. British Journal of Pharmacology. 2008;153:199–215. doi: 10.1038/sj.bjp.0707442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee R, Griffin G, Fernando S, Li X, Hill A, Makriyannis A. AM630, a competitive cannabinoid antagonist. Life Sciences. 1995;56:1949–1955. doi: 10.1016/0024-3205(95)00175-6. [DOI] [PubMed] [Google Scholar]
- Pfizer [accessed November 2008];Pfizer discontinues development program for its phase 3 obesity compound (CP-945-598) 2008 Available at: http://mediaroom.pfizer.com/news/Pfizer/20081105006339/en.
- Rawls SM, Ding Z, Cowan A. Role of TRPV1 and cannabinoid CB1 receptors in AM 404-evoked hypothermia in rats. Pharmacology, Biochemistry, and Behavior. 2006;83:508–516. doi: 10.1016/j.pbb.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Re G, Barbero R, Miolo A, Di Marzo V. Palmitoylethanolamide, endocannabinoids, and related canna-bimimetic compounds in protection against tissue inflammation and pain: Potential use in companion animals. Veterinary Journal. 2007;173:21–30. doi: 10.1016/j.tvjl.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Reggio PH, Basu-Dutt S, Barnett-Norris J, Castro M, Hurst DP, Seltzman HH, et al. The bioactive conformation of aminoalkylindoles at the cannabinoid CB1 and CB2 receptors: Insignts gained from (E)- and (Z )-naphthylidene indenes. Journal of Medicinal Chemistry. 1998;41:5177–5187. doi: 10.1021/jm9801197. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Héaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. Federation of European Biochemical Societies Letters. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR144528, the first potent and selective antagonist of the CB2 receptor. Journal of Pharmacology and Experimental Therapeutics. 1998;284:644–650. [PubMed] [Google Scholar]
- Romero-Sandoval A, Nutile-McMenemy N, DeLeo JA. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology. 2008;108:722–734. doi: 10.1097/ALN.0b013e318167af74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross RA. Allosterism and cannabinoid CB1 receptors: The shape of things to come. Trends in Pharmacological Sciences. 2007;28:567–572. doi: 10.1016/j.tips.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Rumsfeld JS, Nallamothu BK. The hope and fear of rimonabant. Journal of the American Medical Association. 2008;299:1601–1602. doi: 10.1001/jama.299.13.1601. [DOI] [PubMed] [Google Scholar]
- Saario SM, Salo OM, Nevalainen T, Poso A, Laitinen JT, Järvinen T, et al. Characterization of the sulfhydryl-sensitive site in the enzyme responsible for hydrolysis of 2-arachidonoyl-glycerol in rat cerebellar membranes. Chemistry & Biology. 2005;12:649–656. doi: 10.1016/j.chembiol.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Salamone JD, McLaughlin PJ, Sink K, Makriyannis A, Parker LA. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiology & Behavior. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanofi-Aventis. [accessed October 2008];Sanofi-Aventis is complying with the EMEA’s recommendation to temporarily suspend the marketing authorization of Acomplia® in obese and overweight patients. 2008a Available at: www.sanofi-aventis.com.
- Sanofi-Aventis. [accessed December 2008];Sanofi-Aventis to discontinue all clinical trials with rimonabant. 2008b Available at: www.sanofi-aventis.com.
- Schomacher M, Müller HD, Sommer C, Schwab S, Schäbitz WR. Endocannabinoids mediate neuroprotrection after transient focal cerebral ischemia. Brain Research. 2008;1240:213–220. doi: 10.1016/j.brainres.2008.09.019. [DOI] [PubMed] [Google Scholar]
- Sink KS, McLaughlin PJ, Wood JA, Brown C, Fan P, Vemuri VK, et al. The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology. 2008;33:946–55. doi: 10.1038/sj.npp.1301476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solinas M, Tanda G, Justinova Z, Wertheim CE, Yasar S, Piomelli D, et al. The endogenous cannabinoid anandamide produces delta-9-tetrahydrocannabinol-like discriminative and neurochemical effects that are enhanced by inhibition of fatty acid amide hydrolase but not by inhibition of anandamide transport. Journal of Pharmacology and Experimental Therapeutics. 2007;321:370–380. doi: 10.1124/jpet.106.114124. [DOI] [PubMed] [Google Scholar]
- Thakur GA, Duclos RI, Jr, Makriyannis A. Natural cannabinoids: Templates for drug discovery. Life Sciences. 2005;78:454–466. doi: 10.1016/j.lfs.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Sun YX, Okamoto Y, Araki N, Tonai T, Udea N. Moelcular characterization of N-acetylethanolamide-hydrolyzing acid amidase, a novel member of the choloylglycine hydrolase family with structural and functional similarity to acid ceramidase. Journal of Biological Chemistry. 2005;12:11082–11092. doi: 10.1074/jbc.M413473200. [DOI] [PubMed] [Google Scholar]
- University of British Columbia. Cannabinoids in bipolar disorder. [accessed November 2008];Available at. 2006 http://www.clinicaltrials.gov/ct2/show/NCT00397605.
- University of Cologne. [accessed November 2008];A clinical trial on the anti-psychotic properties of cannabidiol. 2006 Available at: http://www.clinicaltrials.gov/ct2/show/NCT00309413.
- Vemuri VK, Janero DR, Makriyannis A. Pharmacotherapeutic targeting of the endocannabinoid system: Drugs for obesity and the metabolic syndrome. Physiology & Behavior. 2008;93:671–686. doi: 10.1016/j.physbeh.2007.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinod KY, Yalamanchili R, Thanos PK, Vadasz C, Cooper TB, Volkow ND, et al. Genetic and pharmacological manipulations of the CB(1) receptor alter ethanol preference and dependence in ethanol preferring and nonpreferring mice. Synapse. 2008;62:574–581. doi: 10.1002/syn.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Collet JP, Shapiro S, Ware MA. Adverse effects of medicinal cannabinoids: A systematic review. Canadian Medical Association Journal. 2008;178:1669–1678. doi: 10.1503/cmaj.071178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woelkart K, Salo-Ahen OMH, Bauer R. CB receptor ligands from plants. Current Topics in Medicinal Chemistry. 2008;8:173–186. doi: 10.2174/156802608783498023. [DOI] [PubMed] [Google Scholar]
- Xie XQ, Eissenstat M, Makriyannis A. Common cannabimimetic pharmacophoric requirements between aminoalkyl indoles and classical cannabinoids. Life Sciences. 1995;56:1963–1970. doi: 10.1016/0024-3205(95)00177-8. [DOI] [PubMed] [Google Scholar]
- Yale University. [accessed November 2008];Cannabidiol treatement of cognitive dysfunction in schizophrenia. 2008 Available at: http://www.clinicaltrials.gov/ct2/show/NCT00588731.
- Zvonok N, Pandarinathan L, Williams J, Johnston M, Karageorgos I, Janero DR, et al. Covalent inhibitors of human monoacylglycerol lipase: Ligand-assisted characterization of the catalytic site by mass spectrometry and mutational analysis. Chemistry & Biology. 2008;15:854–862. doi: 10.1016/j.chembiol.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]