Abstract
Alcoholism is a complex and dynamic disease, punctuated by periods of abstinence and relapse, and influenced by a multitude of vulnerability factors. Chronic excessive alcohol consumption is associated with cognitive deficits, ranging from mild to severe, in executive functions, memory, and metacognitive abilities, with associated impairment in emotional processes and social cognition. These deficits can compromise efforts in initiating and sustaining abstinence by hampering efficacy of clinical treatment and can obstruct efforts in enabling good decision-making, success in interpersonal/social interactions, and awareness of cognitive and behavioral dysfunctions. Despite evidence for differences in recovery levels of selective cognitive processes, certain deficits can persist even with prolonged sobriety. Herein is presented a review of alcohol-related cognitive impairments affecting component processes of executive functioning, memory, and the recently investigated cognitive domains of metamemory, social cognition, and emotional processing; also considered are trajectories of cognitive recovery with abstinence. Finally, in the spirit of critical review, limitations of current knowledge are noted and avenues for new research efforts are proposed that focus on (1) the interaction among emotion-cognition processes and identification of vulnerability factors contributing to the development of emotional and social processing deficits and (2) the time line of cognitive recovery by tracking alcoholism’s dynamic course of sobriety and relapse. Knowledge about the heterochronicity of cognitive recovery in alcoholism has the potential of indicating at which points during recovery intervention may be most beneficial.
Keywords: alcoholism, cognitive impairment, recovery, clinical implications, abstinence
In Alcohol Use Disorder (AUD), individuals commonly exhibit a lack of recognition of their disease, minimizing its harmful consequences in life’s physical, psychological, and social spheres. Supplanting former characterization of the alcoholic as morally flawed, it is now widely recognized that alcoholism is a complex, multi-dimensional, multi-determined disorder requiring adequate cognitive skills to cope with daily life high-risk situations that threaten maintenance of sobriety, whether it is through controlled drinking or complete abstinence.
To initiate behavioral change towards sobriety, an alcoholic patient often has to (1) recognize the reality of this harmful behavior by perceiving the effects of alcohol on the body (i.e., liver cirrhosis, brain damage), family, work, and community; (2) understand its causes and consequences, and match risks and benefits of behavioral change relative to no change by remembering past personal drinking, craving, and abstinence experiences; and (3) identify and assess new behavioral options, learn general information about alcohol dependence and coping strategies, choose adaptive behaviors, and monitor and control their drinking. The components of change and oversight require efficient cognitive skills involving multi-faceted neuropsychological functions to support abilities for self-assessment, social cognition and emotional processing that enable accurate perception and understanding of the social environment, and reliable memory and executive functioning to achieve optimal decision-making. This paper reviews quantitative studies of component cognitive functions, which are subject to compromise in chronic alcoholism, thereby having the potential to undermine efforts towards achieving and maintaining sobriety.
It has been consistently reported that treatment-seeking AUD individuals have detectable cognitive impairment, often involving executive dysfunction and memory deficits (see reviews by Oscar-Berman et al., 2014 and Sullivan et al., 2010). Recently, new considerations to expand the understanding of functional impairment in alcoholism have included the investigation of metacognition (the ability to accurately assess one’s own cognitive abilities), especially metamemory processes, together with emotional and social cognitive abilities (Bora and Zorlu, 2016; Le Berre and Sullivan, 2016; Maurage et al., 2011a; Uekermann and Daum, 2008). In particular, research efforts are warranted for understanding the complex interactions among executive, memory, and social and emotional processing abilities. Also needing explications are how deficits in these processes influence the efficiency of clinical interventions (Blume et al., 2005; Le Berre et al., 2012), cognitive-behavioral therapy (Pitel et al., 2007b) and compromise efforts towards abstinence or controlled drinking (Bates et al., 2002; Bates et al., 2006).
The pattern and extent of cognitive deficits among individuals with chronic alcoholism vary widely, and not all alcoholics demonstrate measurable cognitive impairment (Fein et al., 1990). This heterogeneity is likely at least partly due to the dynamic course of AUD, which is generally marked by periods of withdrawal, abstinence, and relapse. Each of these periods is associated with different levels of functional recovery. Also influential are demographic and disease-related factors such as age, lifetime drinking patterns and amount, and number of withdrawals (cf., Duka et al., 2003). Indeed, recovery of cognitive functions, defined here as the process of returning toward a premorbid level of functioning associated with abstinence can occur; however, cognitive deficits can persist even with prolonged sobriety (Fabian and Parsons, 1983; Fein et al., 1990; Pitel et al., 2009; Rosenbloom et al., 2007; Rourke and Grant, 1999; Stavro et al., 2013; Sullivan et al., 2000a; Yohman et al., 1985). Identifying the pattern, extent, and severity of recovered and persistent cognitive deficits associated with long-term chronic alcoholism with sobriety has the potential to inform brain structure-function plasticity and guide effective management and treatment of AUD.
Reflecting the complexity of normal cognitive functioning, successful performance on most neuropsychological tests requires multiple intact component processes. Parsing complex behavioral functions into their component cognitive processes, their functional building blocks, and examining how alcohol affects these basic processes can indicate which abilities are spared, impaired, recover, or persist with abstinence or continued drinking. Thus, to understand the underlying variation among alcoholism-related cognitive deficits requires a refined characterization of which specific component processes within the broad functional domains implicated are affected.
In the context of the presented overview, the objectives of this paper are three-fold: (1) review evidence for alcohol-related cognitive impairments in affecting component processes of executive functioning, memory, and the recently studied cognitive domains of metamemory, social cognition, and emotional processing; (2) investigate the possible interactions between these functional domains in relation to higher order cognitive processes such as decision making associated with initiation and maintenance of sobriety; and (3) report what is known and not known about the recovery trajectories of these cognitive deficits, and note limitations of our knowledge, thereby identifying avenues for future research.
I- Alcohol-related cognitive impairment
I-1: Executive Functions
Executive functions refer to a number of related but dissociable cognitive processes that enable one to plan, control, and monitor goal-directed and adaptive behaviors in response to novel or non-routine situations (Alvarez and Emory, 2006; Miyake et al., 2000). Specific component executive processes documented as impaired in chronic alcoholism using standard laboratory tasks, including attention, working memory, response inhibition, problem solving, deduction of rules, updating, cognitive flexibility and set shifting, and impulsivity [e.g., (Beatty et al., 1995; Beatty et al., 1993; Chanraud et al., 2007; Fama et al., 2004; Joyce and Robbins, 1991; Le Berre et al., 2012; Loeber et al., 2009; Moriyama et al., 2002; Nixon and Parsons, 1991; Noel et al., 2001; Oscar-Berman et al., 2009; Parsons, 1983; Pitel et al., 2007a; Ratti et al., 2002; Sullivan et al., 2002; Sullivan et al., 1993; Sullivan et al., 2000b; Sullivan et al., 1997; Tarter, 1973; Tarter and Parsons, 1971; Zinn et al., 2004)], each of which can potentially affect higher-order cognitive processes such as decision making and influence initiation and maintenance of sobriety.
Thus, decision-making has been consistently observed to be compromised in AUD under various conditions. These include ambiguous conditions, when the risk associated with a choice is not explicitly stated [e.g., Iowa Gambling Test, (Brevers et al., 2014; Dom et al., 2006; Fernandez-Serrano et al., 2010; Goudriaan et al., 2005; Le Berre et al., 2014; Mazas et al., 2000; Miranda et al., 2009; Noel et al., 2007; Salgado et al., 2009)] and under risky conditions, when the risk associated with a choice is explicitly stated [e.g., Cambridge Gambling Test, (Lawrence et al., 2009); Cups and Coin Flipping Tasks, (Brevers et al., 2014); ambiguous coin task, (Jung et al., 2014)].
To understand the relative contribution of specific cognitive executive processes to higher order cognitive abilities such as decision-making in AUD, the construct of executive function needs to be deconstructed (Miyake et al., 2000). Severity variability in executive function impairment has been observed across studies likely owing to the complexity of these functions and the use of diverse tasks assessing different constellations of component processes. Indeed, most standard tasks assessing executive functioning are multidimensional and involve several executive function component processes.
Individuals with AUD have difficulty with visual-conceptual and visual-motor tracking skills that intersect with motor processing. Such processes involve psychomotor speed, divided attention, mental flexibility, and set shifting as assessed with the Trail Making Test part B (Chanraud et al., 2007; Davies et al., 2005; Loeber et al., 2010; Moriyama et al., 2002; Noel et al., 2012a; Noel et al., 2001; Nowakowska-Domagala et al., 2017; Oscar-Berman et al., 2009; Zinn et al., 2004). Cognitive inhibition is also particularly affected in chronic alcoholism as assessed with the Stroop Color Word test (Dao-Castellana et al., 1998; Le Berre et al., 2012; Noel et al., 2012a; Noel et al., 2001; Nowakowska-Domagala et al., 2017; Pitel et al., 2007a; Pitel et al., 2007b; Ratti et al., 2002; Tedstone and Coyle, 2004) and a semantic inhibition task, the Hayling test, ((Noel et al., 2012b; Noel et al., 2001). Individuals with AUD have difficulty with abstract thinking, cognitive flexibility, set-shifting, concept identification, working memory, problem solving, and ability to use feedback information assessed with measures such as the Wisconsin Card Sorting Test (Chanraud et al., 2007; Fama et al., 2004; Oscar-Berman et al., 2009; Ratti et al., 2002; Sullivan et al., 1993) and the Modified Card Sorting Test (Le Berre et al., 2012). Deficits in other component executive processes have also been associated with AUD, such as difficulties in organization and self-generation of strategies using verbal fluency tasks and the Ruff Figural Fluency task (Dao-Castellana et al., 1998; Fernandez-Serrano et al., 2010; Oscar-Berman et al., 2009; Pitel et al., 2007a), updating abilities using an N-back task (Noel et al., 2012a; Noel et al., 2001; Pitel et al., 2007a), and working memory using the Letter-Number Sequencing test (Chanraud et al., 2007).
Clinically, the ability to change behavioral schemes and make better choices and decisions in life entails the coordination of many component processes of executive functions. Critical ones include attending, consolidating, and retrieving information about change. With respect to AUD, inhibition of automatic drinking habits would enable change toward favoring new healthy behaviors, to resist temptation and make better choices in the face of high-risk situations and selecting and planning a constellation of behavioral avoidance strategies according to different life situations. From a clinical perspective, when transitioning from excessive drinking to sobriety or controlled drinking, alcoholic patients make different decisions to implement new behavioral schemes to maintain their abstinence or reduce alcohol consumption. The tendency to choose short-term gratification at the expense of long-term consequences suggests that alcoholics may suffer from myopia for the future (Camchong et al., 2014; Le Berre et al., 2014). This ‘myopia’ may include patients’ awareness that the problems arise from their substance abuse and keep them in denial (Verdejo-Garcia and Perez-Garcia, 2008) or in a form of anosognosia (Le Berre and Sullivan, 2016) about their disorder.
I-2: Memory and Metamemory
Memory is not a unitary process but comprises a multitude of component mnemonic processes, not all of which have been extensively studied in chronic alcoholism (Squire, 1992; Squire, 2004). Over the last half-century, studies in alcoholism have highlighted impairments affecting episodic memory as well as semantic and cognitive procedural learning (Le Berre et al., 2010; Noel et al., 2012b; Pitel et al., 2007a; Pitel et al., 2007b). By contrast, visuomotor procedural and implicit perceptual learning and memory are relatively preserved (Fama et al., 2004; Fama et al., 2012). More recently, deficits in prospective (Griffiths et al., 2012), autobiographical (D’Argembeau et al., 2006; Nandrino et al., 2016) and source (Schwartz et al., 2002) memory have been reported in individuals with AUD. These component processes of memory are explicated next.
Episodic memory involves the mnemonic system founded on the processes of encoding, storage, and retrieval of personally experienced events, associated with a precise temporal and spatial context (Tulving, 2001; Tulving, 2002). Deficits in encoding and retrieval processes occur in recently abstinent alcoholics (Pitel et al., 2007a) and can affect learning of verbal and nonverbal information (Beatty et al., 1995; Everett et al., 1988; Kopera et al., 2012; Schaeffer and Parsons, 1987; Sherer et al., 1992; Sullivan et al., 1992; Sullivan et al., 2000b; Tivis et al., 1995). Episodic memory deficits have been related to executive dysfunction, with poor generation of spontaneous learning or retrieval strategies indirectly affecting free-recall performance (Noel et al., 2012a; Sullivan et al., 1992). A different perspective considers that a genuine episodic memory impairment exists in alcoholics even after accounting for the contribution of executive dysfunction (Pitel et al., 2007a).
Episodic memory is the foundation for conscious recollection of specific personal events from one’s past and the mental projection of anticipated events into one’s subjective future (Wheeler et al., 1997). Recollection of episodic events includes autonoetic awareness, which is the feeling of re-experiencing or reliving the past and mentally traveling back in subjective time (Tulving, 2001). Sober alcoholics demonstrate a deficit of autonoetic consciousness (Le Berre et al., 2010; Pitel et al., 2007a) associated with difficulties in retrieving the spatiotemporal context of encoding, with studies reporting compromise of temporal ordering ability (e.g., putting events in chronological order) and spatial context recognition (e.g., remembering where I was when I drank too much last time) (Pitel et al., 2007a; Salmon et al., 1986; Sullivan et al., 1997).
Despite evidence of episodic memory deficits, alcoholics as a group have a tendency to overestimate their memory skills (Le Berre et al., 2016; Le Berre et al., 2010). In particular, they have difficulty in accurately predicting how well they will perform on tasks requiring recognition of newly learned information [feeling-of-knowing, FOK (Hart, 1965)]. They are also likely to generate predictions about their cognitive abilities based on semanticized (implicit) and remote memories of self-ability and poor self-reflection (autonoetic), and thus maintain an outdated and unchanged concept of self (Mograbi et al., 2009). The lack of awareness for prospective mnemonic failures suggests a mild form of anosognosia (e.g., you don’t know that you don’t know) for episodic memory dysfunction and is considered a metamemory impairment (Le Berre and Sullivan, 2016). This metamemory deficit differs from retrospective confidence in memory ability, wherein alcoholics accurately judge how well they recognized newly experienced information [i.e., Retrospective Confidence Judgment, RCJ].
It may be that alcoholics fail to consolidate updated information about their level of memory performance into their personal long-term memory and instead base their predictions regarding current memory performance on outdated self-beliefs that their memory skills are good. A mnemonic anosognosia has been proposed to explain the pattern of metamemory impairment observed in alcoholics without the neurological complications associated with the profound memory impairment of Korsakoff’s Syndrome (Hannesdottir and Morris, 2007; Le Berre and Sullivan, 2016; Morris and Mograbi, 2013). These results provide support for the hypothesis that mild mnemonic anosognosia occurs in chronic alcoholism. Overestimation of actual memory abilities can limit benefit from clinical treatment such as cognitive behavioral therapy (CBT) or educational-focused treatment. This recognition disability could place individuals at risk of laboring under the illusion that they have sufficiently consolidated and incorporated into their lexicon essential information acquired during CBT to enable maintenance of their abstinence or reduced alcohol consumption in their daily life.
Semantic memory refers to the ability to recall or recognize facts including personal information, concepts, and general knowledge about the external world, independent of personal experience and spatial/temporal context. In the context of alcoholism, individuals in treatment learn about alcohol and alcohol dependence, the medical and psychiatric consequences associated with excessive alcohol consumption and strategies and techniques to maintain sobriety. Procedural memory for cognitive and behavioral skills that operate at an automatic, unconscious level and independent of episodic memory could also be relevant for successful behavior modification. Over time, AUD individuals supplant new behavioral strategies and procedures to cope with urges and cravings for alcohol and previously entrenched habitual patterns. However, at treatment entry, alcoholic patients with cognitive impairment may exhibit difficulties in acquiring new semantic and procedural information, potentially hampering the efficiency—the essential ability—of cognitive-behavioral therapies (Pitel et al., 2007b), during which patients are taught to anticipate and recognize high-risk situations that could lead to relapse (Assanangkornchai and Srisurapanont, 2007; Berglund et al., 2003; Clay et al., 2008).
Other mnemonic systems impaired in alcoholism include prospective memory (Griffiths et al., 2012), which is the ability to remember to perform an action at a specific point in the future, and autobiographical memory (D’Argembeau et al., 2006), which is memory formed by different types of representation from specific personal events (episodic components) to general knowledge about oneself (semantic component) (Conway, 2001; Tulving et al., 1988). A specific autobiographical memory disorder affecting both the episodic dimension (i.e., long-term memories about specific personal experiences) and the semantic dimension (i.e., general knowledge about past life events) was observed in recently abstinent alcoholic individuals. This deficit persisted after 6 months of abstinence, and was potentially explained by compromised encoding and consolidation of memories during drinking periods (Nandrino et al., 2016). Also potentially impaired is source memory for recently learned information, which is the ability to discriminate and recall the origin or source of information (Schwartz et al., 2002).
Individuals who labor at maintaining sobriety learn and integrate complex information requiring efficient abilities in a number of mnemonic processes. These include the mental re-experiencing and reliving of craving and emotional and personal states of mind during drinking and abstinence periods. Re-experiencing through mentally reliving episodic drinking experiences include spatial contexts (e.g., at home, a favorite bar) and temporal contexts (e.g., alone, with my ‘drinking’ friends, when under job related stressful situations).
I-3: Social cognition and emotional processing
Social cognition refers to processes contributing to the perception and understanding of social environments and social interactions (Frith, 2008). Alcoholic individuals can demonstrate deficits in decoding basic and complex emotional facial expressions (Castellano et al., 2015; D’Hondt et al., 2014; Donadon and Osorio Fde, 2014; Kornreich et al., 2001; Kornreich et al., 2002; Maurage et al., 2009; Maurage et al., 2008; Maurage et al., 2011a; Philippot et al., 1999; Uekermann and Daum, 2008). In particular, recognition of negative affect such as disgust and anger can be affected in alcoholism (Bora and Zorlu, 2016), such compromise having the potential to contribute to interpersonal problems in everyday life, for example, misperceiving a facial grimace for aggression (Kornreich et al., 2002).
Impairment in decoding affective prosody and body postures has also been observed in alcoholics (Maurage et al., 2009; Monnot et al., 2002; Monnot et al., 2001). Emotional prosody deficits may be exacerbated when the affective prosody does not match the semantic content in sentences or when trying to match affective prosody to facial expressions (Uekermann et al., 2005). Some alcoholic patients do not benefit from the crossmodal processing facilitation effect, that is, when affective information is conveyed through multiple sensory modalities [e.g., simultaneous auditory (voices) – visual (faces) processing] (Maurage et al., 2007; Maurage et al., 2013).
Alcoholism has been also associated with difficulties in processing humor and irony (Amenta et al., 2013; Uekermann et al., 2007), identifying and describing emotions, i.e., alexithymia, (Stasiewicz et al., 2012; Uzun et al., 2003), and experiencing empathy (Martinotti et al., 2009; Maurage et al., 2011b). In some alcoholics, the emotional component of empathy (i.e., experiencing and sharing emotional states of another person) can be impaired, while the cognitive component of empathy (i.e., understanding mental states of another person) can be relatively preserved (Maurage et al., 2011b).
Deficits in theory of mind (ToM) are consistently reported in alcoholics (Bosco et al., 2014; Maurage et al., 2015; Maurage et al., 2011b; Nandrino et al., 2014; Onuoha et al., 2016; Thoma et al., 2013; Uekermann et al., 2007). ToM enables individuals to predict, anticipate, and interpret the behavior of others (Frith and Frith, 1999; Premack and Woodruff, 1978). ToM can be divided into (1) affective theory of mind, referring to thinking about affective states, feelings and emotions of others and (2) cognitive theory of mind, referring to thinking about cognitive states, beliefs, thoughts or intentions of others (Shamay-Tsoory et al., 2007). A dissociation between impaired affective ToM but preserved cognitive ToM in alcoholism has been observed (Maurage et al., 2016; Nandrino et al., 2014).
These deficits in social cognition and emotional processing, along with lack of awareness of these deficits, have the potential to contribute to family, social, and career related difficulties exhibited by many alcoholics (Kornreich et al., 2002; Philippot et al., 1999). Alcoholics can misunderstand their relatives’ state of mind, leading to family strife, or have trouble recognizing negative affect such as anger from relatives regarding their drinking behavior (Maurage et al., 2009).
II- Recovery of alcohol-related cognitive impairment with abstinence
Sobriety can result in improvement in brain structure and function, indicative of either damage reversal (i.e., actual recovery) or compensatory mechanisms that can be identified with neuropsychological testing and quantitative structural or functional brain imaging. Tracking alcoholism’s dynamic course of sobriety and relapse reveals the potential for recovery from and accommodation (i.e., compensation) to neural and neuropsychological insult. Functional imaging studies provide evidence for compensation by invoking non-normal sites and circuits to achieve normal performance on tasks typically impaired (Chanraud et al., 2013; Oscar-Berman and Marinkovic, 2007; Sullivan and Pfefferbaum, 2005), occurring at the cost of processing efficiency when patients perform in the normal range but need additional time to achieve this level (Nixon and Parsons, 1991; Sullivan and Pfefferbaum, 2005). Recovery from cognitive impairment in abstinent alcoholics is typically investigated with cross-sectional designs, comparing alcoholic groups with different lengths of sobriety varying from days to several years to each other or with a control group of healthy participants (e.g., Brandt et al., 1983; Hochla et al., 1982; Markowitsch et al., 1986; Munro et al., 2000; Reed et al., 1992). To assess within-subject change, longitudinal designs, retesting the same group of alcoholic patients and control participants at variable time intervals, are preferred (e.g., Fabian and Parsons, 1983; Glenn et al., 1994; Rosenbloom et al., 2004; Rourke and Grant, 1999; Yohman et al., 1985).
Select executive function component processes showed less impairment as a function of abstinence duration (cross-sectional studies) and demonstrate recovery (longitudinal studies) in alcoholics with several years of sobriety (Fein et al., 2006; Rourke and Grant, 1999) or even only a few months after drinking cessation (Loeber et al., 2010; Pitel et al., 2009). Specifically, inhibition, cognitive abstraction/flexibility, updating processes (Fein et al., 2006; Loeber et al., 2010; O’Leary et al., 1977; Pitel et al., 2009), attention (Fein et al., 2006; Loeber et al., 2010; O’Leary et al., 1977; Sullivan et al., 2000a), and short-term/working memory (Fein et al., 2006; Rosenbloom et al., 2004) show less impairment in long-term abstinent alcoholics compared with short-term abstinent alcoholics and exhibit recovery over time. Other studies, however, reported persistent executive impairment in AUD patients after long-term periods of abstinence from months to years (Munro et al., 2000; Nowakowska-Domagala et al., 2017; Yohman et al., 1985). Decision-making deficits may also endure in long-term abstinent alcoholics (Ando et al., 2012; Fein et al., 2004); these deficits have been hypothesized to play a significant role in relapse. Stavro and colleagues (2013) highlighted the absence of studies that track the persistence and resolution of impulsive decision-making impairment in alcoholic individuals abstinent for many years.
Similar to persisting executive dysfunctions, cross-sectional studies report episodic memory deficits a few months to one year after drinking cessation (Munro et al., 2000; Parsons et al., 1990; Rosenbloom et al., 2005) and even after several years of sobriety (Brandt et al., 1983) in AUD patients relative to healthy controls. Short-term retention of verbal and nonverbal information was better in individuals with prolonged (5+ years) abstinence, compared with individuals with shorter durations of abstinence (Brandt et al., 1983); however, learning novel pairs of numbers and symbols was still impaired. By contrast, other studies reported improvement in episodic memory after several years of abstinence in AUD patients, who achieved then comparable performance to those of healthy controls (Fein et al., 2006; Reed et al., 1992; Rourke and Grant, 1999). Even in alcoholic patients with at least 6 months of sobriety, a longitudinal study showed normal levels of episodic memory performance when assessed with a selective reminding list learning test (Pitel et al., 2009). Although there is evidence for recovery in selective episodic memory processes such as list learning, other component episodic memory processes such as associative learning can remain impaired even with long-term abstinence (Brandt et al., 1983). To our knowledge, no study has been conducted to investigate cognitive recovery or improvement in semantic and cognitive procedural learning or in metamemory abilities with abstinence.
Deficits can persist with long-term abstinence in social cognition and emotional processing, which are abilities in decoding emotional facial expressions (Kornreich et al., 2001). Contradictory findings have been reported for processes related to ToM, with persistent deficits reported with prolonged abstinence in some (Bosco et al., 2014; Gizewski et al., 2013) but not all studies (Matyassy et al., 2006). Relapse associated with negative affect (e.g., depression) or social pressure represents approximately 70% of loss of sobriety after detoxification (Zywiak et al., 2003), suggesting that consideration of emotional and interpersonal difficulties is essential in clinical treatment for alcoholics. Additional studies are needed to specify the role of emotional and social cognition impairment in these types of relapses and how duration of sobriety contributes to scope and limits of recovery of emotional and social abilities.
In summary, although a number of cross-sectional and longitudinal studies provide evidence that abstinence is associated with improvement in cognitive functions, a meta-analysis of cognitive deficits in alcoholism suggested persistent dysfunctions in multiple cognitive processes even after weeks or months of abstinence (Stavro et al., 2013). The apparent slowdown in the course of recovery in cognitive functions could potentially be explained by factors such as age (Fein et al., 1990; Munro et al., 2000; Reed et al., 1992; Rourke and Grant, 1999), number of previous detoxifications (Loeber et al., 2010), use of cross-sectional design studies, and lack of consensus about the definition of interim drinking criteria used to classify abstainers and relapsers at follow-up.
III- Limitations of Knowledge to Date and Issues to Consider
Cognitive impairments commonly observed in alcoholism span deficits in executive functions and memory processes, including metacognitive difficulties such as metamemory impairment, and deficits in emotional and social cognitive abilities. Potential interactions among these deficits have yet to be fully appreciated or investigated. A new direction in the exploration of alcohol-related social and emotional processing impairments will further our understanding of the psychological processes underlying functional compromise characterizing alcoholism.
Within cognitive domains there remain debates concerning the varieties of component processes most affected in alcoholism. For example, are memory deficits in alcoholism primarily intrinsically mnemonic, or do they have their origin in executive dysfunction? Contradictory findings have emerged with episodic memory impairment in recently abstinent alcoholics not linked solely to executive dysfunction, suggesting genuine episodic memory deficits (Pitel et al., 2007a). By contrast, another study reported that episodic memory deficits were more related to impaired effortful executive processes in alcoholics than in controls (Noel et al., 2012a). To resolve this controversy, future studies need to investigate the interactions among component episodic memory and executive function processes in alcoholics and take account of alcohol history variables, including length of sobriety (cf., Fein et al., 2006), number of withdrawals (cf., Duka et al., 2003), and total lifetime alcohol consumption.
A few recent studies have explored interactions among component cognitive processes and provide evidence that episodic memory and executive component processes can affect higher-order abilities. Together, these processes may explain unawareness of memory impairment (i.e., metamemory decline) in AUD, as compromise of autonoetic consciousness and strategic mnemonic search abilities were the principal cognitive mechanisms of metamemory decline in recently abstinent alcoholic patients (Le Berre et al., 2010). These compounded deficits could also hamper the learning of new complex semantic and procedural information in alcohol treatment entry and, therefore, potentially could have negative effect on efficiency—the essential ability—of cognitive-behavioral therapies during clinical treatment (Pitel et al., 2007b).
Few studies in AUD have attempted to specify which basic specific cognitive functions support decision-making skills. This ability is reliant on multiple cognitive component processes that can include working memory, inhibition, rule deduction, and reversal learning skills (Dunn et al., 2006), with emotional processes also being potentially influential [for a somatic marker hypothesis of addiction, see (Verdejo-Garcia and Bechara, 2009)]. Among the sparse studies in this realm is one report showing that impaired decision-making performance under conditions of ambiguity could be related to poor response inhibition (Noel et al., 2007). Another study revealed a link between a deficit in decision-making under risky conditions and poor working memory (Brevers et al., 2014). Yet how these compromises affect treatment outcome remains unknown.
With the exception of an identified role of autobiographical memory for ToM abilities (Nandrino et al., 2014) and working memory for humor (Uekermann et al., 2007), the exploration of interactions between cognitive deficits (executive functions and memory) and emotional and social impairments are lacking. Fulfillment of this knowledge gap requires examination with the aim of providing relevant information about cognitive processes underlying these emotional and social alterations. These impairments should be considered as fundamental factors in the emotional and interpersonal difficulties experienced by alcoholic individuals in everyday life and as a consequence in their relapse or maintenance of sobriety.
Regarding the question of cognitive recovery with abstinence in alcoholism, one critical limitation stems from the use of cross-sectional studies to address longitudinal questions. Cross-sectional studies only allow for inferences about cognitive recovery, whereas longitudinal studies provide direct information about cognitive recovery over time. Tracking alcoholism’s dynamic course of sobriety and relapse is essential in revealing the potential and limits for recovery of cognitive abilities over time. Longitudinal studies offer the possibility to control for practice, aging, and sex effects when a matched control group is retested at comparable intervals and yield valuable comparisons between abstainers and relapsers. To our knowledge, only cross-sectional studies have been conducted on emotional and social cognition in alcoholic patients.
It has yet to be determined whether the course of recovery of emotional and social cognitive deficits is associated with duration of abstinence or whether the contribution of alcohol toxicity and risk factors are relevant to social cognitive impairment in alcoholism. Only longitudinal studies can impart new insight to these questions.
Further, interim drinking in itself is not clearly defined in the literature (Pitel et al., 2009) such that while some studies consider patients to still be abstainers (Johnson-Greene et al., 1997) having consumed a moderate amount of alcohol, others consider them as having relapsed (Rosenbloom et al., 2004; Rourke and Grant, 1999). Such a binary classification of relapse induces bias in subsequent observations and does not reflect the potential for recovery of relapsers, who have only resumed a limited amount of alcohol consumption without being at a dependent-level.
Whether cognitive deficits in AUD are the result of harmful consequences of excessive alcohol consumption, premorbid risk factors for addiction, or their combination remains unanswered. Family history studies have informed this area, with social cognitive deficits in high-risk individuals with a family history of alcoholism reported, suggesting that emotional and social impairments could be a risk factor in the development of AUD (Hill et al., 2007). Regarding decision-making deficits, a family history study (Lovallo et al., 2006) supported the premorbid vulnerability hypothesis, with individuals having a positive family history for alcoholism (FH+) demonstrating compromised decision-making abilities as assessed by the Iowa Gambling Task compared with individuals who did not have a family history of alcoholism (FH−). Although this finding suggests that deterioration or poor development of the decision-making processes can occur before the emergence of AUD, it does not negate the hypothesis that decision-making is also negatively affected as a result of chronic heavy drinking. Not all deficits, however, show a relationship with a positive family history of alcohol; for example, ToM impairment was not more prevalent in children of alcohol-dependent parents (Kopera et al., 2014). Exploration of links between cognitive performance and alcohol use variables [i.e., length of alcoholism, usual daily alcohol intake or number of withdrawals (cf., Duka et al., 2003; Loeber et al., 2009) could also constitute other ways to investigate this question. However, alcohol consumption information can only be estimated through historical interview, which is limited by recall accuracy, possibly underlying why such relationships have often eluded detection in alcoholics. Again, longitudinal studies would best inform this area of inquiry.
Finally, metamemory paradigms have successfully highlighted the lack of awareness of memory deficits in alcoholics early in abstinence (Le Berre et al., 2016; Le Berre et al., 2010). Because metamemory is a complex and multidimensional higher-order cognitive function, involving processing components relevant for monitoring and controlling memory (Flavell, 1971; Flavell and Wellman, 1977; Nelson and Narens, 1990), a theoretical framework (Nelson and Narens, 1990) was proposed that involved control and monitoring processes invoked during acquisition, retention, and retrieval of information. The control component refers to regulation applied during a mnemonic activity to improve memory performance, such as selection and use of strategies or decisions on allocation of time and cognitive resources, depending on task demands. The monitoring component assesses the progress and the success of memory functioning. Heretofore, metamemory abilities were essentially investigated by monitoring measures (i.e., FOK and RCJ measures) focused on the memory retrieval phase. Therefore, the exploration of metamemory in alcoholism could be extended by using other monitoring measures such as the ‘judgment of learning’ assessed during the learning phase and by considering the control component of metamemory.
CONCLUSION
Alcoholism is a complex, dynamic disease punctuated by periods of abstinence and relapse, and influenced by multiple vulnerability and resilience factors. This review highlights the cognitive deficits in executive functions, memory, and metacognitive abilities, with associated impairment in emotional processes and social cognition that occur in some alcoholics, and the variable recovery that occurs over time with abstinence. Despite extensive study of cognitive impairment and recovery, knowledge lacunae abound. For example, it is now critical to investigate the emotional and social components contributing to functional impairment in chronic alcoholism. One focus might be on the interaction among emotionally-based cognition processes and identification of vulnerability factors that play a role in the development of emotional and social processing deficits. Treatment focusing on improving level of awareness about impaired and spared cognitive and emotional processing deficits may reveal how an alcoholic compensates for functional compromise, similar to the approach taken by traumatic brain injury (TBI) rehabilitation programs.
To date, studies on cognitive recovery have largely included individuals with long-term sobriety, whereas alcoholics who relapse, who are notoriously difficult to track, are less often studied. Relapses (i.e., the resumption of alcohol drinking following a period of abstinence), however, are a crucial part of the addiction process and deserve attention, especially when after a period of abstinence, alcoholic patients who relapse may experience further decline in cognitive functioning (Pitel et al., 2009). Another factor seldom considered is the population studied. Specifically, most studies have examined treatment-seeking alcoholics, but this group reflects only about one-quarter of individuals with alcoholism (Smith and Fein, 2010).
An evolving aim of neuropsychology with respect to this multifactorial disease is the ability to identify alcoholics who are at particular risk of functional impairments in order to customize clinical treatment to increase the likelihood of sustained abstinence. Research focused on determinants or risk factors of cognitive deficits in alcoholics is even more urgent in light of the potential interactions and relations among vulnerability factors, alcohol consumption variables, and severity of cognitive and emotional impairment that have been elusive to or exclusive of quantitative, objective study. Prospective longitudinal studies, such as the NIH/NIAAA-supported National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA) (Brown et al., 2015), the Collaborative Studies on the Genetics of Alcoholism (COGA) (Begleiter, 1995) that study adolescents before initiating appreciable drinking, and now ABCD which is a longitudinal prospective study starting in preadolescence, can be particularly valuable by providing information to address the question of whether cognitive deficits observed in AUD are the harmful consequences of excessive alcohol consumption or a premorbid risk factor for addiction.
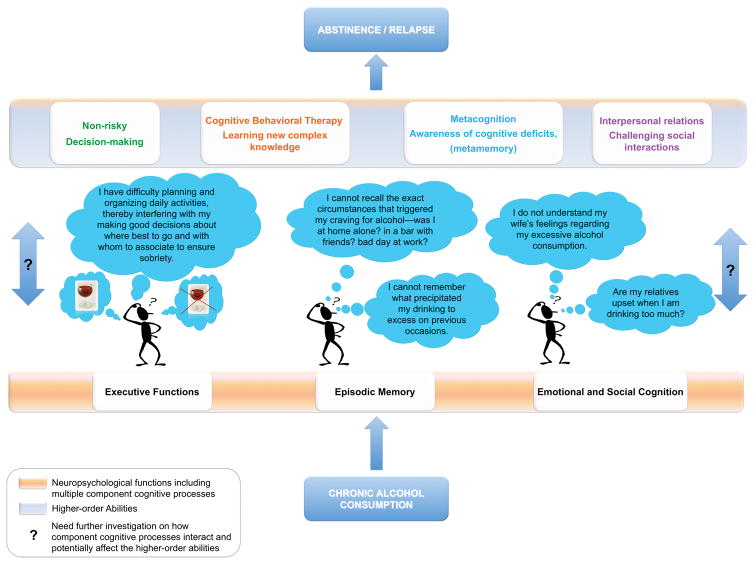
Figure.
Neuropsychological functions affected in chronic alcoholism compromising efforts to initiate and maintain abstinence by potentially hampering efficacy of clinical treatment (e.g., cognitive-behavioral therapy requiring learning new complex knowledge) and potentially obstructing efforts in enabling other higher-order abilities such as non-risky decision-making, success in interpersonal/social interactions, and awareness of cognitive and behavioral dysfunctions (i.e., accurate metacognition).
Blue arrows with question mark = Need of further investigation on how component cognitive processes interact and potentially affect the higher-order abilities.
Acknowledgments
The current analysis, writing, and manuscript preparation were supported by the U.S. National Institute on Alcohol Abuse and Alcoholism grants AA017168, AA017923, AA010723, and the Moldow Women’s Health and Hope Fund.
Footnotes
Conflict of Interest
None of the authors - Anne-Pascale Le Berre, Rosemary Fama, and Edith V. Sullivan - have conflicts of interest with the reported data or their interpretation.
References
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- Amenta S, Noel X, Verbanck P, Campanella S. Decoding of emotional components in complex communicative situations (irony) and its relation to empathic abilities in male chronic alcoholics: an issue for treatment. Alcohol Clin Exp Res. 2013;37:339–347. doi: 10.1111/j.1530-0277.2012.01909.x. [DOI] [PubMed] [Google Scholar]
- Ando B, Must A, Kurgyis E, Szkaliczki A, Drotos G, Rozsa S, Szikszay P, Horvath S, Janka Z, Almos PZ. Personality traits and coping compensate for disadvantageous decision-making in long-term alcohol abstinence. Alcohol Alcohol. 2012;47:18–24. doi: 10.1093/alcalc/agr144. [DOI] [PubMed] [Google Scholar]
- Assanangkornchai S, Srisurapanont M. The treatment of alcohol dependence. Curr Opin Psychiatry. 2007;20:222–227. doi: 10.1097/YCO.0b013e3280fa837d. [DOI] [PubMed] [Google Scholar]
- Bates ME, Bowden SC, Barry D. Neurocognitive impairment associated with alcohol use disorders: implications for treatment. Exp Clin Psychopharmacol. 2002;10:193–212. doi: 10.1037//1064-1297.10.3.193. [DOI] [PubMed] [Google Scholar]
- Bates ME, Pawlak AP, Tonigan JS, Buckman JF. Cognitive impairment influences drinking outcome by altering therapeutic mechanisms of change. Psychol Addict Behav. 2006;20:241–253. doi: 10.1037/0893-164X.20.3.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Moreland VJ, Nixon SJ. Neuropsychological performance of recently abstinent alcoholics and cocaine abusers. Drug Alcohol Depend. 1995;37:247–253. doi: 10.1016/0376-8716(94)01072-s. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Katzung VM, Nixon SJ, Moreland VJ. Problem-solving deficits in alcoholics: evidence from the California Card Sorting Test. J Stud Alcohol. 1993;54:687–692. doi: 10.15288/jsa.1993.54.687. [DOI] [PubMed] [Google Scholar]
- Begleiter H. The Collaborative Study on the Genetics of Alcoholism. Alcohol Health Res World. 1995;19:228–236. [PMC free article] [PubMed] [Google Scholar]
- Berglund M, Thelander S, Salaspuro M, Franck J, Andreasson S, Ojehagen A. Treatment of alcohol abuse: an evidence-based review. Alcohol Clin Exp Res. 2003;27:1645–1656. doi: 10.1097/01.ALC.0000090144.99832.19. [DOI] [PubMed] [Google Scholar]
- Blume AW, Schmaling KB, Marlatt GA. Memory, executive cognitive function, and readiness to change drinking behavior. Addict Behav. 2005;30:301–314. doi: 10.1016/j.addbeh.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Bora E, Zorlu N. Social cognition in alcohol use disorder: a meta-analysis. Addiction. 2016 doi: 10.1111/add.13486. [DOI] [PubMed] [Google Scholar]
- Bosco FM, Capozzi F, Colle L, Marostica P, Tirassa M. Theory of mind deficit in subjects with alcohol use disorder: an analysis of mindreading processes. Alcohol Alcohol. 2014;49:299–307. doi: 10.1093/alcalc/agt148. [DOI] [PubMed] [Google Scholar]
- Brandt J, Butters N, Ryan C, Bayog R. Cognitive loss and recovery in long-term alcohol abusers. Arch Gen Psychiatry. 1983;40:435–442. doi: 10.1001/archpsyc.1983.01790040089012. [DOI] [PubMed] [Google Scholar]
- Brevers D, Bechara A, Cleeremans A, Kornreich C, Verbanck P, Noel X. Impaired decision-making under risk in individuals with alcohol dependence. Alcohol Clin Exp Res. 2014;38:1924–1931. doi: 10.1111/acer.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SA, Brumback T, Tomlinson K, Cummins K, Thompson WK, Nagel BJ, De Bellis MD, Hooper SR, Clark DB, Chung T, Hasler BP, Colrain IM, Baker FC, Prouty D, Pfefferbaum A, Sullivan EV, Pohl KM, Rohlfing T, Nichols BN, Chu W, Tapert SF. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J Stud Alcohol Drugs. 2015;76:895–908. doi: 10.15288/jsad.2015.76.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Endres M, Fein G. PFEFFERBAUM EVSAA, editor. Handbook of Clinical Neurology, Vol. 125, Handbook of Clinical Neurology. Elsevier; 2014. Decision making, risky behavior, and alcoholism; pp. 227–236. [DOI] [PubMed] [Google Scholar]
- Castellano F, Bartoli F, Crocamo C, Gamba G, Tremolada M, Santambrogio J, Clerici M, Carra G. Facial emotion recognition in alcohol and substance use disorders: A meta-analysis. Neurosci Biobehav Rev. 2015;59:147–154. doi: 10.1016/j.neubiorev.2015.11.001. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Martelli C, Delain F, Kostogianni N, Douaud G, Aubin HJ, Reynaud M, Martinot JL. Brain morphometry and cognitive performance in detoxified alcohol-dependents with preserved psychosocial functioning. Neuropsychopharmacology. 2007;32:429–438. doi: 10.1038/sj.npp.1301219. [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Remapping the brain to compensate for impairment in recovering alcoholics. Cereb Cortex. 2013;23:97–104. doi: 10.1093/cercor/bhr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay SW, Allen J, Parran T. A review of addiction. Postgrad Med. 2008;120:E01–07. doi: 10.3810/pgm.2008.07.1802. [DOI] [PubMed] [Google Scholar]
- Conway MA. Sensory-perceptual episodic memory and its context: autobiographical memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1375–1384. doi: 10.1098/rstb.2001.0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Argembeau A, Van Der Linden M, Verbanck P, Noel X. Autobiographical memory in non-amnesic alcohol-dependent patients. Psychol Med. 2006;36:1707–1715. doi: 10.1017/S0033291706008798. [DOI] [PubMed] [Google Scholar]
- D’Hondt F, Campanella S, Kornreich C, Philippot P, Maurage P. Below and beyond the recognition of emotional facial expressions in alcohol dependence: from basic perception to social cognition. Neuropsychiatr Dis Treat. 2014;10:2177–2182. doi: 10.2147/NDT.S74963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dao-Castellana MH, Samson Y, Legault F, Martinot JL, Aubin HJ, Crouzel C, Feldman L, Barrucand D, Rancurel G, Feline A, Syrota A. Frontal dysfunction in neurologically normal chronic alcoholic subjects: metabolic and neuropsychological findings. Psychol Med. 1998;28:1039–1048. doi: 10.1017/s0033291798006849. [DOI] [PubMed] [Google Scholar]
- Davies SJ, Pandit SA, Feeney A, Stevenson BJ, Kerwin RW, Nutt DJ, Marshall EJ, Boddington S, Lingford-Hughes A. Is there cognitive impairment in clinically ‘healthy’ abstinent alcohol dependence? Alcohol Alcohol. 2005;40:498–503. doi: 10.1093/alcalc/agh203. [DOI] [PubMed] [Google Scholar]
- Dom G, De Wilde B, Hulstijn W, van den Brink W, Sabbe B. Decision-making deficits in alcohol-dependent patients with and without comorbid personality disorder. Alcohol Clin Exp Res. 2006;30:1670–1677. doi: 10.1111/j.1530-0277.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- Donadon MF, Osorio Fde L. Recognition of facial expressions by alcoholic patients: a systematic literature review. Neuropsychiatr Dis Treat. 2014;10:1655–1663. doi: 10.2147/NDT.S65376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duka T, Townshend JM, Collier K, Stephens DN. Impairment in cognitive functions after multiple detoxifications in alcoholic inpatients. Alcohol Clin Exp Res. 2003;27:1563–1572. doi: 10.1097/01.ALC.0000090142.11260.D7. [DOI] [PubMed] [Google Scholar]
- Dunn BD, Dalgleish T, Lawrence AD. The somatic marker hypothesis: a critical evaluation. Neurosci Biobehav Rev. 2006;30:239–271. doi: 10.1016/j.neubiorev.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Everett M, Schaeffer KW, Parsons OA. Learning impairment in male and female alcoholics. Arch Clin Neuropsychol. 1988;3:203–211. [PubMed] [Google Scholar]
- Fabian MS, Parsons OA. Differential improvement of cognitive functions in recovering alcoholic women. J Abnorm Psychol. 1983;92:87–95. doi: 10.1037//0021-843x.92.1.87. [DOI] [PubMed] [Google Scholar]
- Fama R, Pfefferbaum A, Sullivan EV. Perceptual learning in detoxified alcoholic men: contributions from explicit memory, executive function, and age. Alcohol Clin Exp Res. 2004;28:1657–1665. doi: 10.1097/01.alc.0000145690.48510.da. [DOI] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Sassoon SA, Pfefferbaum A, Sullivan EV. Differential effect of alcoholism and HIV infection on visuomotor procedural learning and retention. Alcohol Clin Exp Res. 2012;36:1738–1747. doi: 10.1111/j.1530-0277.2012.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Bachman L, Fisher S, Davenport L. Cognitive impairments in abstinent alcoholics. West J Med. 1990;152:531–537. [PMC free article] [PubMed] [Google Scholar]
- Fein G, Klein L, Finn P. Impairment on a simulated gambling task in long-term abstinent alcoholics. Alcohol Clin Exp Res. 2004;28:1487–1491. doi: 10.1097/01.alc.0000141642.39065.9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Torres J, Price LJ, Di Sclafani V. Cognitive performance in long-term abstinent alcoholic individuals. Alcohol Clin Exp Res. 2006;30:1538–1544. doi: 10.1111/j.1530-0277.2006.00185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Serrano MJ, Perez-Garcia M, Schmidt Rio-Valle J, Verdejo-Garcia A. Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. J Psychopharmacol. 2010;24:1317–1332. doi: 10.1177/0269881109349841. [DOI] [PubMed] [Google Scholar]
- Flavell JH. First Discussant’s Comments: What is Memory Development the Development of? Human Development. 1971;14:272–278. [Google Scholar]
- Flavell JH, Wellman HM. Metamemory. In: KAIL RV, HAGEN JW, editors. Perspectives on the development of memory and cognition, Perspectives on the development of memory and cognition. Laurence Erlbaum Associates; Hillsdale, NJ: 1977. pp. 3–33. [Google Scholar]
- Frith CD. Social cognition. Philos Trans R Soc Lond B Biol Sci. 2008;363:2033–2039. doi: 10.1098/rstb.2008.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Frith U. Interacting minds--a biological basis. Science. 1999;286:1692–1695. doi: 10.1126/science.286.5445.1692. [DOI] [PubMed] [Google Scholar]
- Gizewski ER, Muller BW, Scherbaum N, Lieb B, Forsting M, Wiltfang J, Leygraf N, Schiffer B. The impact of alcohol dependence on social brain function. Addict Biol. 2013;18:109–120. doi: 10.1111/j.1369-1600.2012.00437.x. [DOI] [PubMed] [Google Scholar]
- Glenn S, Parsons OA, Sinha R. Assessment of recovery of electrophysiological and neuropsychological functions in chronic alcoholics. Biol Psychiatry. 1994;36:443–452. doi: 10.1016/0006-3223(94)90639-4. [DOI] [PubMed] [Google Scholar]
- Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Brain Res Cogn Brain Res. 2005;23:137–151. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Griffiths A, Hill R, Morgan C, Rendell PG, Karimi K, Wanagaratne S, Curran HV. Prospective memory and future event simulation in individuals with alcohol dependence. Addiction. 2012;107:1809–1816. doi: 10.1111/j.1360-0443.2012.03941.x. [DOI] [PubMed] [Google Scholar]
- Hannesdottir K, Morris RG. Primary and secondary anosognosia for memory impairment in patients with Alzheimer’s disease. Cortex. 2007;43:1020–1030. doi: 10.1016/s0010-9452(08)70698-1. [DOI] [PubMed] [Google Scholar]
- Hart JT. Memory and the feeling-of-knowing experience. J Educ Psychol. 1965;56:208–216. doi: 10.1037/h0022263. [DOI] [PubMed] [Google Scholar]
- Hill SY, Kostelnik B, Holmes B, Goradia D, McDermott M, Diwadkar V, Keshavan M. fMRI BOLD response to the eyes task in offspring from multiplex alcohol dependence families. Alcohol Clin Exp Res. 2007;31:2028–2035. doi: 10.1111/j.1530-0277.2007.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochla NA, Fabian MS, Parsons OA. Brain-age quotients in recently detoxified alcoholic, recovered alcoholic and nonalcoholic women. J Clin Psychol. 1982;38:207–212. doi: 10.1002/1097-4679(198201)38:1<207::aid-jclp2270380135>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Johnson-Greene D, Adams KM, Gilman S, Koeppe RA, Junck L, Kluin KJ, Martorello S, Heumann M. Effects of abstinence and relapse upon neuropsychological function and cerebral glucose metabolism in severe chronic alcoholism. J Clin Exp Neuropsychol. 1997;19:378–385. doi: 10.1080/01688639708403866. [DOI] [PubMed] [Google Scholar]
- Joyce EM, Robbins TW. Frontal lobe function in Korsakoff and non-Korsakoff alcoholics: planning and spatial working memory. Neuropsychologia. 1991;29:709–723. doi: 10.1016/0028-3932(91)90067-i. [DOI] [PubMed] [Google Scholar]
- Jung YC, Schulte T, Muller-Oehring EM, Namkoong K, Pfefferbaum A, Sullivan EV. Compromised frontocerebellar circuitry contributes to nonplanning impulsivity in recovering alcoholics. Psychopharmacology (Berl) 2014;231:4443–4453. doi: 10.1007/s00213-014-3594-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera M, Glass JM, Heitzeg MM, Wojnar M, Puttler LI, Zucker RA. Theory of mind among young adult children from alcoholic families. J Stud Alcohol Drugs. 2014;75:889–894. doi: 10.15288/jsad.2014.75.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopera M, Wojnar M, Brower K, Glass J, Nowosad I, Gmaj B, Szelenberger W. Cognitive functions in abstinent alcohol-dependent patients. Alcohol. 2012;46:666–671. doi: 10.1016/j.alcohol.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Blairy S, Philippot P, Hess U, Noel X, Streel E, Le Bon O, Dan B, Pelc I, Verbanck P. Deficits in recognition of emotional facial expression are still present in alcoholics after mid- to long-term abstinence. J Stud Alcohol. 2001;62:533–542. doi: 10.15288/jsa.2001.62.533. [DOI] [PubMed] [Google Scholar]
- Kornreich C, Philippot P, Foisy ML, Blairy S, Raynaud E, Dan B, Hess U, Noel X, Pelc I, Verbanck P. Impaired emotional facial expression recognition is associated with interpersonal problems in alcoholism. Alcohol Alcohol. 2002;37:394–400. doi: 10.1093/alcalc/37.4.394. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction. 2009;104:1006–1015. doi: 10.1111/j.1360-0443.2009.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Muller-Oehring EM, Kwon D, Serventi MR, Pfefferbaum A, Sullivan EV. Differential compromise of prospective and retrospective metamemory monitoring and their dissociable structural brain correlates. Cortex. 2016;81:192–202. doi: 10.1016/j.cortex.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Pinon K, Vabret F, Pitel AL, Allain P, Eustache F, Beaunieux H. Study of metamemory in patients with chronic alcoholism using a feeling-of-knowing episodic memory task. Alcohol Clin Exp Res. 2010;34:1888–1898. doi: 10.1111/j.1530-0277.2010.01277.x. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Rauchs G, La Joie R, Mezenge F, Boudehent C, Vabret F, Segobin S, Viader F, Allain P, Eustache F, Pitel AL, Beaunieux H. Impaired decision-making and brain shrinkage in alcoholism. Eur Psychiatry. 2014;29:125–133. doi: 10.1016/j.eurpsy.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Le Berre AP, Sullivan EV. Anosognosia for Memory Impairment in Addiction: Insights from Neuroimaging and Neuropsychological Assessment of Metamemory. Neuropsychol Rev. 2016 doi: 10.1007/s11065-016-9323-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Vabret F, Cauvin C, Pinon K, Allain P, Pitel AL, Eustache F, Beaunieux H. Cognitive barriers to readiness to change in alcohol-dependent patients. Alcohol Clin Exp Res. 2012;36:1542–1549. doi: 10.1111/j.1530-0277.2012.01760.x. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel H, Nakovics H, Heinz A, Flor H, Mann K. Impairment of cognitive abilities and decision making after chronic use of alcohol: the impact of multiple detoxifications. Alcohol Alcohol. 2009;44:372–381. doi: 10.1093/alcalc/agp030. [DOI] [PubMed] [Google Scholar]
- Loeber S, Duka T, Welzel Marquez H, Nakovics H, Heinz A, Mann K, Flor H. Effects of repeated withdrawal from alcohol on recovery of cognitive impairment under abstinence and rate of relapse. Alcohol Alcohol. 2010;45:541–547. doi: 10.1093/alcalc/agq065. [DOI] [PubMed] [Google Scholar]
- Lovallo WR, Yechiam E, Sorocco KH, Vincent AS, Collins FL. Working memory and decision-making biases in young adults with a family history of alcoholism: studies from the Oklahoma family health patterns project. Alcohol Clin Exp Res. 2006;30:763–773. doi: 10.1111/j.1530-0277.2006.00089.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ, Kessler J, Denzler P. Recognition memory and psychophysiological responses to stimuli with neutral or emotional content: a study of Korsakoff patients and recently detoxified and longterm abstinent alcoholics. Int J Neurosci. 1986;29:1–35. doi: 10.3109/00207458608985632. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Tedeschi D, Cundari S, Janiri L. Empathy ability is impaired in alcohol-dependent patients. Am J Addict. 2009;18:157–161. doi: 10.1080/10550490802544391. [DOI] [PubMed] [Google Scholar]
- Matyassy A, Kelemen O, Sarkozi Z, Janka Z, Keri S. Recognition of complex mental states in patients with alcoholism after long-term abstinence. Alcohol Alcohol. 2006;41:512–514. doi: 10.1093/alcalc/agl045. [DOI] [PubMed] [Google Scholar]
- Maurage F, de Timary P, Tecco JM, Lechantre S, Samson D. Theory of mind difficulties in patients with alcohol dependence: beyond the prefrontal cortex dysfunction hypothesis. Alcohol Clin Exp Res. 2015;39:980–988. doi: 10.1111/acer.12717. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Charest I, Martin S, de Timary P. Impaired emotional facial expression decoding in alcoholism is also present for emotional prosody and body postures. Alcohol Alcohol. 2009;44:476–485. doi: 10.1093/alcalc/agp037. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Martin S, de Timary P. Face processing in chronic alcoholism: a specific deficit for emotional features. Alcohol Clin Exp Res. 2008;32:600–606. doi: 10.1111/j.1530-0277.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- Maurage P, Campanella S, Philippot P, Pham TH, Joassin F. The crossmodal facilitation effect is disrupted in alcoholism: a study with emotional stimuli. Alcohol Alcohol. 2007;42:552–559. doi: 10.1093/alcalc/agm134. [DOI] [PubMed] [Google Scholar]
- Maurage P, D’Hondt F, de Timary P, Mary C, Franck N, Peyroux E. Dissociating Affective and Cognitive Theory of Mind in Recently Detoxified Alcohol-Dependent Individuals. Alcohol Clin Exp Res. 2016;40:1926–1934. doi: 10.1111/acer.13155. [DOI] [PubMed] [Google Scholar]
- Maurage P, Grynberg D, Noel X, Joassin F, Hanak C, Verbanck P, Luminet O, de Timary P, Campanella S, Philippot P. The “Reading the Mind in the Eyes” test as a new way to explore complex emotions decoding in alcohol dependence. Psychiatry Res. 2011a;190:375–378. doi: 10.1016/j.psychres.2011.06.015. [DOI] [PubMed] [Google Scholar]
- Maurage P, Grynberg D, Noel X, Joassin F, Philippot P, Hanak C, Verbanck P, Luminet O, de Timary P, Campanella S. Dissociation between affective and cognitive empathy in alcoholism: a specific deficit for the emotional dimension. Alcohol Clin Exp Res. 2011b;35:1662–1668. doi: 10.1111/j.1530-0277.2011.01512.x. [DOI] [PubMed] [Google Scholar]
- Maurage P, Joassin F, Pesenti M, Grandin C, Heeren A, Philippot P, de Timary P. The neural network sustaining crossmodal integration is impaired in alcohol-dependence: an fMRI study. Cortex. 2013;49:1610–1626. doi: 10.1016/j.cortex.2012.04.012. [DOI] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Alcohol Clin Exp Res. 2000;24:1036–1040. [PubMed] [Google Scholar]
- Miranda R, Jr, MacKillop J, Meyerson LA, Justus A, Lovallo WR. Influence of antisocial and psychopathic traits on decision-making biases in alcoholics. Alcohol Clin Exp Res. 2009;33:817–825. doi: 10.1111/j.1530-0277.2009.00901.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mograbi DC, Brown RG, Morris RG. Anosognosia in Alzheimer’s disease--the petrified self. Conscious Cogn. 2009;18:989–1003. doi: 10.1016/j.concog.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Monnot M, Lovallo WR, Nixon SJ, Ross E. Neurological basis of deficits in affective prosody comprehension among alcoholics and fetal alcohol-exposed adults. J Neuropsychiatry Clin Neurosci. 2002;14:321–328. doi: 10.1176/jnp.14.3.321. [DOI] [PubMed] [Google Scholar]
- Monnot M, Nixon S, Lovallo W, Ross E. Altered emotional perception in alcoholics: deficits in affective prosody comprehension. Alcohol Clin Exp Res. 2001;25:362–369. [PubMed] [Google Scholar]
- Moriyama Y, Mimura M, Kato M, Yoshino A, Hara T, Kashima H, Kato A, Watanabe A. Executive dysfunction and clinical outcome in chronic alcoholics. Alcohol Clin Exp Res. 2002;26:1239–1244. doi: 10.1097/01.ALC.0000026103.08053.86. [DOI] [PubMed] [Google Scholar]
- Morris RG, Mograbi DC. Anosognosia, autobiographical memory and self knowledge in Alzheimer’s disease. Cortex. 2013;49:1553–1565. doi: 10.1016/j.cortex.2012.09.006. [DOI] [PubMed] [Google Scholar]
- Munro CA, Saxton J, Butters MA. The neuropsychological consequences of abstinence among older alcoholics: a cross-sectional study. Alcohol Clin Exp Res. 2000;24:1510–1516. [PubMed] [Google Scholar]
- Nandrino JL, El Haj M, Torre J, Naye D, Douchet H, Danel T, Cottencin O. Autobiographical Memory Deficits in Alcohol-Dependent Patients with Short- and Long-Term Abstinence. Alcohol Clin Exp Res. 2016;40:865–873. doi: 10.1111/acer.13001. [DOI] [PubMed] [Google Scholar]
- Nandrino JL, Gandolphe MC, Alexandre C, Kmiecik E, Yguel J, Urso L. Cognitive and affective theory of mind abilities in alcohol-dependent patients: the role of autobiographical memory. Drug Alcohol Depend. 2014;143:65–73. doi: 10.1016/j.drugalcdep.2014.07.010. [DOI] [PubMed] [Google Scholar]
- Nelson TO, Narens L. Metamemory: a theoretical framework and new finding. In: GHB, editor. The Psychology of Learning and Motivation, The Psychology of Learning and Motivation. Academic Press; New York: 1990. pp. 125–141. [Google Scholar]
- Nixon SJ, Parsons OA. Alcohol-related efficiency deficits using an ecologically valid test. Alcohol Clin Exp Res. 1991;15:601–606. doi: 10.1111/j.1530-0277.1991.tb00567.x. [DOI] [PubMed] [Google Scholar]
- Noel X, Bechara A, Dan B, Hanak C, Verbanck P. Response inhibition deficit is involved in poor decision making under risk in nonamnesic individuals with alcoholism. Neuropsychology. 2007;21:778–786. doi: 10.1037/0894-4105.21.6.778. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, Brevers D, Campanella S, Hanak C, Kornreich C, Verbanck P. The contribution of executive functions deficits to impaired episodic memory in individuals with alcoholism. Psychiatry Res. 2012a;198:116–122. doi: 10.1016/j.psychres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, Brevers D, Campanella S, Hanak C, Kornreich C, Verbanck P. The contribution of executive functions deficits to impaired episodic memory in individuals with alcoholism. Psychiatry Research. 2012b;198:116–122. doi: 10.1016/j.psychres.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Noel X, Van der Linden M, Schmidt N, Sferrazza R, Hanak C, Le Bon O, De Mol J, Kornreich C, Pelc I, Verbanck P. Supervisory attentional system in nonamnesic alcoholic men. Arch Gen Psychiatry. 2001;58:1152–1158. doi: 10.1001/archpsyc.58.12.1152. [DOI] [PubMed] [Google Scholar]
- Nowakowska-Domagala K, Jablkowska-Gorecka K, Mokros L, Koprowicz J, Pietras T. Differences in the verbal fluency, working memory and executive functions in alcoholics: Short-term vs. long-term abstainers. Psychiatry Res. 2017;249:1–8. doi: 10.1016/j.psychres.2016.12.034. [DOI] [PubMed] [Google Scholar]
- O’Leary MR, Radford LM, Chaney EF, Schau EJ. Assessment of cognitive recovery in alcoholics by use of the trail-making test. J Clin Psychol. 1977;33:579–582. doi: 10.1002/1097-4679(197704)33:2<579::aid-jclp2270330254>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Onuoha RC, Quintana DS, Lyvers M, Guastella AJ. A Meta-analysis of Theory of Mind in Alcohol Use Disorders. Alcohol Alcohol. 2016;51:410–415. doi: 10.1093/alcalc/agv137. [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Marinkovic K. Alcohol: effects on neurobehavioral functions and the brain. Neuropsychol Rev. 2007;17:239–257. doi: 10.1007/s11065-007-9038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Kirkley SM, Gansler DA, Merritt D, Couture A. Frontal brain dysfunction in alcoholism with and without antisocial personality disorder. Neuropsychiatr Dis Treat. 2009;5:309–326. doi: 10.2147/ndt.s4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, Gravitz ZR. Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol. 2014;125:183–210. doi: 10.1016/B978-0-444-62619-6.00012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons OA. Cognitive dysfunction and recovery in alcoholics. Subst Alcohol Actions Misuse. 1983;4:175–190. [PubMed] [Google Scholar]
- Parsons OA, Schaeffer KW, Glenn SW. Does neuropsychological test performance predict resumption of drinking in posttreatment alcoholics? Addict Behav. 1990;15:297–307. doi: 10.1016/0306-4603(90)90073-7. [DOI] [PubMed] [Google Scholar]
- Philippot P, Kornreich C, Blairy S, Baert I, Den Dulk A, Le Bon O, Streel E, Hess U, Pelc I, Verbanck P. Alcoholics’ deficits in the decoding of emotional facial expression. Alcohol Clin Exp Res. 1999;23:1031–1038. [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, Quinette P, Desgranges B, Eustache F. Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol Clin Exp Res. 2007a;31:1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Rivier J, Beaunieux H, Vabret F, Desgranges B, Eustache F. Changes in the episodic memory and executive functions of abstinent and relapsed alcoholics over a 6-month period. Alcohol Clin Exp Res. 2009;33:490–498. doi: 10.1111/j.1530-0277.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- Pitel AL, Witkowski T, Vabret F, Guillery-Girard B, Desgranges B, Eustache F, Beaunieux H. Effect of episodic and working memory impairments on semantic and cognitive procedural learning at alcohol treatment entry. Alcohol Clin Exp Res. 2007b;31:238–248. doi: 10.1111/j.1530-0277.2006.00301.x. [DOI] [PubMed] [Google Scholar]
- Premack D, Woodruff G. Does the chimpanzee have a theory of mind? Behav Brain Sci. 1978;1:512–526. [Google Scholar]
- Ratti MT, Bo P, Giardini A, Soragna D. Chronic alcoholism and the frontal lobe: which executive functions are imparied? Acta Neurol Scand. 2002;105:276–281. doi: 10.1034/j.1600-0404.2002.0o315.x. [DOI] [PubMed] [Google Scholar]
- Reed RJ, Grant I, Rourke SB. Long-term abstinent alcoholics have normal memory. Alcohol Clin Exp Res. 1992;16:677–683. doi: 10.1111/j.1530-0277.1992.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, O’Reilly A, Sassoon SA, Sullivan EV, Pfefferbaum A. Persistent cognitive deficits in community-treated alcoholic men and women volunteering for research: limited contribution from psychiatric comorbidity. J Stud Alcohol. 2005;66:254–265. doi: 10.15288/jsa.2005.66.254. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, Sullivan EV. Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology. 2004;18:589–597. doi: 10.1037/0894-4105.18.3.589. [DOI] [PubMed] [Google Scholar]
- Rosenbloom MJ, Rohlfing T, O’Reilly AW, Sassoon SA, Pfefferbaum A, Sullivan EV. Improvement in memory and static balance with abstinence in alcoholic men and women: selective relations with change in brain structure. Psychiatry Res. 2007;155:91–102. doi: 10.1016/j.pscychresns.2006.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Grant I. The interactive effects of age and length of abstinence on the recovery of neuropsychological functioning in chronic male alcoholics: a 2-year follow-up study. J Int Neuropsychol Soc. 1999;5:234–246. doi: 10.1017/s1355617799533067. [DOI] [PubMed] [Google Scholar]
- Salgado JV, Malloy-Diniz LF, Campos VR, Abrantes SS, Fuentes D, Bechara A, Correa H. Neuropsychological assessment of impulsive behavior in abstinent alcohol-dependent subjects. Rev Bras Psiquiatr. 2009;31:4–9. doi: 10.1590/s1516-44462009000100003. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Butters N, Schuckit M. Memory for temporal order and frequency of occurrence in detoxified alcoholics. Alcohol. 1986;3:323–329. doi: 10.1016/0741-8329(86)90009-1. [DOI] [PubMed] [Google Scholar]
- Schaeffer KW, Parsons OA. Learning impairment in alcoholics using an ecologically relevant test. J Nerv Ment Dis. 1987;175:213–218. doi: 10.1097/00005053-198704000-00004. [DOI] [PubMed] [Google Scholar]
- Schwartz BL, Parker ES, Deutsch SI, Rosse RB, Kaushik M, Isaac A. Source monitoring in alcoholism. J Clin Exp Neuropsychol. 2002;24:806–817. doi: 10.1076/jcen.24.6.806.8406. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Shur S, Barcai-Goodman L, Medlovich S, Harari H, Levkovitz Y. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res. 2007;149:11–23. doi: 10.1016/j.psychres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Sherer M, Nixon SJ, Parsons OA, Adams RL. Performance of alcoholic and brain-damaged subjects on the Luria Memory Words test. Arch Clin Neuropsychol. 1992;7:499–504. [PubMed] [Google Scholar]
- Smith S, Fein G. Cognitive performance in treatment-naive active alcoholics. Alcohol Clin Exp Res. 2010;34:2097–2105. doi: 10.1111/j.1530-0277.2010.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Declarative and nondeclarative memory: multiple brain systems supporting learning and memory. J Cogn Neurosci. 1992;4:232–243. doi: 10.1162/jocn.1992.4.3.232. [DOI] [PubMed] [Google Scholar]
- Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol Learn Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Stasiewicz PR, Bradizza CM, Gudleski GD, Coffey SF, Schlauch RC, Bailey ST, Bole CW, Gulliver SB. The relationship of alexithymia to emotional dysregulation within an alcohol dependent treatment sample. Addict Behav. 2012;37:469–476. doi: 10.1016/j.addbeh.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, Potvin S. Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol. 2013;18:203–213. doi: 10.1111/j.1369-1600.2011.00418.x. [DOI] [PubMed] [Google Scholar]
- Sullivan E, Harris R, Pfefferbaum A. Alcohol’s effects on brain and behavior. Alcohol Research & Health. 2010;33:127–143. [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Fama R, Rosenbloom MJ, Pfefferbaum A. A profile of neuropsychological deficits in alcoholic women. Neuropsychology. 2002;16:74–83. doi: 10.1037//0894-4105.16.1.74. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Ha CN, Zipursky RB, Pfefferbaum A. The contribution of constructional accuracy and organizational strategy to nonverbal recall in schizophrenia and chronic alcoholism. Biol Psychiatry. 1992;32:312–333. doi: 10.1016/0006-3223(92)90036-y. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Mathalon DH, Zipursky RB, Kersteen-Tucker Z, Knight RT, Pfefferbaum A. Factors of the Wisconsin Card Sorting Test as measures of frontal-lobe function in schizophrenia and in chronic alcoholism. Psychiatry Res. 1993;46:175–199. doi: 10.1016/0165-1781(93)90019-d. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Pfefferbaum A. Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl) 2005;180:583–594. doi: 10.1007/s00213-005-2267-6. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Lim KO, Pfefferbaum A. Longitudinal changes in cognition, gait, and balance in abstinent and relapsed alcoholic men: relationships to changes in brain structure. Neuropsychology. 2000a;14:178–188. [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom MJ, Pfefferbaum A. Pattern of motor and cognitive deficits in detoxified alcoholic men. Alcohol Clin Exp Res. 2000b;24:611–621. [PubMed] [Google Scholar]
- Sullivan EV, Shear PK, Zipursky RB, Sagar HJ, Pfefferbaum A. Patterns of content, contextual, and working memory impairments in schizophrenia and nonamnesic alcoholism. Neuropsychology. 1997;11:195–206. doi: 10.1037//0894-4105.11.2.195. [DOI] [PubMed] [Google Scholar]
- Tarter RE. An analysis of cognitive deficits in chronic alcoholics. J Nerv Ment Dis. 1973;157:138–147. doi: 10.1097/00005053-197308000-00006. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Parsons OA. Conceptual shifting in chronic alcoholics. J Abnorm Psychol. 1971;77:71–75. doi: 10.1037/h0030491. [DOI] [PubMed] [Google Scholar]
- Tedstone D, Coyle K. Cognitive impairments in sober alcoholics: performance on selective and divided attention tasks. Drug Alcohol Depend. 2004;75:277–286. doi: 10.1016/j.drugalcdep.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Thoma P, Winter N, Juckel G, Roser P. Mental state decoding and mental state reasoning in recently detoxified alcohol-dependent individuals. Psychiatry Res. 2013;205:232–240. doi: 10.1016/j.psychres.2012.08.042. [DOI] [PubMed] [Google Scholar]
- Tivis R, Beatty WW, Nixon SJ, Parsons OA. Patterns of cognitive impairment among alcoholics: are there subtypes? Alcohol Clin Exp Res. 1995;19:496–500. doi: 10.1111/j.1530-0277.1995.tb01537.x. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory and common sense: how far apart? Philos Trans R Soc Lond B Biol Sci. 2001;356:1505–1515. doi: 10.1098/rstb.2001.0937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Tulving E, Schacter DL, McLachlan DR, Moscovitch M. Priming of semantic autobiographical knowledge: a case study of retrograde amnesia. Brain Cogn. 1988;8:3–20. doi: 10.1016/0278-2626(88)90035-8. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Channon S, Winkel K, Schlebusch P, Daum I. Theory of mind, humour processing and executive functioning in alcoholism. Addiction. 2007;102:232–240. doi: 10.1111/j.1360-0443.2006.01656.x. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I. Social cognition in alcoholism: a link to prefrontal cortex dysfunction? Addiction. 2008;103:726–735. doi: 10.1111/j.1360-0443.2008.02157.x. [DOI] [PubMed] [Google Scholar]
- Uekermann J, Daum I, Schlebusch P, Trenckmann U. Processing of affective stimuli in alcoholism. Cortex. 2005;41:189–194. doi: 10.1016/s0010-9452(08)70893-1. [DOI] [PubMed] [Google Scholar]
- Uzun O, Ates A, Cansever A, Ozsahin A. Alexithymia in male alcoholics: study in a Turkish sample. Compr Psychiatry. 2003;44:349–352. doi: 10.1016/S0010-440X(03)00009-9. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56(Suppl 1):48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Perez-Garcia M. Substance abusers’ self-awareness of the neurobehavioral consequences of addiction. Psychiatry Res. 2008;158:172–180. doi: 10.1016/j.psychres.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Wheeler MA, Stuss DT, Tulving E. Toward a theory of episodic memory: the frontal lobes and autonoetic consciousness. Psychol Bull. 1997;121:331–354. doi: 10.1037/0033-2909.121.3.331. [DOI] [PubMed] [Google Scholar]
- Yohman JR, Parsons OA, Leber WR. Lack of recovery in male alcoholics’ neuropsychological performance one year after treatment. Alcohol Clin Exp Res. 1985;9:114–117. doi: 10.1111/j.1530-0277.1985.tb05530.x. [DOI] [PubMed] [Google Scholar]
- Zinn S, Stein R, Swartzwelder HS. Executive functioning early in abstinence from alcohol. Alcohol Clin Exp Res. 2004;28:1338–1346. doi: 10.1097/01.alc.0000139814.81811.62. [DOI] [PubMed] [Google Scholar]
- Zywiak WH, Westerberg VS, Connors GJ, Maisto SA. Exploratory findings from the Reasons for Drinking Questionnaire. J Subst Abuse Treat. 2003;25:287–292. doi: 10.1016/s0740-5472(03)00118-1. [DOI] [PubMed] [Google Scholar]