Abstract
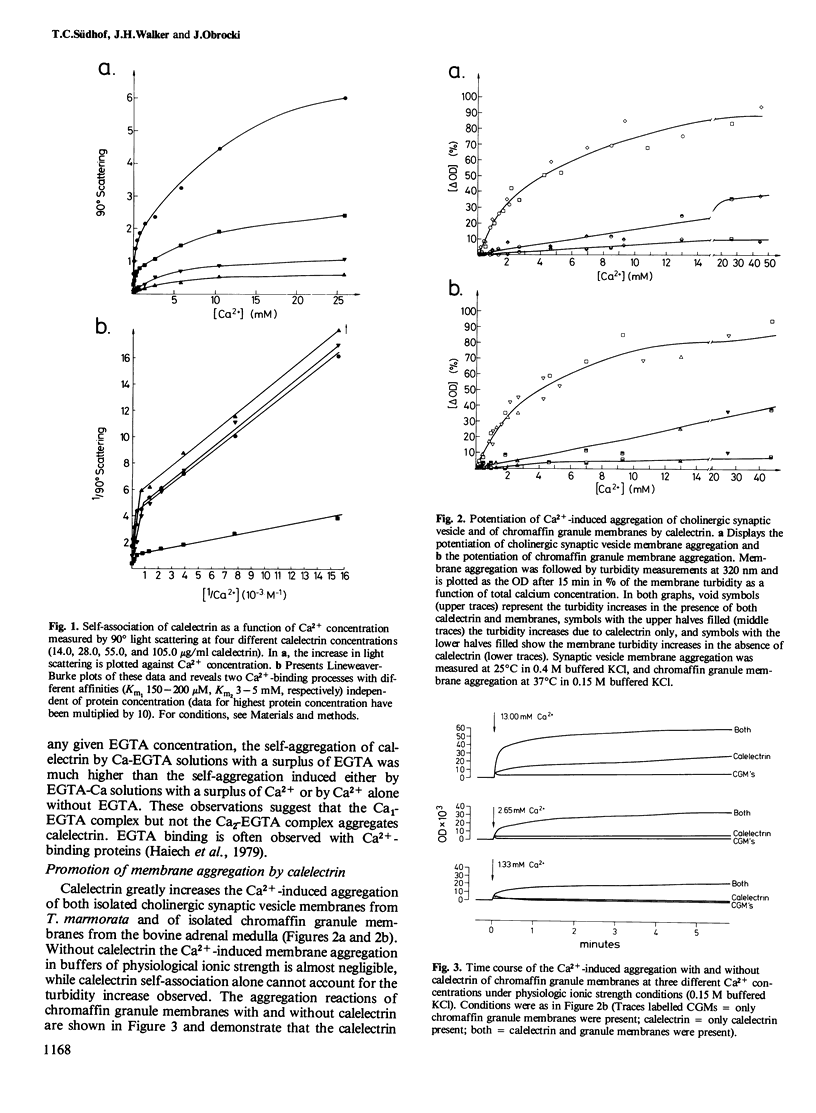
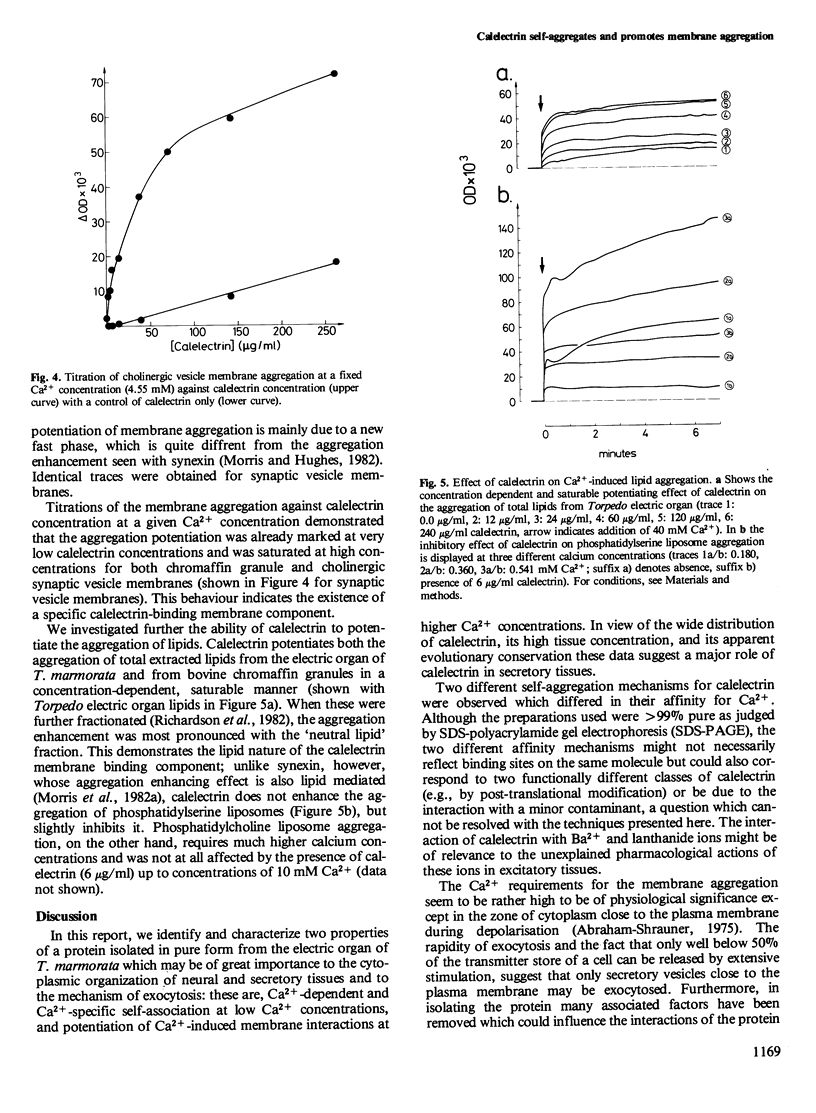
Calelectrin is a protein that can be purified to homogeneity from the cholinergically innervated electric organ of Torpedo marmorata where it is present in large amounts. It has been shown to bind to the membranes of the electric organ in a Ca2+-dependent and specific manner. Using the purified protein we now report that it is specifically self-aggregated by Ca2+ in micromolar concentrations but not by Mg2+ at much higher concentrations. Sr2+ is also completely inactive, while Ba2+ and the trivalent lanthanides Tb3+, Eu3 +, and La3+ can substitute for Ca2+. Calelectrin also greatly enhances the Ca2+-induced aggregation of isolated synaptic vesicle membranes from the cholinergic nerve terminals of T. marmorata and of chromaffin granule membranes from the bovine adrenal medulla. The potentiation of membrane aggregation is mainly due to the appearance of a fast aggregatory phase in the presence of calelectrin . It is saturable with respect to calelectrin and can be demonstrated at very low calelectrin concentrations, suggesting a specific calelectrin membrane-binding component. This component seems to be of lipid nature since the aggregation of total extracted lipids from Torpedo electric organ and from chromaffin granules could also be enhanced by calelectrin . The Ca2+-induced self-association of calelectrin and its aggregation enhancing effect may be of great importance to the structural organization of neural and secretory cells and the mechanism of exocytosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlson S. S., Wagner J. A., Kelly R. B. Purification of synaptic vesicles from elasmobranch electric organ and the use of biophysical criteria to demonstrate purity. Biochemistry. 1978 Apr 4;17(7):1188–1199. doi: 10.1021/bi00600a009. [DOI] [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Identification and purification of an adrenal medullary protein (synexin) that causes calcium-dependent aggregation of isolated chromaffin granules. J Biol Chem. 1978 Apr 25;253(8):2858–2866. [PubMed] [Google Scholar]
- Creutz C. E., Pazoles C. J., Pollard H. B. Self-association of synexin in the presence of calcium. Correlation with synexin-induced membrane fusion and examination of the structure of synexin aggregates. J Biol Chem. 1979 Jan 25;254(2):553–558. [PubMed] [Google Scholar]
- Creutz C. E., Pollard H. B. A Cell-free Model for Protein-Lipid Interactions in Exocytosis: Aggregation and Fusion of Chromaffin Granules in the Presence of Calcium, Synexin, and CIS-Unsaturated Fatty Acids. Biophys J. 1982 Jan;37(1):119–120. doi: 10.1016/S0006-3495(82)84630-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiech J., Derancourt J., Pechère J. F., Demaille J. G. Magnesium and calcium binding to parvalbumins: evidence for differences between parvalbumins and an explanation of their relaxing function. Biochemistry. 1979 Jun 26;18(13):2752–2758. doi: 10.1021/bi00580a010. [DOI] [PubMed] [Google Scholar]
- Haynes D. H., Lansman J., Cahill A. L., Morris S. J. Kinetics of cation-induced aggregation of Torpedo electric organ synaptic vesicles. Biochim Biophys Acta. 1979 Nov 2;557(2):340–353. doi: 10.1016/0005-2736(79)90332-8. [DOI] [PubMed] [Google Scholar]
- Hong K., Düzgüneş N., Papahadjopoulos D. Modulation of membrane fusion by calcium-binding proteins. Biophys J. 1982 Jan;37(1):297–305. doi: 10.1016/S0006-3495(82)84678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris S. J., Chiu V. C., Haynes D. H. Divalent cation-induced aggregation of chromaffin granule membranes. Membr Biochem. 1979;2(2):163–201. doi: 10.3109/09687687909063864. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Hellweg M. A., Haynes D. H. Light scattering turbidity changes as a measure of the kinetics of Ca2+ -promoted aggregation of chromaffin granule membrane ghosts. Biochim Biophys Acta. 1979 May 17;553(2):342–350. doi: 10.1016/0005-2736(79)90237-2. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Hughes J. M. Synexin protein is non-selective in its ability to increase Ca2+-dependent aggregation of biological and artificial membranes. Biochem Biophys Res Commun. 1979 Nov 14;91(1):345–350. doi: 10.1016/0006-291x(79)90624-7. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Hughes J. M., Whittaker V. P. Purification and mode of action of synexin: a protein enhancing calcium-induced membrane aggregation. J Neurochem. 1982 Aug;39(2):529–536. doi: 10.1111/j.1471-4159.1982.tb03977.x. [DOI] [PubMed] [Google Scholar]
- Morris S. J., Südhof T. C., Haynes D. H. Lipid and protein interactions in ca-promoted aggregation and fusion of chromaffin granule membranes. Biophys J. 1982 Jan;37(1):117–118. doi: 10.1016/S0006-3495(82)84629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portis A., Newton C., Pangborn W., Papahadjopoulos D. Studies on the mechanism of membrane fusion: evidence for an intermembrane Ca2+-phospholipid complex, synergism with Mg2+, and inhibition by spectrin. Biochemistry. 1979 Mar 6;18(5):780–790. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- Richardson P. J., Walker J. H., Jones R. T., Whittaker V. P. Identification of a cholinergic-specific antigen Chol-1 as a ganglioside. J Neurochem. 1982 Jun;38(6):1605–1614. doi: 10.1111/j.1471-4159.1982.tb06640.x. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. Core structure, internal osmotic pressure and irreversible structural changes of chromaffin granules during osmometer behaviour. Biochim Biophys Acta. 1982 Jan 4;684(1):27–39. doi: 10.1016/0005-2736(82)90045-1. [DOI] [PubMed] [Google Scholar]
- Tashiro T., Stadler H. Chemical composition of cholinergic synaptic vesicles from Torpedo marmorata based on improved purification. Eur J Biochem. 1978 Oct 16;90(3):479–487. doi: 10.1111/j.1432-1033.1978.tb12627.x. [DOI] [PubMed] [Google Scholar]
- Walker J. H. Isolation from cholinergic synapses of a protein that binds to membranes in a calcium-dependent manner. J Neurochem. 1982 Sep;39(3):815–823. doi: 10.1111/j.1471-4159.1982.tb07965.x. [DOI] [PubMed] [Google Scholar]


