Abstract
Recent studies suggest that the presence of a KRAS mutation may be insufficient for defining a clinically homogenous molecular group, as many KRAS mutant tumors lose reliance on K-Ras for survival. Identifying pathways that support K-Ras dependency may define clinically relevant KRAS sub-groups and lead to the identification of new drug targets. We have analyzed a panel of 17 KRAS mutant lung cancer cell lines classified as K-Ras dependent or independent, for co-dependency on PKCδ. We show that functional dependency on K-Ras and PKCδ co-segregate, and that dependency correlates with a more epithelial-like phenotype. Furthermore, we show that the pro-apoptotic and pro-tumorigenic functions of PKCδ also segregate based on K-Ras dependency, as K-Ras independent cells are more sensitive to topoisomerase inhibitors, and depletion of PKCδ in this sub-group suppresses apoptosis through increased activation of ERK. In contrast, K-Ras dependent lung cancer cells are largely insensitive to topoisomerase inhibitors, and depletion of PKCδ can increase apoptosis and decrease activation of ERK in this sub-group. We have previously shown that nuclear translocation of PKCδ is necessary and sufficient for pro-apoptotic signaling. Our current studies show that K-Ras dependent cells are refractive to PKCδ driven apoptosis. Analysis of this sub-group showed increased PKCδ expression and an increase in the nuclear:cytoplasmic ratio of PKCδ. In addition, targeting PKCδ to the nucleus induces apoptosis in K-Ras independent, but not K-Ras dependent NSCLC cells. Our studies provide tools for identification of the subset of patients with KRAS mutant tumors most amenable to targeting of the K-Ras pathway, and identify PKCδ as a potential target in this tumor population. These sub-groups are likely to be of clinical relevance, as high PKCδ expression correlates with increased overall survival and a more epithelial tumor phenotype in patients with KRAS mutant lung adenocarcinomas.
Keywords: KRAS, lung cancer, protein kinase C δ, apoptosis, cytotoxic drugs
INTRODUCTION
Oncogenic mutations in KRAS occur in about 25% of lung adenocarcinomas resulting in constitutive activation of survival pathways including MEK/ERK and PI3K/Akt (1). Recently, sub-groups of KRAS mutant lung and pancreatic tumors have been defined that differ in their reliance on K-Ras for survival (2, 3). K-Ras dependent tumors are fully reliant on K-Ras, while K-Ras independent tumors have lost their addiction to K-Ras and presumably depend on alternative pathways for survival (2, 3). Direct targeting of K-Ras has been largely ineffective, and indirect targeting of K-Ras effectors, such as RAF, MEK and PI3K, has yielded mixed results (4, 5). A better understanding of the molecular co-dependencies that promote survival of K-Ras dependent tumors is important if additional drug targets are to be identified. Previous studies have shown that some cancer cells with oncogenic K-Ras are dependent on PKCδ for survival through a mechanism that involves regulation of ERK and/or Akt (6–9). This suggests that PKCδ could represent a key pathway influencing outcomes from K-Ras directed therapy.
The PKC family of serine/threonine kinases contributes to numerous biological processes, including proliferation, survival, and apoptosis (10–12). Studies in PKCδ knock-out mice have confirmed a role for this kinase in cell death in response to irradiation and during mammary gland involution (13, 14). Likewise, many in vitro studies show that non-transformed cells use PKCδ for apoptotic signaling (12). The finding that apoptotic pathways are often disabled in cancer cells may underlie the somewhat paradoxical observation that PKCδ activation may drive proliferation and survival in many tumor cells, and in in vivo tumor models. In mouse mammary gland cancer PKCδ is a tumor promoter, and increased PKCδ expression is a negative prognostic indicator in Her+ and other subtypes of human breast cancer (15). PKCδ also promotes tumor progression in human pancreatic and lung cancer (9, 16). Other studies have defined roles for PKCδ in the invasion and migration of tumor cells (17, 18), the regulation of integrin expression, proliferation downstream of the epidermal growth factor receptor (EGFR) (8, 19, 20), and endocytic recycling of growth factor receptors (21–23).
Here we show that the pro-apoptotic and pro-tumorigenic functions of PKCδ segregate based on K-Ras dependency, and define parameters for identification of sub-groups of K-Ras mutant tumors. Importantly, in patients with lung adenocarcinoma, high PKCδ expression correlates with a better prognosis, underscoring the clinical importance of our findings. Our studies may have implications for the selection of patients with KRAS mutant tumors that are more or less likely to respond to targeting of the K-Ras pathway, and support investigation of PKCδ as a therapeutic target in this patient population.
RESULTS
K-Ras dependent NSCLC cells require PKCδ for survival
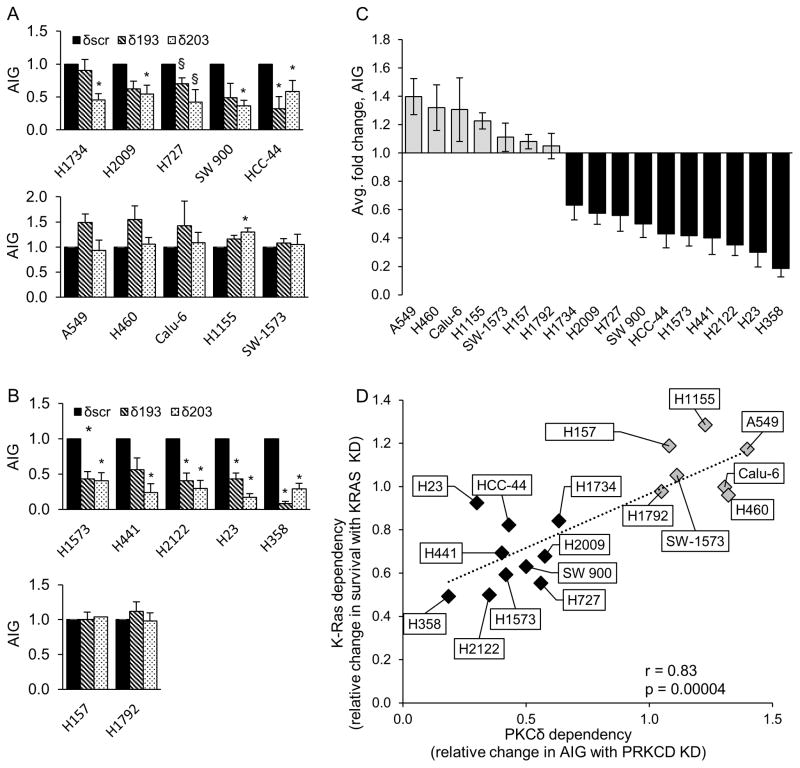
While many tumor cells with oncogenic KRAS mutations require K-Ras for survival (i.e. are “K-Ras dependent”), a subset of KRAS mutant NSCLC cell lines are able to proliferate in the absence of K-Ras (i.e. are “K-Ras independent”)(2). We have previously shown that PKCδ is required for the transformed phenotype and in vivo tumor growth of K-Ras dependent NSCLC cells, and that PKCδ regulates ERK activation and integrin αVβ3 expression in K-Ras dependent NSCLC cells (8, 9). As PKCδ is also a well-established regulator of DNA damage-induced apoptosis (12, 26, 27), a critical question is whether the pro-tumorigenic and pro-apoptotic functions of PKCδ segregate with functional dependency on K-Ras. For these studies we used a panel of 17 KRAS mutant lung cancer cell lines which include 10 K-Ras dependent cell lines (H1734, H23, H441, H358, H1573, H2122, SW 900, H727, HCC-44 and H2009) and 7 K-Ras independent cell lines (H157, SW-1573, Calu-6, A549, H460, H1792, H1155) in which depletion of K-Ras has no effect on cell survival (Figure S1). We first determined the contribution of PKCδ to the tumorigenic growth of KRAS mutant NSCLC cells by assaying AIG in cells stably depleted of PKCδ by expression of shRNAs (δ193 or δ203) or a scrambled control shRNA (δscr). Depletion of PKCδ using δ193 was ≥90% and ≥50% for δ203 (see Figure S2). Depletion of PKCδ with either shRNA significantly reduced the ability of all 10 K-Ras dependent cell lines to form colonies in soft agar (Figure 1A). Of these, H358 cells were the most dependent on PKCδ (>80% decrease in AIG), while H1734 cells were the least dependent. In contrast, depletion of PKCδ had no effect, or in some cases significantly increased AIG in K-Ras independent cells (Figure 1B). The relative change in AIG across our cell line panel is depicted graphically in Figure 1C with numbers <1 indicating a requirement for PKCδ for tumorigenic growth. Plotting K-Ras dependency for survival (see Figure S1) versus PKCδ dependent AIG (Figure 1C) reveals two distinct sub-groups of NSCLC cells (Figure 1D) and clearly demonstrates that dependency on oncogenic K-Ras and PKCδ are highly correlated (Pearson coefficient, r = 0.83, p < 0.00004).
Figure 1. K-Ras dependent NSCLC cells require PKCδ for survival.
Control NSCLC cells (δscr=solid bars), and cells expressing PKCδ shRNA (δ193=diagonal bars, δ203=dotted bars), were assayed for anchorage independent growth (AIG) as described in Materials and Methods; (A) K-Ras dependent and (B), K-Ras independent cell lines. Graphs represent the average of 3 or more experiments +/− SEM; * = p < 0.05 and § = p < 0.09 as determined by Student’s 2 tailed t-test. (C) Relative change in AIG in K-Ras independent (gray bars) and K-Ras dependent (black bars) NSCLC cell lines with depletion of PKCδ. For each cell line, AIG in δscr cells was set as one; PKCδ dependency is expressed as the average relative change in AIG in δ193 and δ203 cells. Data represents the average of 3 or more experiments +/− SEM. (D) Linear regression was performed between K-Ras and PKCδ dependency. K-Ras dependency is the relative change in survival with depletion of K-Ras (see Figure S1) and PKCδ dependency is the relative change in AIG growth with depletion of PKCδ (see Figure 1C). Three or more experiments were averaged for each data point. Pearson correlation r=0.83, p=0.0004. (E) q-RT PCR for ITGAV, ITGB3, PRKCD and KRAS was performed on the indicated cells following transient depletion with either lentiviral KRAS shrna (gray bars) or a control lentivirus (black bars). For each cell line, control cells were set as one and fold change is represented. Graphs represent the average of 2–3 experiments +/− SEM; * = p < 0.05. (F) q-RT PCR for ITGAV, ITGB3 and PRKCD was performed on indicated NSCLC lines stably depleted of PKCδ (δ193, gray bars) or a δscr control (black bars). For each cell line, δscr cells were set as one and fold change is represented. Graphs represent the average of 2–3 experiments +/− SEM; * = p < 0.05 and § = p < 0.09.
To explore the relationship between K-Ras and PKCδ further, A549, H2009 and H441 cells were transiently depleted of K-Ras by expression of shRNA (Figure 1E, gray bars) or a scrambled control shRNA (Figure 1E, black bars) and PKCδ mRNA expression was assayed. Depletion of K-Ras had no effect on expression of PKCδ in any of the cell lines analyzed (Figure 1E, top left). Similarly, we have shown that PKCδ depletion has no effect on K-Ras activation in NSCLC cells (9). We next asked whether PKCδ supports AIG in K-ras dependent cells through a collateral mechanism independent of K-Ras. We have previously shown that PKCδ regulates AIG in K-Ras dependent NSCLC cells through regulation of integrin αV and β3 expression (Figure 1F and (8)). To determine if PKCδ regulation of αV and β3 requires K-Ras, we assayed mRNA expression in H2009 and H441 cells after depletion of K-Ras. In contrast to depletion of PKCδ (Figure 1F), depletion of K-Ras had no effect on integrin αV expression in K-Ras dependent cells, however integrin αV expression was reduced in K-Ras depleted A549 cells (Figure 1E, bottom left). Integrin β3 expression was more variable but followed a similar trend (Figure 1E, bottom right). Our data is consistent with a role for PKCδ in supporting AIG and survival signaling in K-Ras dependent cells through a mechanism that does not require K-Ras.
PKCδ drives apoptosis in K-Ras independent, but not dependent NSCLC cells
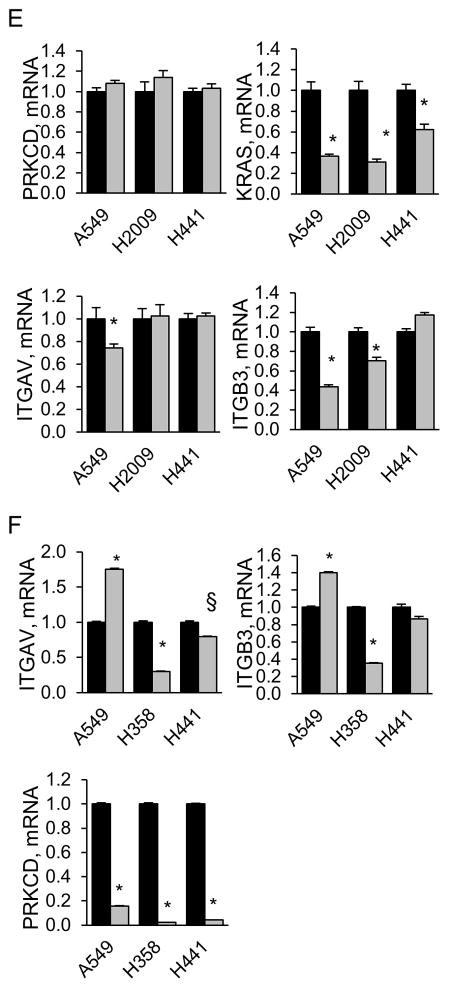
Our studies identify PKCδ as a potential therapeutic target in lung cancer cells that are functionally dependent on K-Ras. However, many non-transformed cells require PKCδ for DNA damage induced apoptosis, which is also important for the therapeutic response of tumor cells to genotoxins (12, 26–28). To determine if the pro-apoptotic function and pro-tumorigenic properties of PKCδ are mutually exclusive, our cell panel was treated with chemotherapeutic agents and apoptosis was assayed using a DNA fragmentation assay. As shown in Figures 2A and 2B, the pro-tumorigenic PKCδ phenotype of K-Ras dependent cells is strongly associated with resistance to the topoisomerase inhibitors, etoposide and SN38. A similar, albeit less significant, trend is seen when cells were treated with carboplatin, a DNA alkylating agent (Figure 2C). The response among K-Ras independent cells to DNA damaging agents was variable, although overall they were nearly 4 times more responsive to etoposide and twice as responsive to SN-38 as K-Ras dependent cells (Figures 2A and 2B). When cells were treated with the microtubule disruption agent, paclitaxel, some cell lines in both the K-Ras dependent and independent sub-groups responded, with no significant difference in response between groups (Figure 2D).
Figure 2. KRAS dependent NSCLC cells show reduced sensitivity to DNA damaging agents.
(A–D), K-Ras independent (gray bars) or K-Ras dependent (black bars) cells were treated for 24 hours with 50 μM etoposide (A), 10 μM SN38 (B), or for 48 hours with 20 μg/ml carboplatin (C), or 10 nM pacilitaxel (D), and DNA fragmentation was assayed as described in Materials and Methods. The range of DNA fragmentation values from K-Ras independent (I, gray bars) and dependent (D, black bars) cell lines is shown in the inset for each graph with the black line indicating the median. Data shown is the average of 3 or more independent experiments; * = p <0.05, ** p <0.09. (E) NSCLC cells were treated with 50 μM etoposide for 24 hours. Phosphorylation of p53 at Ser15 and expression of p21 was assayed by immunoblot as described in Materials and Methods. Blots were stripped and probed for actin and a total p53 antibody which detects WT p53 and some forms of mutant p53.
Many tumor cells inactivate DNA damage induced apoptotic pathways, suggesting a possible mechanism for the resistance of K-Ras dependent cells to apoptosis. TP53, which encodes the tumor suppressor protein p53, is mutated in about 50% of NSCLC, and mutation of TP53 predicts resistance to chemotherapeutic drugs in lung and other types of cancer (29). Analysis of TP53 mutations through the COSMIC (http://cancer.sanger.ac.uk/cosmic) and UMD TP53 mutation databases (http://p53.fr) indicates that 4/7 K-Ras independent cell lines have mutant TP53 (H157, H1792, H1155 and CaLu-6), while all 10 K-Ras dependent cell lines have TP53 mutations (30). Specific TP53 mutations are summarized in Table S1. As not all TP53 mutations are inactivating, we verified the functional status of p53 by analyzing p53 stability, Ser15 phosphorylation, and expression of the p53 target gene, p21. In a few cell lines with mutant TP53 (see H2009 and H727, Figure 2E), treatment with etoposide increased p53 protein stability and/or Ser15 phosphorylation, as well as p21 expression, indicating a partially functional p53 protein. Notably, H2009 and H727 cells are among the least PKCδ dependent of the K-Ras dependent NSCLC cells (see Figure 1C). Of the K-Ras independent cell lines, those with WT TP53 (A549, H460 and SW-1573) are among the most sensitive to etoposide, however some K-Ras independent cells with mutant TP53, particularly H157 cells, still showed sensitivity. Thus, while mutations in TP53 correlate with resistance to apoptosis and with a pro-tumorigenic function for PKCδ in K-Ras dependent cells, in the K-Ras independent sub-group, wild type TP53 alone does not predict sensitivity to apoptosis.
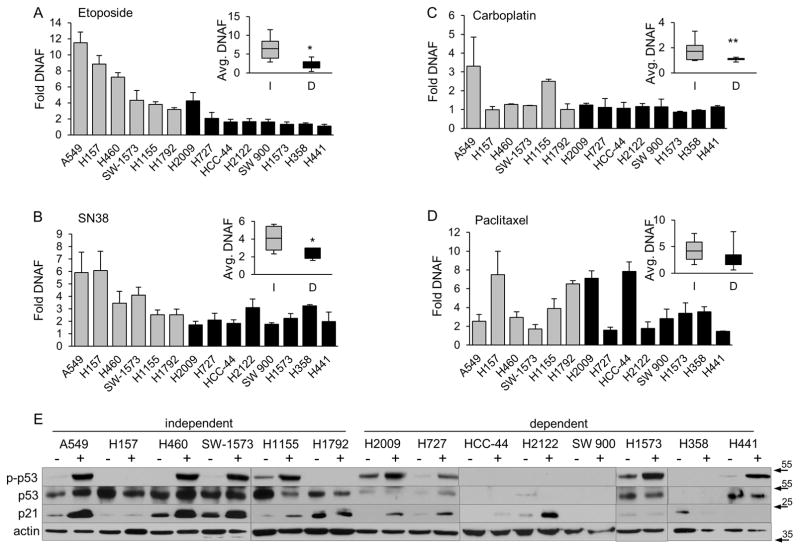
Our data suggests that the pro-apoptotic function of PKCδ, especially in the context of topoisomerase inhibitors, is lost or suppressed in K-Ras dependent NSCLC cells, relative to K-Ras independent cells. To explore this directly, we analyzed etoposide-induced apoptosis in cells depleted of PKCδ. Depletion of PKCδ with either δ193 or δ203 resulted in suppression of apoptosis in K-Ras independent A549 and H460 cells compared to cells expressing δscr (Figure 3A). In contrast, depletion of PKCδ had little effect on etoposide-induced apoptosis, or synergized with etoposide to further increase DNA fragmentation in K-Ras dependent H2009 and HCC-44 cells (Figure 3C).
Figure 3. PKCδ drives apoptosis in K-Ras independent, but not dependent NSCLC cells.
(A) and (C), A549, H460 (A), H2009 or HCC-44 (C) control cells (δscr, black bars), and cells expressing δ193 (gray bars) or δ203 (white bars) were treated with etoposide for 24 hours and DNA fragmentation was assayed as described in Materials and Methods. This experiment was repeated 3 or more times; a representative experiment +/− SEM is shown; * = p < 0.05 as compared to δscr treated with etoposide. (B) and (D), A549 (B) or H2009 (D) cells were transiently depleted of PKCδ with siRNA to PKCδ (siPKCδ) or a control non-targeting siRNA (siNT) and treated with the indicated dose (μM) of etoposide for 24 hours. Cell lysates were probed for expression of the indicated proteins by immunoblot as described in Materials and Methods. (E) and (F), A549 δscr or δ193 cells (E), or A549 cells transiently transfected with siPKCδ or a non-targeting control (siNT) (F), were treated without (black bars) or with (gray bars) 10 μM PD98059 for 30 min, followed by the addition of 5 μM etoposide (gray and white bars) for an additional 24 hours. DNA fragmentation was assayed as described in Materials and Methods. This experiment was repeated 3 or more times; a representative experiment +/− SEM is shown; * = p < 0.05.
We next explored the hypothesis that PKCδ is differentially linked to survival or apoptotic pathways in K-Ras dependent and independent NSCLC cells. We found no consistent differences in basal ERK or Akt activation between these NSCLC subsets (data not shown). However, our previous studies suggested that ERK activation is differentially regulated by PKCδ depletion in K-Ras dependent versus independent NSCLC cells (9). In our present study we explored this further using our panel of cell lines. Our results show that basal ERK activation either increases, or does not change with depletion of PKCδ in the 7 K-Ras independent NSCLC cell lines (Figure 2S, panel A). In contrast, depletion of PKCδ suppresses basal ERK activation in 9/10 K-Ras dependent NSCLC cell lines (Figure S2, panel B). Interestingly, ERK activation in response to EGF stimulation did not differ in PKCδ depleted A549 (K-Ras independent) or H2009 (K-Ras dependent) cells (Figure 2S, panel C), suggesting that PKCδ does not regulate EGFR activation, and consistent with a role for PKCδ in regulating AIG and survival signaling through a mechanism that does not require K-Ras.
As ERK can be activated by DNA damage agents in some cells, and is thought to provide a survival signal (31), we asked if differential basal activation of ERK could account for the different apoptotic phenotypes we observe upon depletion of PKCδ. Activation of ERK and its downstream kinase, pRSK90, was assayed in etoposide-treated A549 (K-Ras independent) and H2009 (K-Ras dependent) cells depleted of PKCδ with siRNA (Figure 3B and 3D). In A549 cells, treatment with etoposide transiently increased activation of ERK and RSK90, and this was more robust when A549 cells are depleted of PKCδ (siNT versus siPKCδ, Figure 3B). In contrast, in H2009 cells expressing siPKCδ, basal and etoposide induced pERK and pRSK90 were greatly reduced compared to siNT (Figure 3D). As increased ERK and RSK90 activation correlate with decreased apoptosis in A549 cells depleted of PKCδ, we hypothesized that activation of the ERK pathway may contribute to the suppression apoptosis observed (see Figure 3A). To test this, A549 cells depleted of PKCδ by stable expression of δ193 (Figure 3E) or by transfection of siPKCδ (Figure 3F), were pretreated with the MEK inhibitor, PD98059, prior to the addition of etoposide. Pre-treatment with PD98059 resulted in a nearly complete rescue of the apoptotic response in both δ193 (Figure 3E) and siPKCδ A549 cells (Figure 3F). We conclude that PKCδ is a negative regulator of basal ERK activity in K-Ras independent cells, and that increased activation of ERK in A549 cells depleted of PKCδ (Figure 3B) contributes to the suppression of apoptosis observed (Figure 3A). In contrast, in K-Ras dependent NSCLC cells our data indicates that PKCδ is a positive regulator of ERK, as basal ERK activation is reduced with depletion of PKCδ (Figures S2 and 3D).
K-Ras dependent NSCLC cells are refractory to PKCδ driven apoptosis
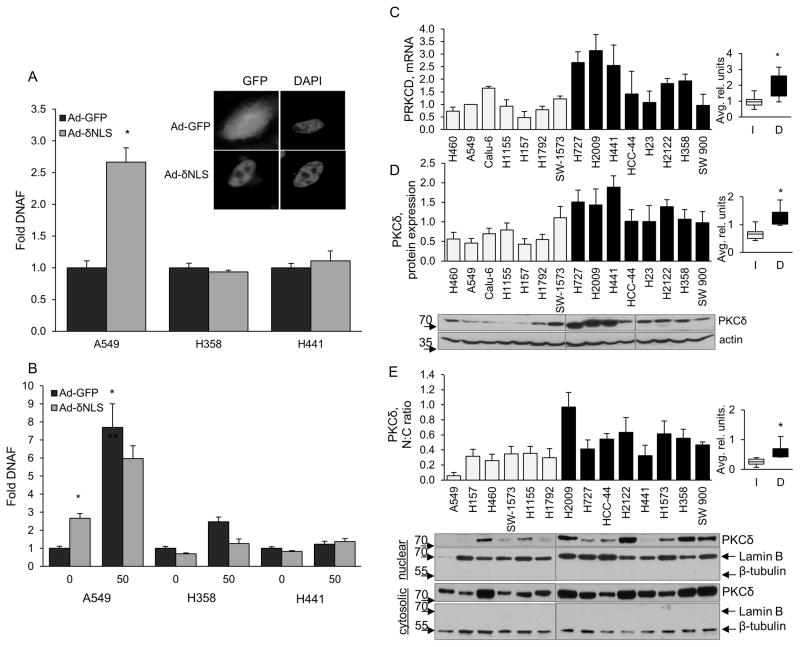
As a group, K-Ras dependent NSCLC cells are largely resistant to DNA damage induced apoptosis, especially cell death induced by topoisomerase inhibitors (Figure 2). We have previously shown that apoptosis induced by topoisomerase inhibitors and irradiation requires nuclear import of PKCδ, and that a nuclear targeted form of PKCδ is a potent inducer of apoptosis (25, 32–34). Thus, a possible explanation for the resistance of K-Ras dependent NSCLC cells to apoptosis from topoisomerase inhibitors in our models could be impairment of PKCδ activated apoptotic signaling. To address this, NSCLC cells were transduced with an adenovirus that expresses an SV40-NLS tagged PKCδ (Ad-δNLS) or an Ad-GFP control adenovirus. This form of PKCδ is constitutively active and targeted to the nucleus (25, 35) (inset, Figure 4A). Expression of Ad-δNLS induced apoptosis in K-Ras independent A549 cells, but not in H441 or H358 K-Ras dependent cells (Figure 4A). Similarly, expression of Ad-δNLS was unable to sensitize cells K-Ras dependent cells H358 and H441 cells to etoposide-induced apoptosis (Figure 4B). These data demonstrate that K-Ras dependent NSCLC cells are refractory to apoptosis induced by nuclear-targeted PKCδ, and suggest that nuclear localization of PKCδ may be impaired or that nuclear localization and induction of apoptosis may be uncoupled in K-Ras dependent cells. To explore this further we assayed PKCδ expression and nuclear localization across our NSCLC cell line panel. Surprisingly, PKCδ mRNA (Figure 4C) and protein expression (Figure 4D) are both significantly increased in K-Ras dependent cells as compared to K-Ras independent cells. Likewise, the ratio of nuclear to cytoplasmic PKCδ was significantly higher in K-Ras dependent cells compared to independent cells (Figure 4E). Our studies suggest that K-Ras dependent NSCLC cells are able to accumulate large amounts of nuclear PKCδ without inducing apoptosis, supporting our finding that nuclear targeting of PKCδ occurs but does not drive apoptosis in this cell population (Figures 4A and 4B). Taken together, our data suggests that PKCδ provides an essential pro-survival signal in NSCLC cells dependent on K-Ras, however PKCδ is no longer able to regulate DNA damage-induced apoptosis in this cell population. Furthermore our studies identify high PKCδ expression and an increased PKCδ nuclear:cytoplasmic ratio as potential biomarkers for identification of K-Ras dependent NSCLC cancers.
Figure 4. K-Ras dependent NSCLC cells are refractive to apoptosis downstream of PKCδ.
(A) Indicated cell lines were transduced with Ad-δNLS (gray bars) or Ad-GFP (black bars) and DNA fragmentation was assayed after 72 hours. Inset shows immunofluorescence of GFP tagged PKCδ (GFP) or DAPI (DAPI). (B) Cells transduced for 72 hours as in (A) were treated with 50 μM etoposide for the last 24 hours prior to harvesting for DNA fragmentation. This experiment was repeated 3 or more times; a representative experiment +/− SEM is shown; * = p < 0.05 as compared to Ad-GFP control. (C) q-RT PCR for PKCδ mRNA; light gray bars= K-Ras independent, black bars= K-Ras dependent cells. (D and E) Immunoblot for PKCδ protein expression in whole cell lysates (D) or cytoplasmic and nuclear fractions (E). Immunoblots in (D) were stripped and re-probed for actin; immunoblots in (E) were stripped and re-probed for lamin B or β-tubulin as a marker of the nuclear and cytosolic compartments, respectively. Immunoblots were quantified by densitometry and values normalized to actin. Graphs (D) PKCδ protein expression and (E) PKCδ nuclear:cytoplasmic ratio, show the average of 3 or more experiments For C, D and E, the average range of values from K-Ras independent (I, light gray bars) and dependent (D, black bars) cell lines are shown in the inset for each graph with theblack line indicating the median. Error bars for both graphs are SEM, * indicates p < 0.05. (C), p=0.013, (D), p=0.0008, (C), p=0.005
High PKCδ expression correlates with markers of epithelial differentiation and predicts increased patient survival
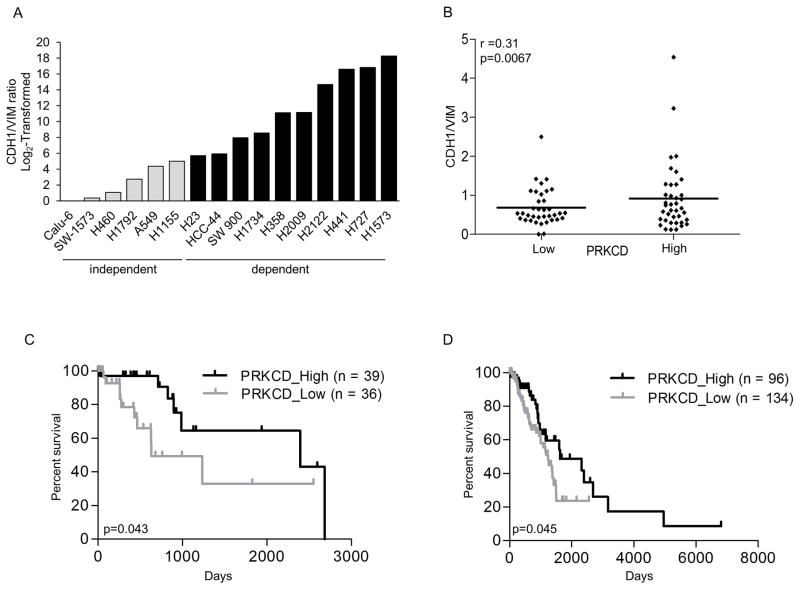
In lung, colon and pancreatic cancer cells, K-Ras dependency correlates with an epithelial phenotype (2, 3). As our studies link high PKCδ expression to K-Ras dependency, we asked if PKCδ dependency and/or expression correlates with a more epithelial-like phenotype in KRAS mutant NSCLC as indicated by increased expression of E-cadherin (CDH1) and decreased expression of vimentin (VIM). Analysis of publically available microarray data shows that the ratio of CDH1 mRNA to VIM mRNA is higher in all PKCδ dependent cell lines compared to PKCδ independent cell lines (r=0.73) (Figure 5A), and nearly identical to the correlation between K-Ras dependency and CDH1/VIM mRNA (r=0.78) in this NSCLC cell line panel. Likewise, we show that high PKCδ expression correlates with an increased ratio of CDH1/VIM mRNA in human lung adenocarcinomas, indicating a more epithelial phenotype in this tumor population (Figure 5B). PKCδ does not regulate regulate E-cadherin or vimentin protein expression in NSCLC cells as there is no change in expression of either protein in K-Ras independent or K-Ras dependent NSCLC cells depleted of PKCδ (Figure S3).
Figure 5. High PKCδ expression correlates with markers of epithelial differentiation and predicts increased patient survival.
(A) CDH1/VIM mRNA expression ratio of K-Ras independent (gray bars) and K-Ras dependent (black bars) NSCLC cell lines (see Material and Methods). Data shown was normalized to the lowest value (Calu-6) and log2-transformed. (B) KRAS mutant lung adenocarcinomas in the TCGA database were binned into PKCδ high (mRNA expression > median) and PKCδ low (mRNA expression < median). Graph shows CDH1/VIM mRNA ratio in PKCδ low versus PKCδ high mRNA expression. (C) Kaplan-Meier curve of patients with KRAS mutant lung adenocarcinomas stratified by high (black line) and low (gray line) PKCδ mRNA expression. (D) Kaplan-Meier curve of patients with lung adenocarcinomas stratified by high (black line) and low (gray line) PKCδ mRNA expression.
Our studies suggest that PKCδ expression could potentially be a biomarker for identification of K-Ras dependent NSCLC tumors. To address this, we asked if PKCδ mRNA expression can be used to identify clinically significant subpopulations of patients with lung adenocarcinomas. Tumors in the TCGA database were binned into PKCδ high and PKCδ low expressers, and further into those with KRAS mutations. High PKCδ expression was found to be a predictor of increased overall survival in KRAS mutant adenocarcinomas (Figure 5C). We propose that high PKCδ expression is a marker of K-Ras dependence in KRAS mutant tumors, and that together with PKCδ nuclear:cytoplasmic ratio, may be useful for identifying patients most likely to benefit from K-Ras and/or PKCδ directed therapy. Interestingly, high PKCδ expression also predicted better overall survival when all lung adenocarcinomas were analyzed (Figure 5D), suggesting that PKCδ may cooperate with additional oncogenic drivers in lung tumors.
DISCUSSION
Oncogenic mutation of KRAS is commonly observed in NSCLC, however attempts at direct or indirect targeting of the KRAS oncogene itself have, to date, failed to generate any K-Ras specific clinical therapies (4) (36). Beyond the issues associated with the druggability of K-Ras itself, it is also likely that the presence of a KRAS mutation may be insufficient for defining a clinically homogenous molecular grouping. Based on prior in vitro data, K-Ras dependency versus independency represents an obvious additional filter that might need to be employed to direct K-Ras specific therapies towards clinically relevant KRAS molecular sub-groups [2, 3]. Here we show that continued reliance on K-Ras for survival (K-Ras “addiction”) is highly correlated with dependency on PKCδ. We propose that PKCδ represents a secondary, non-oncogene co-addiction in tumor cells that are also addicted to oncogenic K-Ras. In K-Ras dependent cells, TP53 appears to be uniformly mutant, CDH1:VIM ratios suggest an epithelial phenotype, PKCδ expression levels are increased with an increased nuclear:cytoplasmic ratio, and basal ERK signaling is PKCδ dependent. This spectrum of changes results in reduced sensitivity to key cytotoxic agents, most notably topoisomerase inhibitors. Our findings support further exploration of PKCδ as a drug target in this patient population, and suggest that dependency on PKCδ may define the subset of KRAS mutant tumors most amenable to targeting of the K-Ras pathway and/or suitable for specific cytotoxic therapy.
The development of targeted therapies for cancer has exploited the finding that many tumor cells are reliant on the function of a specific activated oncogene for survival (“oncogene addiction”)(37). However, cancer cells can also become dependent on proteins that are non-essential for the survival of normal cells, a condition referred to as “non-oncogene addiction” (38). Identification of such functionally important pathways is critical for new target identification, and may enable the development of drugs with greater tumor specificity. Such pathways may also provide additional opportunities for simultaneous targeting if they provide collateral support for oncogenic signaling. We have previously shown that depletion of PKCδ does not suppress K-Ras activation in K-Ras dependent NSCLC cells, however these studies did not address a role for K-Ras in regulation of PKCδ (9). Here we show that depletion of K-Ras has no effect on the expression of PKCδ in any of the NSCLC cell lines analyzed (Figure 1E), supporting a function for PKCδ independent of K-Ras. Our previous studies also identified the integrin pair αVβ3 as a downstream target of PKCδ specifically in K-Ras dependent NSCLC cells, and showed that PKCδ regulation of integrin αVβ3 is required for AIG (8). Here we show that while αV and β3 expression in K-Ras dependent NSCLC cells requires PKCδ, it does not require K-Ras (Figure 1E). We conclude that even though dependency on K-Ras and PKCδ co-segregate in K-Ras dependent NSCLC cells, PKCδ likely supports AIG in K-Ras dependent NSCLC cells through a collateral mechanism, and not as a downstream effector of K-Ras. In contrast, studies from Ueda et al suggests that PKCδ can regulate ERK signaling downstream of Ras through activation of Raf (39), while studies from Koo et al suggest that PKCδ may increase the stability of the K-Ras protein downstream of estrogen (40).
We propose that the reliance of K-Ras dependent NSCLC cells on PKCδ for survival represents a non-oncogene addiction. This is consistent with the observation that PKCδ is a tumor promoter in vivo, but that functional genetic alterations in PKCδ are only rarely found in human cancers (11, 41). However, changes in PKCδ expression have been reported in some human tumors (42, 43) and analysis of TCGA data shows increased expression of PKCδ in a sub-group of lung adenocarcinomas (44). A future goal will be to use PKCδ expression/localization, possibly in combination with other genetic markers of K-Ras dependency, to classify patient’s tumors by K-Ras dependent and independent status. As our studies suggest that PKCδ functions in a collateral pathway to support oncogenic K-Ras driven tumorigenesis, co-targeting of K-Ras and PKCδ may likewise be an important strategy in K-Ras dependent tumors. Notably, changes in PKCδ expression in lung adenocarcinomas are co-occurrent (odds ratio > 2 and <10) with K-Ras activation (p<0.008) (www.cbioportal.org), supporting a link between these signaling pathways.
Our current data shows that lung cancer cells addicted to oncogenic K-Ras are co-dependent on PKCδ for maintenance of basal ERK signaling, as PKCδ is required for basal activation of ERK specifically in this sub-group of cells (Figure S2, panels A and B). Similarly, McCormick and colleagues have shown that oncogenic K-Ras, but not wild type K-Ras, regulates basal ERK signaling in KRAS mutant cancer cells (45). An interesting observation is that in our studies PKCδ does not regulate epidermal growth factor (EGF) induced ERK activation in either K-Ras sub-group (Figure S2, panel C). As a non-oncogene addiction pathway, PKCδ may regulate survival pathways independent of K-Ras that impinge on ERK activation. For example, we have shown that PKCδ regulation of the integrin pair, αVβ3, mediates ERK activation in K-Ras dependent NSCLC cells (8). PKCδ can also suppress ERK activation via negative regulation of hedgehog signaling (46), and contributes to ERK activation through regulation of recycling of activated cell surface receptors as has been shown for ErbB2, KIT and EGFR (21, 23). Other studies by Xia et al show that PKCδ is required for survival of NIH3T3 and pancreatic cancer cells expressing activated K-Ras through a mechanism resulting in activation of Akt (6, 7).
In contrast to the strong correlation between K-Ras dependency and a pro-survival role for PKCδ, K-Ras independent NSCLC cells are much more sensitive to DNA damaging agents, particularly the topoisomerase inhibitors etoposide and SN38. Hence K-Ras independent NSCLC cells appear to be similar to non-transformed cells in their utilization of PKCδ for apoptosis (12). In contrast, K-Ras dependent NSCLC cells are highly refractory to pro-apoptotic signaling by PKCδ. The identification of sub-groups of KRAS mutant cells with distinct PKCδ functions may explain previous reports that PKCδ can be either pro or anti-apoptotic in cancer cells with aberrant K-Ras activation (6, 47). We show that resistance of K-Ras dependent NSCLC cells to DNA damage induced apoptosis is not due to impaired nuclear localization or reduced expression/activation of PKCδ. In fact, K-Ras dependent NSCLC cells have higher PKCδ expression and increased basal nuclear localization of PKCδ, suggesting that nuclear localization of PKCδ and apoptotic signaling are dissociated in this sub-group. Furthermore, our studies suggest that high nuclear PKCδ may be an indicator of apoptotic resistance. One mechanism for apoptosis resistance may be genetic inactivation of DNA damage response or apoptosis pathways. In this regard, all K-Ras dependent cells have point mutations in TP53, while TP53 mutations occur in only 4/7 K-Ras -independent cell lines. Interrogation of the COSMIC database also shows increased mutation/copy number alterations in the damage sensors ATM/ATR in K-Ras dependent cells (7/10) compared to K-Ras independent cells (2/6), and mutations in caspases 7, 8 and 10 in 3/10 of the K-Ras dependent cell lines, but not in K-Ras independent cells (http://cancer.sanger.ac.uk/cosmic). Finally, our studies suggest that the ERK pathway is important for determining sensitivity to apoptosis as pre-treatment with MEK inhibitors increases etoposide-induced apoptosis in K-Ras independent NSCLC cells (Figure 3E and 3F).
Our studies show that K-Ras dependency is highly correlated with dependency on PKCδ in a sub-group of KRAS mutant NSCLC cells, supporting exploration of PKCδ as a drug target in KRAS mutant lung cancer. Other studies have demonstrated a synthetic lethal interaction between K-Ras and PKCδ (48), and a recent report shows that activation of PKCδ confers resistance to ALK inhibitors in ALK dependent NSCLC cells (49). The plasticity of PKCδ signaling in lung cancer cells illustrates the need to better understand the genetic context of specific cancer subtypes so as to more accurately deploy targeted therapies. Interestingly, analysis of specific KRAS codon mutations shows that mutation of codon 61, which occurs in <1% of lung cancers, is frequently found in K-Ras independent NSCLC cells (3/7 cell lines), but not in K-Ras dependent NSCLC cell lines (0/10) (http://cancer.sanger.ac.uk/cosmic) (50). In patients with lung adenocarcinomas, and in specifically in KRAS mutant lung adenocarcinomas, we show that high PKCδ expression is predictor of increased overall survival, and of a more epithelial phenotype. In addition, our studies suggest that K-Ras dependent lung cancers can further be distinguished by increased PKCδ expression and nuclear localization, resistance to DNA damage induced apoptosis, and PKCδ dependent activation of ERK signaling pathways. Hence our current studies provide parameters for selection of patients most likely to benefit from targeting the K-Ras pathway and/or from specific cytotoxic drugs and provide support for development of PKCδ directed therapies for use in this patient population.
MATERIALS AND METHODS
Cell Culture
NSCLC cell lines, A549, NCI-H460, Calu-6, H1155, SW1-573, NCI-H157, H1792, H1734, NCI-H2009, NCI-H727, SW 900, HCC-44, H1573, NCI-H441, H2122, H23, NCI-H358 were acquired from the University of Colorado Denver lung SPORE cell bank. Cell line profiling for authentication was done at the DNA sequencing Core at University of Colorado Anschutz Medical Campus using the AmpFLSTR® Identifiler® PCR Amplification Kit from Applied Biosystems. Cells were maintained in RPMI-1640 with 2 mM L-glutamine and 10% fetal bovine serum. See Table S1 for the histology, KRAS and TP53 mutational status of the cell panel.
siRNA and shRNA
Transient depletion of PKCδ by siRNA was done using ON-TARGETplus SMARTpool siRNA for human PKCδ (L-003524-00-0010, Dharmacon, Thermo Fisher, Lafayette, CO), and the ON-TARGETplus non-targeting Pool (D-001810-10-05). Stable depletion of PKCδ was performed as previously described using lentiviral constructs containing shRNA to human PKCδ (Open Biosystems, pLKO-TRC00010193 or pLKO-TRC00010203) or an shRNA control (pLKO-scrambled) (9). Cell lines were maintained in selection media with 1 μg/ml puromycin and used at low passage (<8). Stable and transient depletion of K-Ras was performed using lentiviral constructs as previously described (9, 24). Cell proliferation was assayed using WST-1 reagent (Sigma).
Adenoviral transduction
Cells were plated at 2.5 × 103 cells per well of a 96 well plate. The following day cells were transduced with either Ad-GFP control or an SV40-NLS and GFP tagged PKCδ (Ad- δNLS) at an MOI (multiplicity of infection) of 100 and incubated with virus for 72 hours or 48 hours with the addition of 50μM etoposide for the last 24 hours (25). Nuclear localization of δNLS was verified by fluorescent microscopy using an Olympus BX51 microscope and a 100× oil immersion objective.
Immunoblot analysis
Immunoblot analysis was done as previously described (14). Antibodies to PKCδ (sc-937) and lamin B (sc-6217) were purchased from Santa Cruz Biotech (Santa Cruz, CA); antibodies to phospho-ERK1/2 (pERK1/2; #9101), ERK1/2 (#4695), P-P53 (#9284), p53 (#2524), p21 (#2946) and vimentin (#5741) were purchased from Cell Signaling Technologies (Danvers, MA). Antibodies to pRSK90 (#AF889) and RSK90 (#AF992) were obtained from R&D Systems. The antibody to β-actin (ab49900) was purchased from Abcam. Antibodies to β-tubulin (556321) and e-cadherin (610182) were obtained from BD Biosciences. For some experiments nuclear and cytosol fractions were isolated using a nuclear/cytosol fractionation kit (BioVision Incorporated, # K266-100) according to the manufacturer’s instructions, except that Triton X-100 was added to the nuclear extraction buffer at a final concentration of 1%. Protein concentration was determined using the DC Protein Assay Kit (Bio-Rad Laboratories, # 500-0111).
Quantitative Real Time Polymerase Chain Reaction (qRT-PCR)
Total RNA was purified from NSCLC cells using a Zymo Research Quick RNA miniprep kit (R1055) and 125ng was reverse transcribed using Thermo Verso cDNA kit (AB1453). Biological triplicates of cDNA for each cell line were analyzed by PCR using Taqman Universal Master Mix II with UNG (Applied Biosystems 4440038) and Taqman probes recognizing PRKCD (Hs01090047_m1) or RNA18s control (Hs03928990_g1). Analysis was performed using RNA18s as the endogenous control and A549 cDNA as the reference. Relative mRNA levels for ITGAV, ITGB3, PRKCD, KRAS and GAPDH were determined using the following primer sets and Applied Biosystems SYBR Select Master Mix (#4472897): ITGAV, forward 5′-AGGAGAAGGTGCCTACGAAGCT-3′, reverse 5′-GCACAGGAAAGTCTTGCTAAGGC-3′, ITGB3, forward 5′-AAGCAGAGTGTGTCACGGAACC-3′, reverse 5′-CCTCCAGCCAATCTTTTCATCAC-3′; PRKCD, 5′-CAGACCAAGGACCACCTGTT-3′, reverse 5′-GCATAAAACGTGGCACGGTA-3′; KRAS, forward 5′-ATTTTGTGGACGAATATGATCCAAC-3′, reverse 5′ GCTGTGTCGAGAATATCCAAGAGAC3′ and GAPDH, forward 5′-GCCAAATATGATGACATCAAG-3′, reverse, 5′-GGTGTCGCTGTTGAAGTCAGAG-3′. For these reactions gene expression was normalized to GAPDH. All reactions were performed using the StepOnePlus Real-Time PCR system and a comparative CT (ΔΔCT).
DNA fragmentation and anchorage independent growth
DNA fragmentation was assayed using a Cell Death Detection ELISA PLUS (Sigma Aldrich, St. Louis, MO). Anchorage-independent growth (AIG) was assayed as described previously (9). For each cell line, 1 × 104 cells were suspended in a top layer of 0.36% noble agar (USB Corporation) and a bottom layer of 0.4% agar both in RPMI-1640 + 10% FBS in a 6-well culture dish. After 1–3 weeks, colonies were stained with Nitrotetrazolium Blue chloride (NBT; Sigma) and photographed with a S600 Canon camera. Colonies were quantified with ImageJ analysis software.
Analysis of TCGA data
RNA-seq gene expression and mutational data from lung adenocarcinoma patients was obtained from the The Cancer Genome Atlas (TCGA) through cBioPortal (https://www.cbioportal.org). Clinical data was obtained from the TCGA data portal (https://tcga-data.nci.nih.gov/tcga). In total, 230 patients that have matched clinical data and RNA-seq/mutational data were used in this analysis. Patients were classified into high or low PKCδ groups if the PKCδ expression of a patient was higher or lower than the mean expression. Kaplan-Meier survival analysis was conducted using PRISM software, and log-rank test p < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
We gratefully acknowledge the intellectual contributions of Dr. Ross Camidge. This work was supported by a United Against Lung Cancer research award, a pilot grant from NIH Lung SPORE grant P50 CA58187, and R01DE015648 to MER.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc).
Conflict of interest: The authors declare no conflict of interest
References
- 1.Beau-Faller M, Legrain M, Voegeli AC, Guerin E, Lavaux T, Ruppert AM, et al. Detection of K-Ras mutations in tumour samples of patients with non-small cell lung cancer using PNA-mediated PCR clamping. Br J Cancer. 2009;100(6):985–92. doi: 10.1038/sj.bjc.6604925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh A, Greninger P, Rhodes D, Koopman L, Violette S, Bardeesy N, et al. A gene expression signature associated with “K-Ras addiction” reveals regulators of EMT and tumor cell survival. Cancer Cell. 2009;15(6):489–500. doi: 10.1016/j.ccr.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Collisson EA, Sadanandam A, Olson P, Gibb WJ, Truitt M, Gu S, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17(4):500–3. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick F. KRAS as a Therapeutic Target. Clinical Can Res. 2015;21(8):1797–801. doi: 10.1158/1078-0432.CCR-14-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janne PA, Shaw AT, Pereira JR, Jeannin G, Vansteenkiste J, Barrios C, et al. Selumetinib plus docetaxel for KRAS-mutant advanced non-small-cell lung cancer: a randomised, multicentre, placebo-controlled, phase 2 study. Lancet Oncol. 2013;14(1):38–47. doi: 10.1016/S1470-2045(12)70489-8. [DOI] [PubMed] [Google Scholar]
- 6.Xia S, Chen Z, Forman LW, Faller DV. PKCdelta survival signaling in cells containing an activated p21Ras protein requires PDK1. Cell Signal. 2009;21(4):502–8. doi: 10.1016/j.cellsig.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xia S, Forman LW, Faller DV. Protein kinase C delta is required for survival of cells expressing activated p21RAS. J Biol Chem. 2007;282(18):13199–210. doi: 10.1074/jbc.M610225200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Symonds JM, Ohm AM, Tan AC, Reyland ME. PKCdelta regulates integrin alphaVbeta3 expression and transformed growth of K-ras dependent lung cancer cells. Oncotarget. 2016;7(14):17905–19. doi: 10.18632/oncotarget.7560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Symonds JM, Ohm AM, Carter CJ, Heasley LE, Boyle TA, Franklin WA, et al. Protein kinase C delta is a downstream effector of oncogenic K-ras in lung tumors. Cancer Res. 2011;71(6):2087–97. doi: 10.1158/0008-5472.CAN-10-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reyland ME, Jones DN. Multifunctional roles of PKCdelta: Opportunities for targeted therapy in human disease. Pharmacol Ther. 2016;165:1–13. doi: 10.1016/j.pharmthera.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garg R, Benedetti LG, Abera MB, Wang H, Abba M, Kazanietz MG. Protein kinase C and cancer: what we know and what we do not. Oncogene. 2014;33(45):5225–37. doi: 10.1038/onc.2013.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen HM, Reyland ME, Barlow LA. Mechanisms of taste bud cell loss after head and neck irradiation. J Neuroscience. 2012;32(10):3474–84. doi: 10.1523/JNEUROSCI.4167-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen-Petersen BL, Miller MR, Neville MC, Anderson SM, Nakayama KI, Reyland ME. Loss of protein kinase C delta alters mammary gland development and apoptosis. Cell Death Dis. 2010;1:e17. doi: 10.1038/cddis.2009.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Humphries MJ, Limesand KH, Schneider JC, Nakayama KI, Anderson SM, Reyland ME. Suppression of apoptosis in the protein kinase Cdelta null mouse in vivo. J Biol Chem. 2006;281(14):9728–37. doi: 10.1074/jbc.M507851200. [DOI] [PubMed] [Google Scholar]
- 15.Allen-Petersen BL, Carter CJ, Ohm AM, Reyland ME. Protein kinase Cdelta is required for ErbB2-driven mammary gland tumorigenesis and negatively correlates with prognosis in human breast cancer. Oncogene. 2014;33(10):1306–15. doi: 10.1038/onc.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mauro LV, Grossoni VC, Urtreger AJ, Yang C, Colombo LL, Morandi A, et al. PKC Delta (PKCdelta) promotes tumoral progression of human ductal pancreatic cancer. Pancreas. 2010;39(1):e31–41. doi: 10.1097/MPA.0b013e3181bce796. [DOI] [PubMed] [Google Scholar]
- 17.Jackson D, Zheng Y, Lyo D, Shen Y, Nakayama K, Nakayama KI, et al. Suppression of cell migration by protein kinase Cdelta. Oncogene. 2005;24(18):3067–72. doi: 10.1038/sj.onc.1208465. [DOI] [PubMed] [Google Scholar]
- 18.Kho DH, Bae JA, Lee JH, Cho HJ, Cho SH, Lee JH, et al. KITENIN recruits Dishevelled/PKC delta to form a functional complex and controls the migration and invasiveness of colorectal cancer cells. Gut. 2009;58(4):509–19. doi: 10.1136/gut.2008.150938. [DOI] [PubMed] [Google Scholar]
- 19.Paugh BS, Paugh SW, Bryan L, Kapitonov D, Wilczynska KM, Gopalan SM, et al. EGF regulates plasminogen activator inhibitor-1 (PAI-1) by a pathway involving c-Src, PKCdelta, and sphingosine kinase 1 in glioblastoma cells. FASEB J. 2008;22(2):455–65. doi: 10.1096/fj.07-8276com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kharait S, Dhir R, Lauffenburger D, Wells A. Protein kinase Cdelta signaling downstream of the EGF receptor mediates migration and invasiveness of prostate cancer cells. Biochem Biophys Res Commun. 2006;343(3):848–56. doi: 10.1016/j.bbrc.2006.03.044. [DOI] [PubMed] [Google Scholar]
- 21.Bailey TA, Luan H, Tom E, Bielecki TA, Mohapatra B, Ahmad G, et al. A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells. J Biol Chem. 2014;289(44):30443–58. doi: 10.1074/jbc.M114.608992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu CT, Cheng CC, Pan SM, Wu JR, Wu WS. PKC mediates fluctuant ERK-paxillin signaling for hepatocyte growth factor-induced migration of hepatoma cell HepG2. Cell Signal. 2013;25(6):1457–67. doi: 10.1016/j.cellsig.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 23.Park M, Kim WK, Song M, Park M, Kim H, Nam HJ, et al. Protein kinase C-delta-mediated recycling of active KIT in colon cancer. Clinical Cancer Res. 2013;19(18):4961–71. doi: 10.1158/1078-0432.CCR-13-0131. [DOI] [PubMed] [Google Scholar]
- 24.Singh A, Settleman J. Oncogenic K-ras “addiction” and synthetic lethality. Cell Cycle. 2009;8(17):2676–7. doi: 10.4161/cc.8.17.9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeVries-Seimon TA, Ohm AM, Humphries MJ, Reyland ME. Induction of apoptosis is driven by nuclear retention of protein kinase C delta. J Biol Chem. 2007;282(31):22307–14. doi: 10.1074/jbc.M703661200. [DOI] [PubMed] [Google Scholar]
- 26.Reyland ME, Barzen KA, Anderson SM, Quissell DO, Matassa AA. Activation of PKC is sufficient to induce an apoptotic program in salivary gland acinar cells. Cell Death Differ. 2000;7(12):1200–9. doi: 10.1038/sj.cdd.4400744. [DOI] [PubMed] [Google Scholar]
- 27.Matassa AA, Carpenter L, Biden TJ, Humphries MJ, Reyland ME. PKCdelta is required for mitochondrial-dependent apoptosis in salivary epithelial cells. J Biol Chem. 2001;276(32):29719–28. doi: 10.1074/jbc.M100273200. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida K. Nuclear trafficking of pro-apoptotic kinases in response to DNA damage. Trends Mol Med. 2008;14(7):305–13. doi: 10.1016/j.molmed.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Mogi A, Kuwano H. TP53 mutations in nonsmall cell lung cancer. J Biomed Biotechnol. 2011;2011:583929. doi: 10.1155/2011/583929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osaki S, Nakanishi Y, Takayama K, Pei XH, Ueno H, Hara N. Alteration of drug chemosensitivity caused by the adenovirus-mediated transfer of the wild-type p53 gene in human lung cancer cells. Cancer Gene Ther. 2000;7(2):300–7. doi: 10.1038/sj.cgt.7700096. [DOI] [PubMed] [Google Scholar]
- 31.Kolb RH, Greer PM, Cao PT, Cowan KH, Yan Y. ERK1/2 signaling plays an important role in topoisomerase II poison-induced G2/M checkpoint activation. PloS one. 2012;7(11):e50281. doi: 10.1371/journal.pone.0050281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adwan TS, Ohm AM, Jones DN, Humphries MJ, Reyland ME. Regulated binding of importin-alpha to protein kinase Cdelta in response to apoptotic signals facilitates nuclear import. J Biol Chem. 2011;286(41):35716–24. doi: 10.1074/jbc.M111.255950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Humphries MJ, Ohm AM, Schaack J, Adwan TS, Reyland ME. Tyrosine phosphorylation regulates nuclear translocation of PKCdelta. Oncogene. 2008;27(21):3045–53. doi: 10.1038/sj.onc.1210967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wie SM, Adwan TS, DeGregori J, Anderson SM, Reyland ME. Inhibiting tyrosine phosphorylation of protein kinase Cdelta (PKCdelta) protects the salivary gland from radiation damage. J Biol Chem. 2014;289(15):10900–8. doi: 10.1074/jbc.M114.551366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeVries TA, Neville MC, Reyland ME. Nuclear import of PKCdelta is required for apoptosis: identification of a novel nuclear import sequence. EMBO J. 2002;21(22):6050–60. doi: 10.1093/emboj/cdf606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riely GJ, Marks J, Pao W. KRAS mutations in non-small cell lung cancer. Proc Am Thorac Soc. 2009;6(2):201–5. doi: 10.1513/pats.200809-107LC. [DOI] [PubMed] [Google Scholar]
- 37.Pagliarini R, Shao W, Sellers WR. Oncogene addiction: pathways of therapeutic response, resistance, and road maps toward a cure. EMBO rep. 2015;16(3):280–96. doi: 10.15252/embr.201439949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.LaGory EL, Sitailo LA, Denning MF. The protein kinase Cdelta catalytic fragment is critical for maintenance of the G2/M DNA damage checkpoint. J Biol Chem. 2010;285(3):1879–87. doi: 10.1074/jbc.M109.055392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ueda Y, Hirai S, Osada S, Suzuki A, Mizuno K, Ohno S. Protein kinase C activates the MEK-ERK pathway in a manner independent of Ras and dependent on Raf. J Biol Chem. 1996;271(38):23512–9. doi: 10.1074/jbc.271.38.23512. [DOI] [PubMed] [Google Scholar]
- 40.Koo KH, Jeong WJ, Cho YH, Park JC, Min DS, Choi KY. K-Ras stabilization by estrogen via PKCdelta is involved in endometrial tumorigenesis. Oncotarget. 2015 doi: 10.18632/oncotarget.4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antal CE, Hudson AM, Kang E, Zanca C, Wirth C, Stephenson NL, et al. Cancer-associated protein kinase C mutations reveal kinase’s role as tumor suppressor. Cell. 2015;160(3):489–502. doi: 10.1016/j.cell.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.D’Costa AM, Robinson JK, Maududi T, Chaturvedi V, Nickoloff BJ, Denning MF. The proapoptotic tumor suppressor protein kinase C-delta is lost in human squamous cell carcinomas. Oncogene. 2006;25(3):378–86. doi: 10.1038/sj.onc.1209065. [DOI] [PubMed] [Google Scholar]
- 43.Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP. Analysis of protein kinase C delta (PKC delta) expression in endometrial tumors. Human Pathology. 2008;39(1):21–9. doi: 10.1016/j.humpath.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imielinski M, Berger AH, Hammerman PS, Hernandez B, Pugh TJ, Hodis E, et al. Mapping the hallmarks of lung adenocarcinoma with massively parallel sequencing. Cell. 2012;150(6):1107–20. doi: 10.1016/j.cell.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Young A, Lou D, McCormick F. Oncogenic and wild-type Ras play divergent roles in the regulation of mitogen-activated protein kinase signaling. Cancer Discov. 2013;3(1):112–23. doi: 10.1158/2159-8290.CD-12-0231. [DOI] [PubMed] [Google Scholar]
- 46.Cai Q, Li J, Gao T, Xie J, Evers BM. Protein kinase Cdelta negatively regulates hedgehog signaling by inhibition of Gli1 activity. J Biol Chem. 2009;284(4):2150–8. doi: 10.1074/jbc.M803235200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu T, Tsuji T, Chen C. Roles of PKC isoforms in the induction of apoptosis elicited by aberrant Ras. Oncogene. 2010;29(7):1050–61. doi: 10.1038/onc.2009.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, et al. A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell. 2009;137(5):835–48. doi: 10.1016/j.cell.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wilson FH, Johannessen CM, Piccioni F, Tamayo P, Kim JW, Van Allen EM, et al. A functional landscape of resistance to ALK inhibition in lung cancer. Cancer Cell. 2015;27(3):397–408. doi: 10.1016/j.ccell.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prior IA, Lewis PD, Mattos C. A comprehensive survey of Ras mutations in cancer. Cancer Res. 2012;72(10):2457–67. doi: 10.1158/0008-5472.CAN-11-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.