Abstract
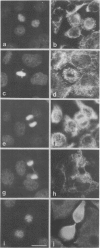
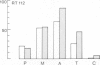
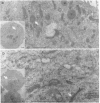
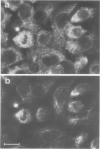
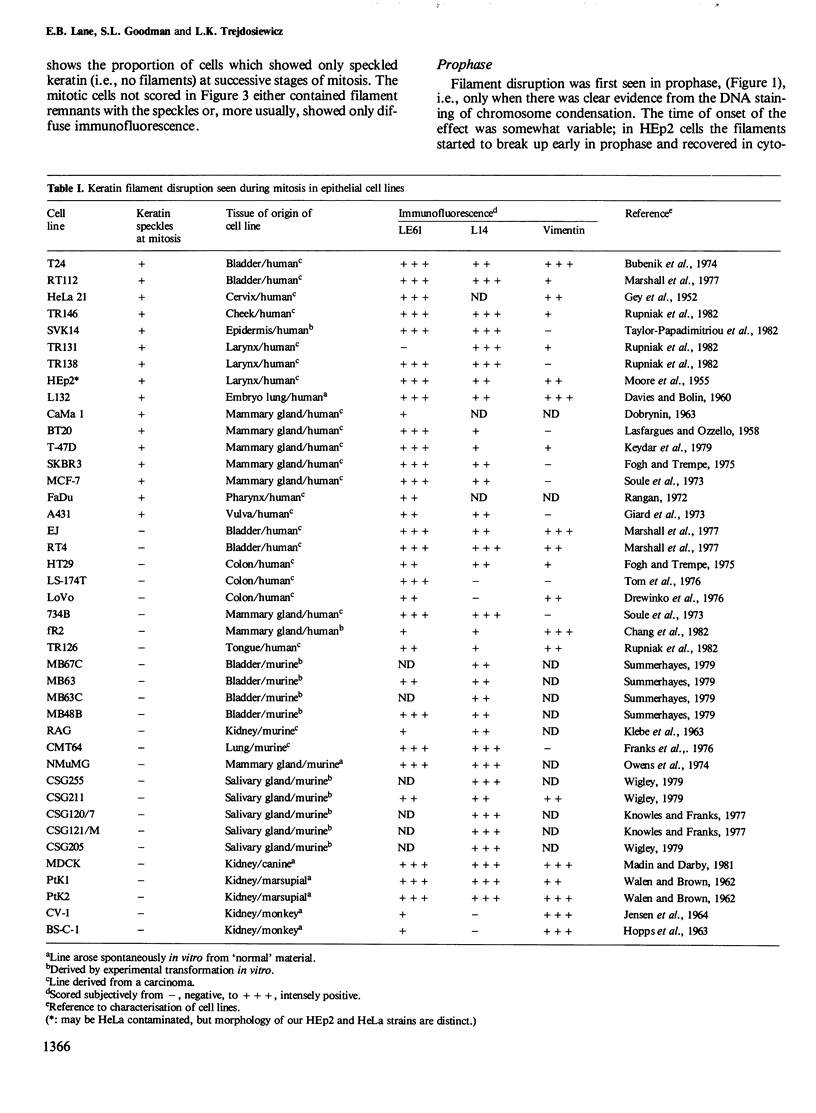
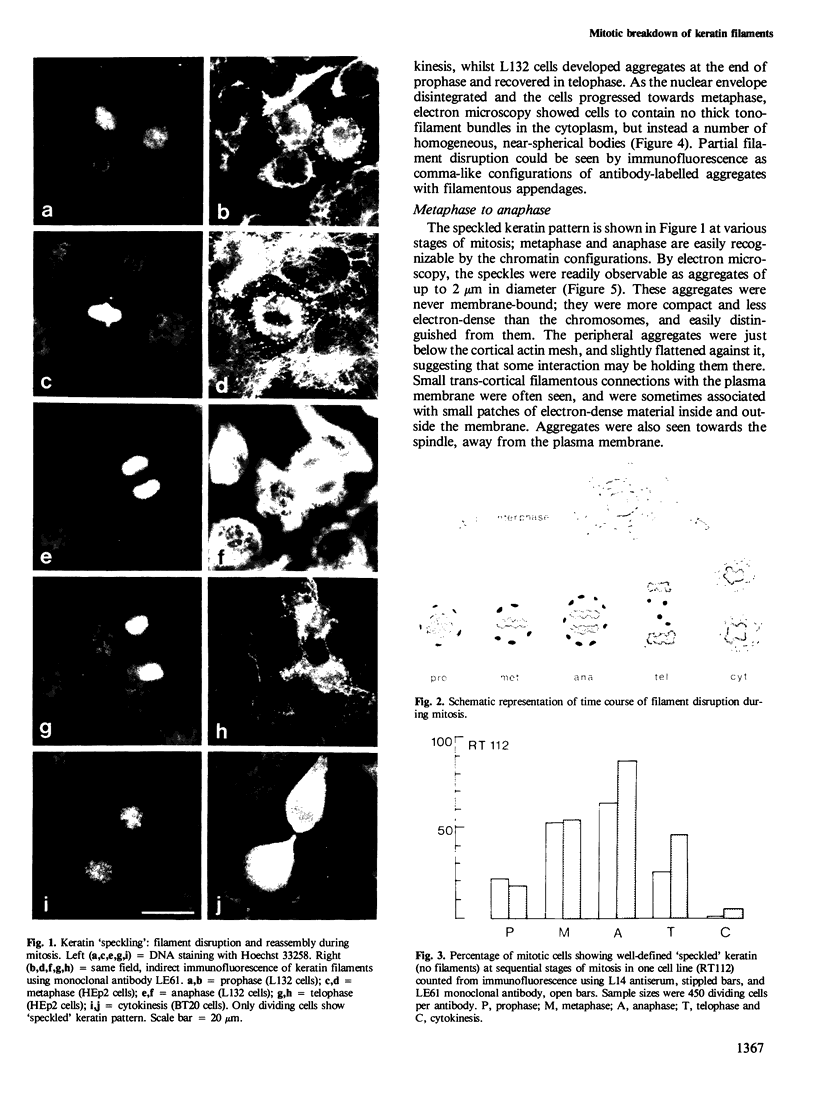
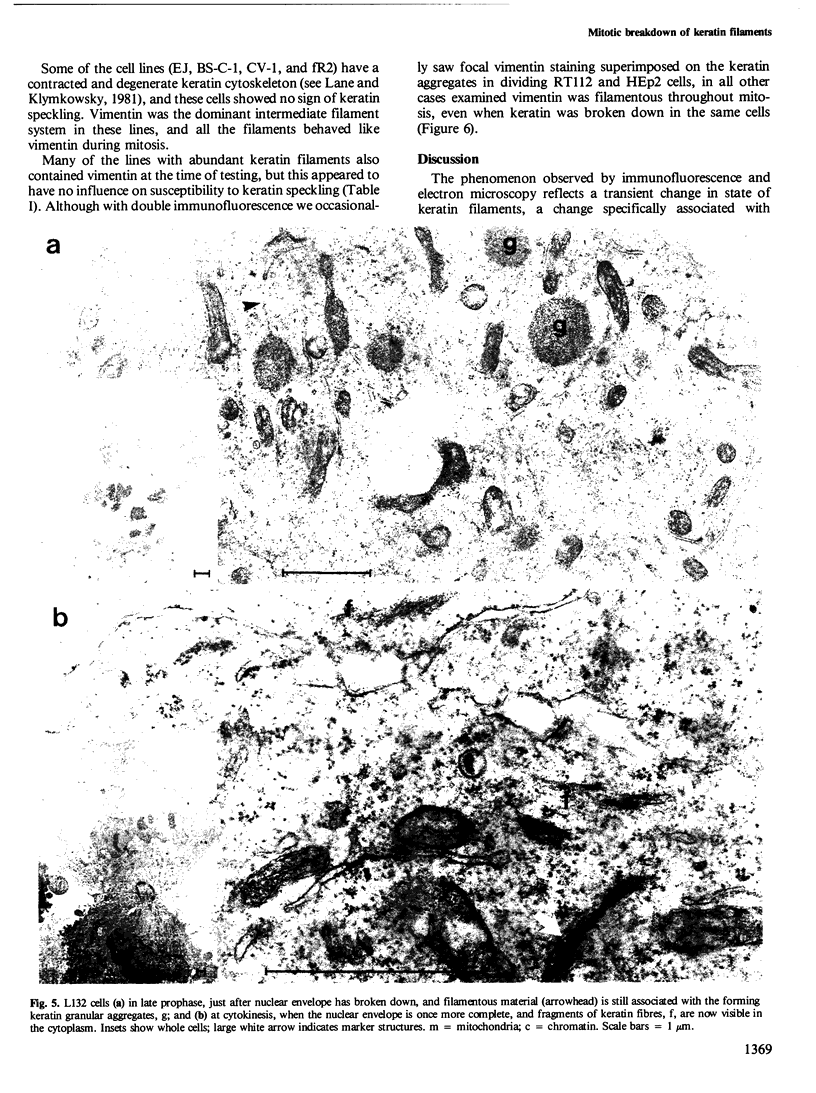
The behaviour of keratin filaments during cell division was examined in a wide range of epithelial lines from several species. Almost half of them show keratin disruption as described previously: by immunofluorescence, filaments are replaced during mitosis by a 'speckled' pattern of discrete cytoplasmic dots. In the electron microscope these ' speckles ' are seen as granules around the cell periphery, just below the actin cortical mesh, with no detectable 10 nm filament structure inside them and no keratin filament bundles in the rest of the cytoplasm. A time course of the filament reorganization was constructed from double immunofluorescence data; filaments are disrupted in prophase, and the filament network is intact again by cytokinesis. The phenomenon is restricted to cells rich in keratin filaments, such as keratinocytes; it is unrelated to the co-existence of vimentin in many of these cells, and vimentin is generally maintained as filaments while the keratin is restructured. Some resistance to the effect may be conferred by an extended cycle time. Filament reorganization takes place within minutes, so that a reversible mechanism seems more likely than one involving de novo protein synthesis, at this metabolically quiet stage of the cell cycle.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H. Intermediate filaments: a family of homologous structures. J Muscle Res Cell Motil. 1981 Jun;2(2):141–166. doi: 10.1007/BF00711866. [DOI] [PubMed] [Google Scholar]
- Aubin J. E., Osborn M., Franke W. W., Weber K. Intermediate filaments of the vimentin-type and the cytokeratin-type are distributed differently during mitosis. Exp Cell Res. 1980 Sep;129(1):149–165. doi: 10.1016/0014-4827(80)90340-7. [DOI] [PubMed] [Google Scholar]
- Blose S. H. Ten-nanometer filaments and mitosis: maintenance of structural continuity in dividing endothelial cells. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3372–3376. doi: 10.1073/pnas.76.7.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Brecher S. The occurrence and possible role of 80-100 A filaments in PtKl cells. Exp Cell Res. 1975 Dec;96(2):303–310. doi: 10.1016/0014-4827(75)90261-x. [DOI] [PubMed] [Google Scholar]
- Bubeník J., Baresová M., Viklický V., Jakoubková J., Sainerová H., Donner J. Established cell line of urinary bladder carcinoma (T24) containing tumour-specific antigen. Int J Cancer. 1973 May;11(3):765–773. doi: 10.1002/ijc.2910110327. [DOI] [PubMed] [Google Scholar]
- Chang S. E., Keen J., Lane E. B., Taylor-Papadimitriou J. Establishment and characterization of SV40-transformed human breast epithelial cell lines. Cancer Res. 1982 May;42(5):2040–2053. [PubMed] [Google Scholar]
- DOBRYNIN Y. V. ESTABLISHMENT AND CHARACTERISTICS OF CELL STRAINS FROM SOME EPITHELIAL TUMORS OF HUMAN ORIGIN. J Natl Cancer Inst. 1963 Nov;31:1173–1195. [PubMed] [Google Scholar]
- Drewinko B., Romsdahl M. M., Yang L. Y., Ahearn M. J., Trujillo J. M. Establishment of a human carcinoembryonic antigen-producing colon adenocarcinoma cell line. Cancer Res. 1976 Feb;36(2 Pt 1):467–475. [PubMed] [Google Scholar]
- Evans R. M., Fink L. M. An alteration in the phosphorylation of vimentin-type intermediate filaments is associated with mitosis in cultured mammalian cells. Cell. 1982 May;29(1):43–52. doi: 10.1016/0092-8674(82)90088-5. [DOI] [PubMed] [Google Scholar]
- Fan H., Penman S. Regulation of protein synthesis in mammalian cells. II. Inhibition of protein synthesis at the level of initiation during mitosis. J Mol Biol. 1970 Jun 28;50(3):655–670. doi: 10.1016/0022-2836(70)90091-4. [DOI] [PubMed] [Google Scholar]
- Frank E. D., Warren L. Aortic smooth muscle cells contain vimentin instead of desmin. Proc Natl Acad Sci U S A. 1981 May;78(5):3020–3024. doi: 10.1073/pnas.78.5.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franks L. M., Carbonell A. W., Hemmings V. J., Riddle P. N. Metastasizing tumors from serum-supplemented and serum-free cell lines from a C57BL mouse lung tumor. Cancer Res. 1976 Mar;36(3):1049–1055. [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Giard D. J., Aaronson S. A., Todaro G. J., Arnstein P., Kersey J. H., Dosik H., Parks W. P. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973 Nov;51(5):1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Grosset L., Odartchenko N. Duration of mitosis and separate mitotic phases compared to nuclear DNA content in erythroblasts of four vertebrates;. Cell Tissue Kinet. 1975 Jan;8(1):91–96. doi: 10.1111/j.1365-2184.1975.tb01210.x. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- HOPPS H. E., BERNHEIM B. C., NISALAK A., TJIO J. H., SMADEL J. E. BIOLOGIC CHARACTERISTICS OF A CONTINUOUS KIDNEY CELL LINE DERIVED FROM THE AFRICAN GREEN MONKEY. J Immunol. 1963 Sep;91:416–424. [PubMed] [Google Scholar]
- Horwitz B., Kupfer H., Eshhar Z., Geiger B. Reorganization of arrays of prekeratin filaments during mitosis. Immunofluorescence microscopy with multiclonal and monoclonal prekeratin antibodies. Exp Cell Res. 1981 Aug;134(2):281–290. doi: 10.1016/0014-4827(81)90427-4. [DOI] [PubMed] [Google Scholar]
- Hynes R. O., Destree A. T. 10 nm filaments in normal and transformed cells. Cell. 1978 Jan;13(1):151–163. doi: 10.1016/0092-8674(78)90146-0. [DOI] [PubMed] [Google Scholar]
- JENSEN F. C., GIRARDI A. J., GILDEN R. V., KOPROWSKI H. INFECTION OF HUMAN AND SIMIAN TISSUE CULTURES WITH ROUS SARCOMA VIRUS. Proc Natl Acad Sci U S A. 1964 Jul;52:53–59. doi: 10.1073/pnas.52.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Klebe R. J., Chen T., Ruddle F. H. Controlled production of proliferating somatic cell hybrids. J Cell Biol. 1970 Apr;45(1):74–82. doi: 10.1083/jcb.45.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles M. A., Franks L. M. Stages in neoplastic transformation of adult epithelial cells by 7,12-dimethylbenz(a)anthracene in vitro. Cancer Res. 1977 Nov;37(11):3917–3924. [PubMed] [Google Scholar]
- LASFARGUES E. Y., OZZELLO L. Cultivation of human breast carcinomas. J Natl Cancer Inst. 1958 Dec;21(6):1131–1147. [PubMed] [Google Scholar]
- Lane E. B., Klymkowsky M. W. Epithelial tonofilaments: investigating their form and function using monoclonal antibodies. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):387–402. doi: 10.1101/sqb.1982.046.01.038. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOORE A. E., SABACHEWSKY L., TOOLAN H. W. Culture characteristics of four permanent lines of human cancer cells. Cancer Res. 1955 Oct;15(9):598–602. [PubMed] [Google Scholar]
- Marshall C. J., Franks L. M., Carbonell A. W. Markers of neoplastic transformation in epithelial cell lines derived from human carcinomas. J Natl Cancer Inst. 1977 Jun;58(6):1743–1751. doi: 10.1093/jnci/58.6.1743. [DOI] [PubMed] [Google Scholar]
- Matsui S. I., Seon B. K., Sandberg A. A. Disappearance of a structural chromatin protein A24 in mitosis: implications for molecular basis of chromatin condensation. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6386–6390. doi: 10.1073/pnas.76.12.6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milcarek C., Zahn K. The synthesis of ninety proteins including actin throughout the HeLa cell cycle. J Cell Biol. 1978 Dec;79(3):833–838. doi: 10.1083/jcb.79.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Traub P. Properties of Ca2+-activated protease specific for the intermediate-sized filament protein vimentin in Ehrlich-ascites-tumour cells. Eur J Biochem. 1981 May;116(1):51–57. doi: 10.1111/j.1432-1033.1981.tb05299.x. [DOI] [PubMed] [Google Scholar]
- Obara Y., Yoshida H., Chai L. S., Weinfeld H., Sandberg A. A. Contrast between the environmental pH dependencies of prophasing and nuclear membrane formation in interphase-metaphase cells. J Cell Biol. 1973 Sep;58(3):608–617. doi: 10.1083/jcb.58.3.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens R. B., Smith H. S., Hackett A. J. Epithelial cell cultures from normal glandular tissue of mice. J Natl Cancer Inst. 1974 Jul;53(1):261–269. doi: 10.1093/jnci/53.1.261. [DOI] [PubMed] [Google Scholar]
- Piras R., Piras M. M. Changes in microtubule phosphorylation during cell cycle of HeLa cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1161–1165. doi: 10.1073/pnas.72.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintart J., Leroy-Houyet M. A., Trouet A., Baudhuin P. Endocytosis and chloroquine accumulation during the cell cycle of hepatoma cells in culture. J Cell Biol. 1979 Sep;82(3):644–653. doi: 10.1083/jcb.82.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaekers F. C., Puts J. J., Kant A., Moesker O., Jap P. H., Vooijs G. P. Use of antibodies to intermediate filaments in the characterization of human tumors. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):331–339. doi: 10.1101/sqb.1982.046.01.034. [DOI] [PubMed] [Google Scholar]
- Rangan S. R. A new human cell line (FaDu) from a hypopharyngeal carcinoma. Cancer. 1972 Jan;29(1):117–121. doi: 10.1002/1097-0142(197201)29:1<117::aid-cncr2820290119>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B., Kaufmann S., Vogelstein B. Increased phosphorylation rate of intermediate filaments during mitotic arrest. Exp Cell Res. 1981 Jun;133(2):445–448. doi: 10.1016/0014-4827(81)90338-4. [DOI] [PubMed] [Google Scholar]
- Schlaepfer W. W., Hasler M. B. Characterization of the calcium-induced disruption of neurofilaments in rat peripheral nerve. Brain Res. 1979 May 25;168(2):299–309. doi: 10.1016/0006-8993(79)90171-9. [DOI] [PubMed] [Google Scholar]
- Schliwa M., Euteneuer U. Structural transformation of epidermal tonofilaments upon cold treatment. Exp Cell Res. 1979 Aug;122(1):93–101. doi: 10.1016/0014-4827(79)90564-0. [DOI] [PubMed] [Google Scholar]
- Soule H. D., Vazguez J., Long A., Albert S., Brennan M. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973 Nov;51(5):1409–1416. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Taylor-Papadimitriou J., Purkis P., Lane E. B., McKay I. A., Chang S. E. Effects of SV40 transformation on the cytoskeleton and behavioural properties of human keratinocytes. Cell Differ. 1982 May;11(3):169–180. doi: 10.1016/0045-6039(82)90008-2. [DOI] [PubMed] [Google Scholar]
- Tom B. H., Rutzky L. P., Jakstys M. M., Oyasu R., Kaye C. I., Kahan B. D. Human colonic adenocarcinoma cells. I. Establishment and description of a new line. In Vitro. 1976 Mar;12(3):180–191. doi: 10.1007/BF02796440. [DOI] [PubMed] [Google Scholar]
- Trejdosiewicz L. K., Smolira M. A., Hodges G. M., Goodman S. L., Livingston D. C. Cell surface distribution of fibronectin in cultures of fibroblasts and bladder derived epithelium: SEM-immunogold localization compared to immunoperoxidase and immunofluorescence.. J Microsc. 1981 Aug;123(Pt 2):227–236. doi: 10.1111/j.1365-2818.1981.tb01297.x. [DOI] [PubMed] [Google Scholar]
- WALEN K. H., BROWN S. W. Chromosomes in a marsupial (Potorous tridactylis) tissue culture. Nature. 1962 Apr 28;194:406–406. doi: 10.1038/194406a0. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T., Shinitzky M. Microviscosity modulation during the cell cycle of neuroblastoma cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4458–4461. doi: 10.1073/pnas.74.10.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]