Abstract
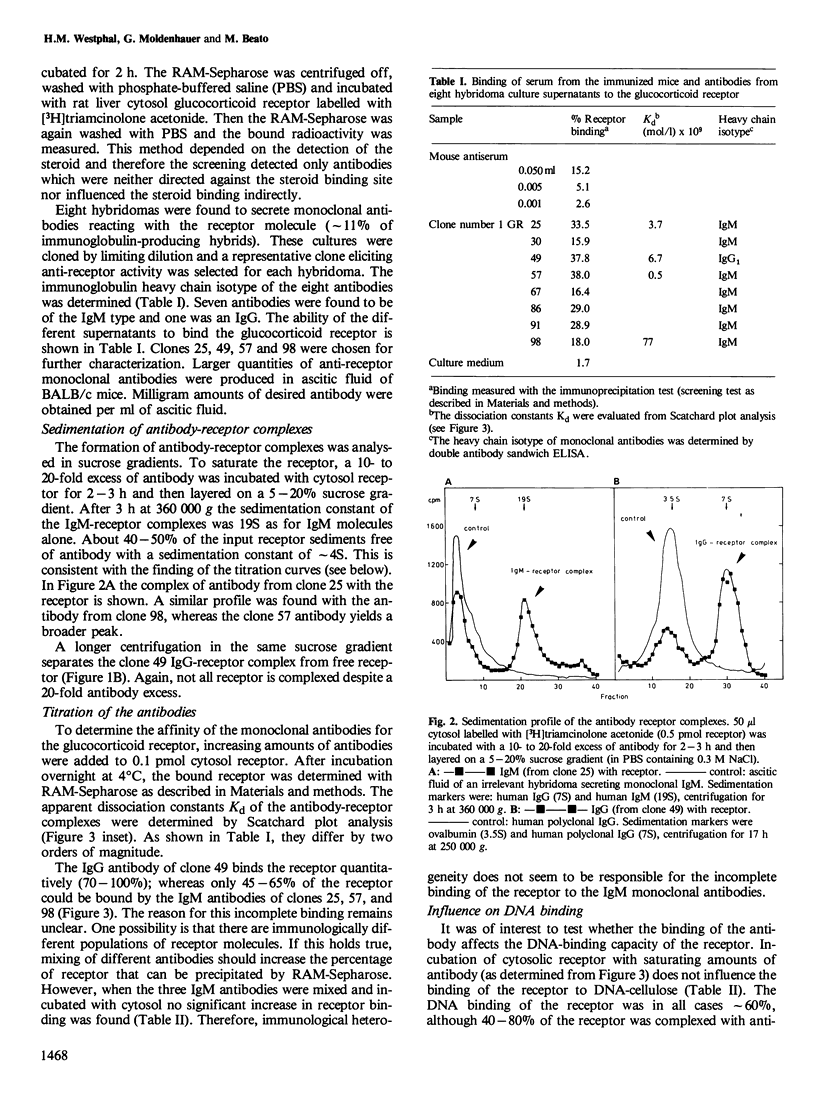
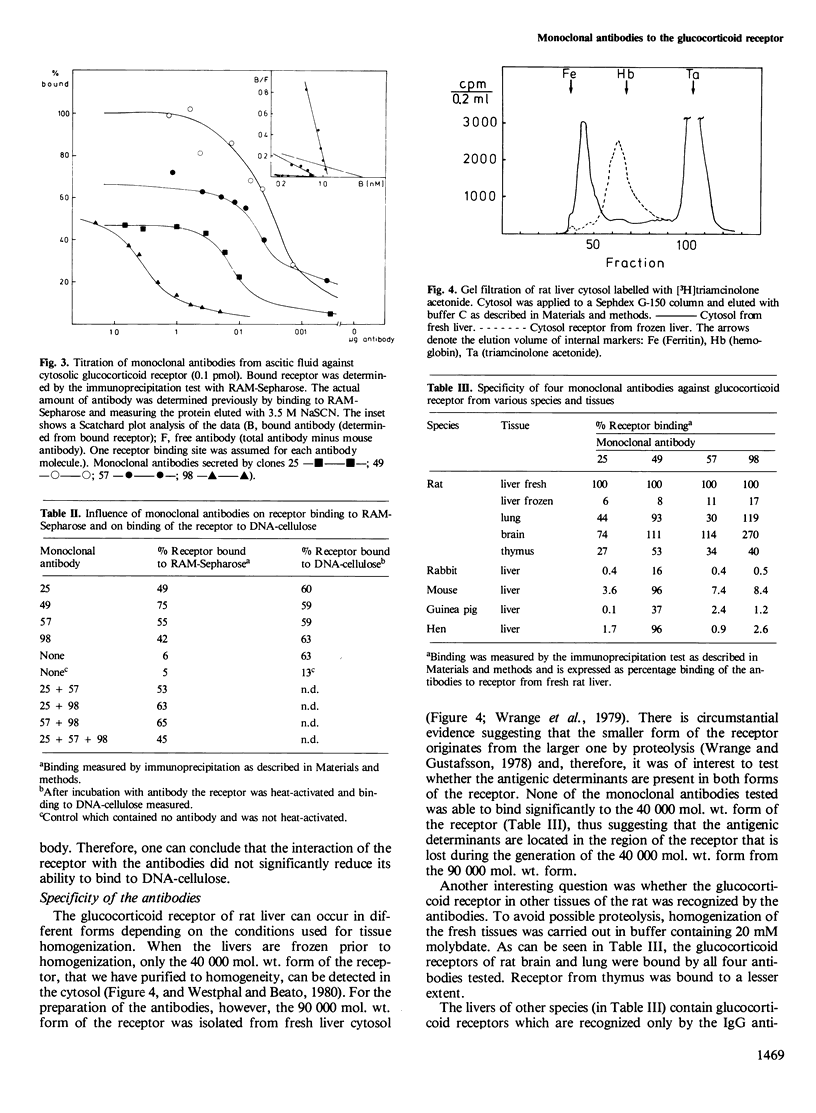
Monoclonal antibodies against the 90 000 mol. wt. form of the activated rat liver glucocorticoid receptor were generated from mice immunized with a partially purified receptor preparation. The screening assay was based on the precipitation of liver cytosol, labelled with [3H]triamcinolone acetonide, with monoclonal antibodies bound to immobilized rabbit anti-mouse IgG. Out of 102 hybridomas obtained, 76 produced immunoglobulin and eight of them were found to react with the receptor molecule. Only one of the positive clones secreted IgG whereas the other seven produced IgM. The complexes of receptor and antibodies were identified by sucrose density gradient centrifugation. All seven monoclonal antibodies tested reacted with the 90 000 mol. wt. form of the receptor but not with the 40 000 mol. wt. form that contains the steroid and DNA binding domains. None of the monoclonal antibodies interfered with the binding of the receptor to DNA cellulose, thus suggesting that the antigenic determinants are located in a region of the receptor that is not directly implicated in either steroid binding or DNA binding. These antigenic determinants were common to glucocorticoid receptors from several tissues of the rat, whereas glucocorticoid receptors from other species react only with some of the antibodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carlstedt-Duke J., Okret S., Wrange O., Gustafsson J. A. Immunochemical analysis of the glucocorticoid receptor: identification of a third domain separate from the steroid-binding and DNA-binding domains. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4260–4264. doi: 10.1073/pnas.79.14.4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Eisen H. J. An antiserum to the rat liver glucocorticoid receptor. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3893–3897. doi: 10.1073/pnas.77.7.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galfre G., Howe S. C., Milstein C., Butcher G. W., Howard J. C. Antibodies to major histocompatibility antigens produced by hybrid cell lines. Nature. 1977 Apr 7;266(5602):550–552. doi: 10.1038/266550a0. [DOI] [PubMed] [Google Scholar]
- Govindan M. V. Purification of glucocorticoid receptors from rat liver cytosol. Preparation of antibodies against the major receptor proteins and application of immunological techniques to study activation and translocation. J Steroid Biochem. 1979 Jul;11(1A):323–332. doi: 10.1016/0022-4731(79)90315-7. [DOI] [PubMed] [Google Scholar]
- Greene G. L., Fitch F. W., Jensen E. V. Monoclonal antibodies to estrophilin: probes for the study of estrogen receptors. Proc Natl Acad Sci U S A. 1980 Jan;77(1):157–161. doi: 10.1073/pnas.77.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronemeyer H., Pongs O. Localization of ecdysterone on polytene chromosomes of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2108–2112. doi: 10.1073/pnas.77.4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huet J., Sentenac A., Fromageot P. Spot-immunodetection of conserved determinants in eukaryotic RNA polymerases. Study with antibodies to yeast RNA polymerases subunits. J Biol Chem. 1982 Mar 10;257(5):2613–2618. [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- Okret S., Carlstedt-Duke J., Wrange O., Carlström K., Gustafsson J. A. Characterization of an antiserum against the glucocorticoid receptor. Biochim Biophys Acta. 1981 Oct 12;677(2):205–219. doi: 10.1016/0304-4165(81)90087-8. [DOI] [PubMed] [Google Scholar]
- Schaffner W., Weissmann C. A rapid, sensitive, and specific method for the determination of protein in dilute solution. Anal Biochem. 1973 Dec;56(2):502–514. doi: 10.1016/0003-2697(73)90217-0. [DOI] [PubMed] [Google Scholar]
- Tsawdaroglou N. G., Govindan M. V., Schmid W., Sekeris C. E. Dexamethasone-binding proteins in cytosol and nucleus of rat thymocytes. Purification of three receptor proteins. Eur J Biochem. 1981;114(2):305–313. doi: 10.1111/j.1432-1033.1981.tb05150.x. [DOI] [PubMed] [Google Scholar]
- Westphal H. M., Beato M. The activated glucocorticoid receptor of rat liver. Purification and physical characterization. Eur J Biochem. 1980 May;106(2):395–403. doi: 10.1111/j.1432-1033.1980.tb04585.x. [DOI] [PubMed] [Google Scholar]
- Westphal H. M., Fleischmann G., Beato M. Photoaffinity labeling of steroid binding proteins with unmodified ligands. Eur J Biochem. 1981 Sep;119(1):101–106. doi: 10.1111/j.1432-1033.1981.tb05582.x. [DOI] [PubMed] [Google Scholar]
- Wrange O., Carlstedt-Duke J., Gustafsson J. A. Purification of the glucocorticoid receptor from rat liver cytosol. J Biol Chem. 1979 Sep 25;254(18):9284–9290. [PubMed] [Google Scholar]
- Wrange O., Gustafsson J. A. Separation of the hormone- and DNA-binding sites of the hepatic glucocorticoid receptor by means of proteolysis. J Biol Chem. 1978 Feb 10;253(3):856–865. [PubMed] [Google Scholar]