Abstract
We have cloned the structural genes for a regulated ( PHO5 ) and a constitutive ( PHO3 ) acid phosphatase from yeast by transformation and complementation of a yeast pho3 , pho5 double mutant. Both genes are located on a 5.1-kb BamHI fragment. The cloned genes were identified on the basis of genetic evidence and by hybrid selection of mRNA coupled with in vitro translation and immunoprecipitation. Subcloning of partial Sau3A digests and functional in vivo analysis by transformation together with DNA sequence analysis showed that the two genes are oriented in the order (5') PHO5 , PHO3 (3'). While the nucleotide sequences of the two coding regions are quite similar, the putative promoter regions show a lower degree of sequence homology. Partly divergent promoter sequences may explain the different regulation of the two genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
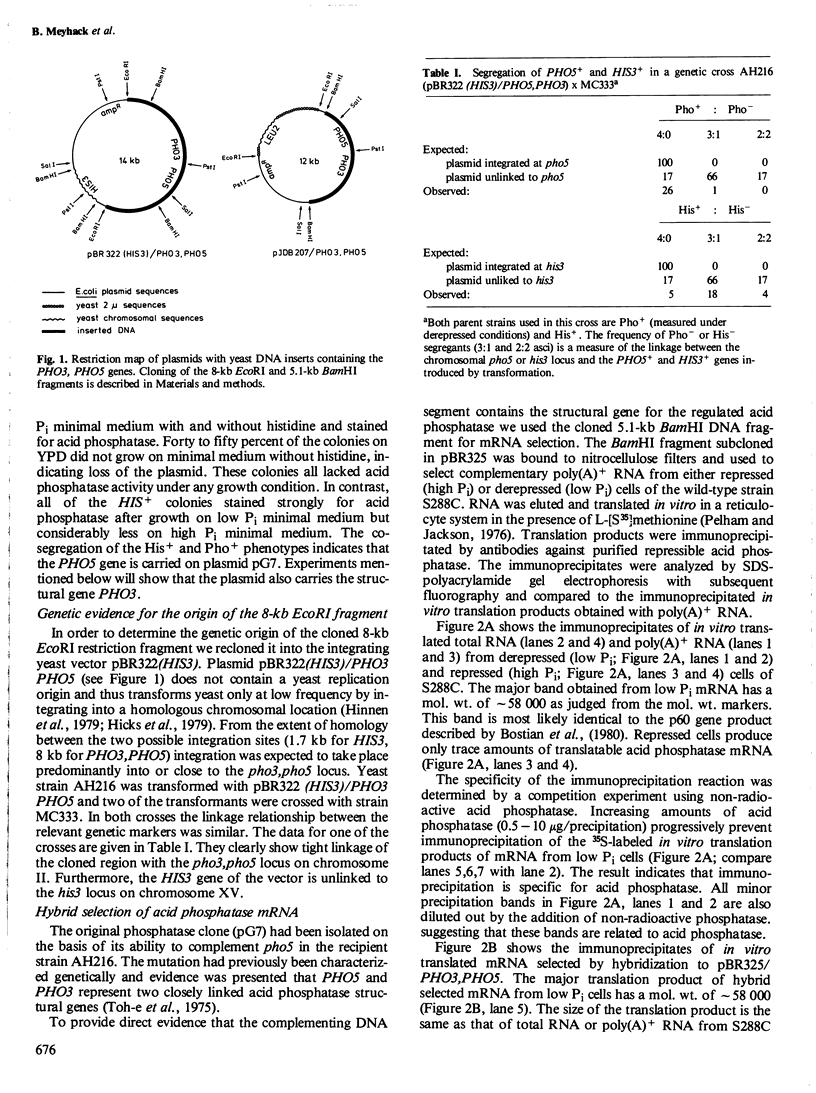
- Bostian K. A., Lemire J. M., Cannon L. E., Halvorson H. O. In vitro synthesis of repressible yeast acid phosphatase: identification of multiple mRNAs and products. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4504–4508. doi: 10.1073/pnas.77.8.4504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansche P. E., Beres V., Lange P. Gene duplication in Saccharomyces cerevisiae. Genetics. 1978 Apr;88(4 Pt 1):673–687. [PMC free article] [PubMed] [Google Scholar]
- Hicks J. B., Hinnen A., Fink G. R. Properties of yeast transformation. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1305–1313. doi: 10.1101/sqb.1979.043.01.149. [DOI] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Kramer R. A., Andersen N. Isolation of yeast genes with mRNA levels controlled by phosphate concentration. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6541–6545. doi: 10.1073/pnas.77.11.6541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange P., Hansche P. E. Mapping of a centromere-linked gene responsible for constitutive acid phosphatase synthesis in yeast. Mol Gen Genet. 1980;180(3):605–607. doi: 10.1007/BF00268067. [DOI] [PubMed] [Google Scholar]
- Lauer G. D., Roberts T. M., Klotz L. C. Determination of the nuclear DNA content of Saccharomyces cerevisiae and implications for the organization of DNA in yeast chromosomes. J Mol Biol. 1977 Aug 25;114(4):507–526. doi: 10.1016/0022-2836(77)90175-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Mayer H. Optimization of the EcoRI-activity of EcoRI endonuclease. FEBS Lett. 1978 Jun 15;90(2):341–344. doi: 10.1016/0014-5793(78)80400-1. [DOI] [PubMed] [Google Scholar]
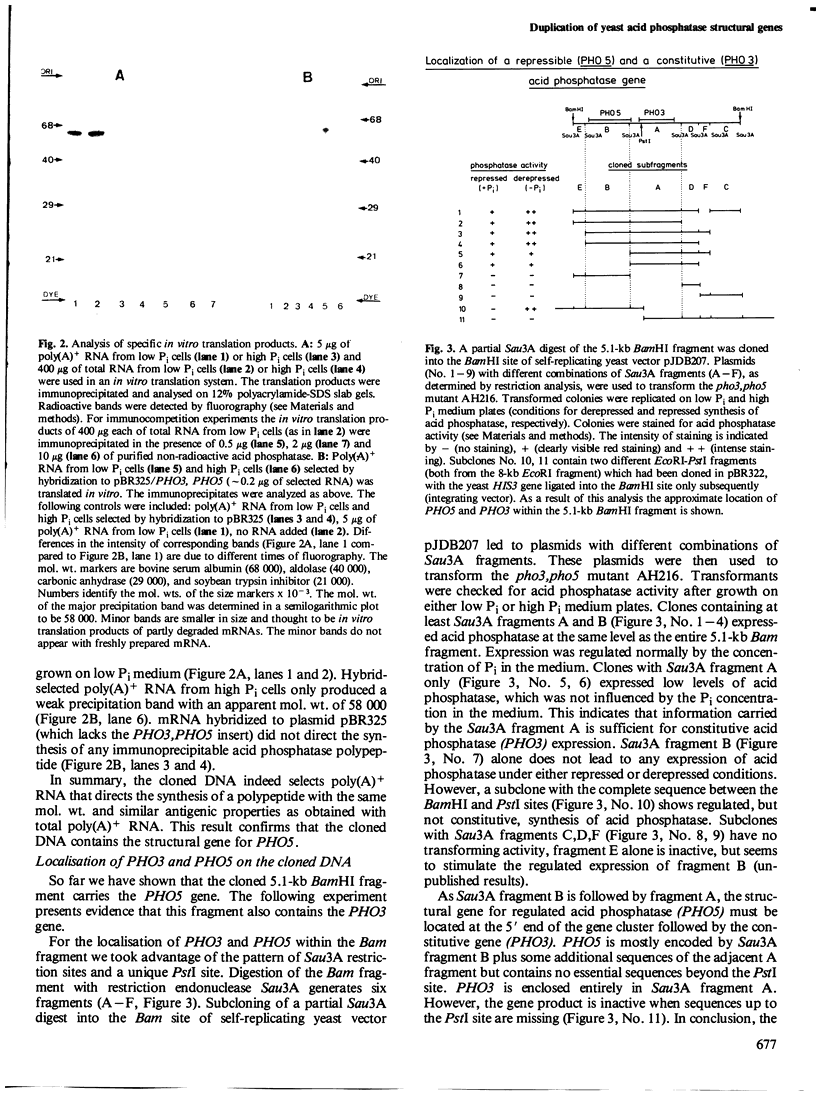
- Mildner P., Barbarić S., Golubić Z., Ries B. Purification of protoplast-secreted acid phosphatase from baker's yeast. Effect on adenosine triphosphatase activity. Biochim Biophys Acta. 1976 Mar 11;429(1):274–282. doi: 10.1016/0005-2744(76)90050-4. [DOI] [PubMed] [Google Scholar]
- Olson M. V., Loughney K., Hall B. D. Identification of the yeast DNA sequences that correspond to specific tyrosine-inserting nonsense suppressor loci. J Mol Biol. 1979 Aug 15;132(3):387–410. doi: 10.1016/0022-2836(79)90267-5. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rogers D. T., Lemire J. M., Bostian K. A. Acid phosphatase polypeptides in Saccharomyces cerevisiae are encoded by a differentially regulated multigene family. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2157–2161. doi: 10.1073/pnas.79.7.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schurr A., Yagil E. Regulation and characterization of acid and alkaline phosphatase in yeast. J Gen Microbiol. 1971 Mar;65(3):291–303. doi: 10.1099/00221287-65-3-291. [DOI] [PubMed] [Google Scholar]
- To-E A., Ueda Y., Kakimoto S. I., Oshima Y. Isolation and characterization of acid phosphatase mutants in Saccharomyces cerevisiae. J Bacteriol. 1973 Feb;113(2):727–738. doi: 10.1128/jb.113.2.727-738.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-E A. Genetic Mapping of the pho2, PHO82-pho4 and pho85 Loci of Yeast. Genetics. 1980 Apr;94(4):929–932. doi: 10.1093/genetics/94.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toh-e A., Kobayashi S., Oshima Y. Disturbance of the machinery for the gene expression by acidic pH in the repressible acid phosphatase system of Saccharomyces cerevisiae. Mol Gen Genet. 1978 Jun 14;162(2):139–149. doi: 10.1007/BF00267870. [DOI] [PubMed] [Google Scholar]
- Ueda Y., To-E A., Oshima Y. Isolation and characterization of recessive, constitutive mutations for repressible acid phosphatase synthesis in Saccharomyces cerevisiae. J Bacteriol. 1975 Jun;122(3):911–922. doi: 10.1128/jb.122.3.911-922.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]