Abstract
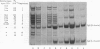
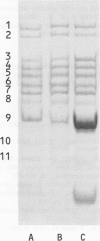
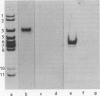
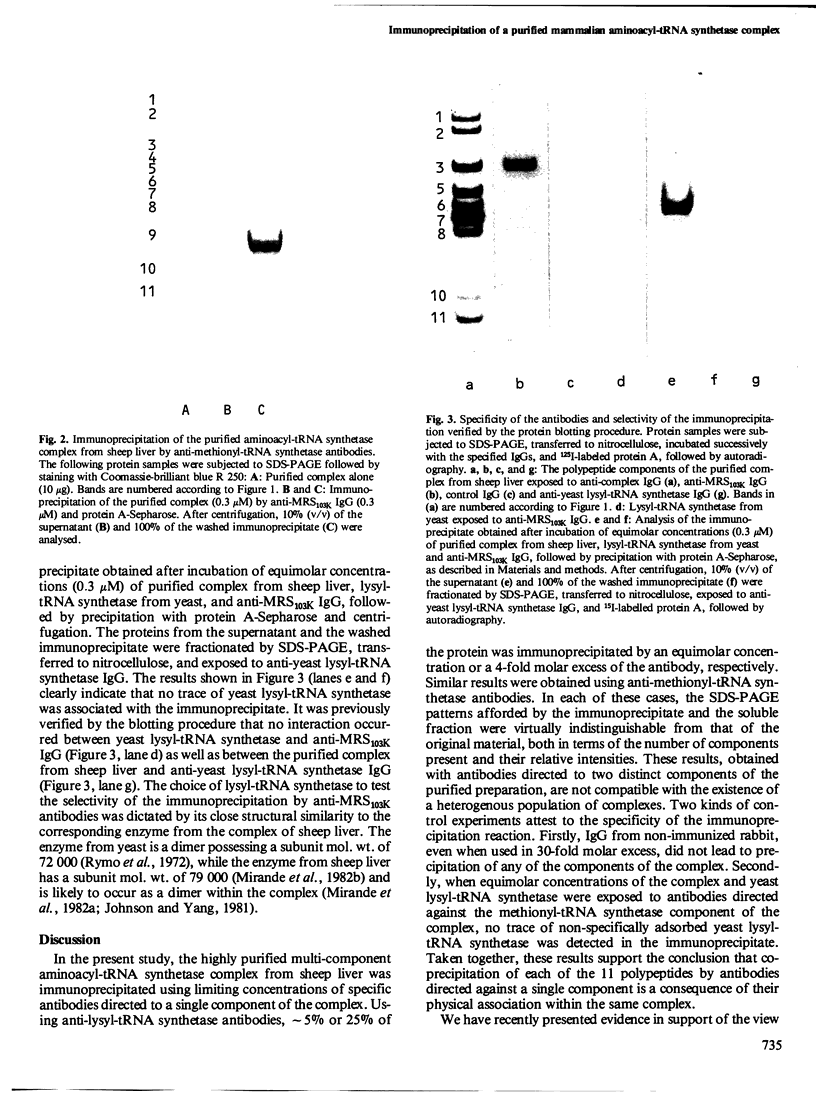
Seven aminoacyl-tRNA synthetases from sheep liver were co-purified as high mol. wt. entities to constant specific activities. The purified multienzyme preparation displayed an apparent mol. wt. of approximately 10(6) and was composed of 11 distinct polypeptides, as revealed by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). To test the assumption that all of these components were physically associated within the same complex, the purified preparation was subjected to immunoprecipitation by antibodies raised against its lysyl- or methionyl-tRNA synthetase component. Depending on the limiting concentrations of the specific antibodies used, from 5 to 40% of the input protein was recovered in the immunoprecipitate. Its polypeptide composition, as revealed by SDS-PAGE, was indistinguishable from that of the original material. The immunoprecipitation reaction was highly specific, as attested by the observation that IgG from nonimmunized rabbit failed to precipitate any of the 11 polypeptides, even when used in 30-fold molar excess over input protein. We conclude that co-precipitation of all of these polypeptides by antibodies directed against a single component of the purified preparation is a consequence of their physical association within the same multienzyme complex.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bandyopadhyay A. K., Deutscher M. P. Complex of aminoacyl-transfer RNA synthetases. J Mol Biol. 1971 Aug 28;60(1):113–122. doi: 10.1016/0022-2836(71)90451-7. [DOI] [PubMed] [Google Scholar]
- Brevet A., Geffrotin C., Kellermann O. Macromolecular complex of aminoacyl-tRNA synthetases from sheep liver. Identification of the methionyl-tRNA synthetase component by affinity labeling. Eur J Biochem. 1982 Jun;124(3):483–488. doi: 10.1111/j.1432-1033.1982.tb06619.x. [DOI] [PubMed] [Google Scholar]
- Charezinski M., Borkowski T. Occurrence of aminoacyl-tRNA synthetase complexes in calf brain. Arch Biochem Biophys. 1981 Apr 1;207(2):241–247. doi: 10.1016/0003-9861(81)90030-8. [DOI] [PubMed] [Google Scholar]
- Denney R. M. Detection and partial purification of rapidly sedimenting forms of aminoacyl-transfer ribonucleic acid synthetases from human placenta. Arch Biochem Biophys. 1977 Sep;183(1):156–167. doi: 10.1016/0003-9861(77)90430-1. [DOI] [PubMed] [Google Scholar]
- Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70(A):419–439. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- Hele P., Hebert L. Occurrence of a complex of aminoacryl-tRNA synthetases in lactating rat mammary gland. Biochim Biophys Acta. 1977 Dec 2;479(3):311–321. doi: 10.1016/0005-2787(77)90113-7. [DOI] [PubMed] [Google Scholar]
- Johnson D. L., Yang D. C. Stoichiometry and composition of an aminoacyl-tRNA synthetase complex from rat liver. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4059–4062. doi: 10.1073/pnas.78.7.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellermann O., Brevet A., Tonetti H., Waller J. P. Macromolecular complexes of aminoacyl-tRNA synthetases from eukaryotes. 1. Extensive purification and characterization of the high-molecular-weight complex(es) of seven aminoacyl-tRNA synthetases from sheep liver. Eur J Biochem. 1979 Sep;99(3):541–550. doi: 10.1111/j.1432-1033.1979.tb13286.x. [DOI] [PubMed] [Google Scholar]
- Livingston D. M. Immunoaffinity chromatography of proteins. Methods Enzymol. 1974;34:723–731. doi: 10.1016/s0076-6879(74)34094-3. [DOI] [PubMed] [Google Scholar]
- Rymo L., Lundvik L., Lagerkvist U. Subunit structure and binding properties of three amino acid transfer ribonucleic acid ligases. J Biol Chem. 1972 Jun 25;247(12):3888–3897. [PubMed] [Google Scholar]
- Saxholm H. J., Pitot H. C. Characterization of a proteolipid complex of aminoacyl-tRNA synthetases and transfer RNA from rat liver. Biochim Biophys Acta. 1979 May 24;562(3):386–399. doi: 10.1016/0005-2787(79)90103-5. [DOI] [PubMed] [Google Scholar]
- Som K., Hardesty B. Isolation and partial characterization of an aminoacyl-tRNA synthetase complex from rabbit reticulocytes. Arch Biochem Biophys. 1975 Feb;166(2):507–517. doi: 10.1016/0003-9861(75)90414-2. [DOI] [PubMed] [Google Scholar]
- Vadeboncoeur C., Lapointe J. Slow diffusion of glutamate and ATP-Mg into high-molecular-weight complexes containing the glutamyl-tRNA synthetase from bovine brain. Eur J Biochem. 1980 Aug;109(2):581–587. doi: 10.1111/j.1432-1033.1980.tb04831.x. [DOI] [PubMed] [Google Scholar]
- Van Dang C., Yang D. C. Disassembly and gross structure of particulate aminoacyl-tRNA synthetases from rat liver. Isolation and the structural relationship of synthetase complexes. J Biol Chem. 1979 Jun 25;254(12):5350–5356. [PubMed] [Google Scholar]
- Vennegoor C., Bloemendal H. Occurrence and particle character of aminoacyl-tRNA synthetases in the post-microsomal fraction from rat liver. Eur J Biochem. 1972 Apr 24;26(4):462–473. doi: 10.1111/j.1432-1033.1972.tb01788.x. [DOI] [PubMed] [Google Scholar]