Abstract
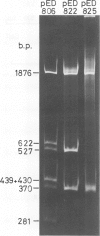
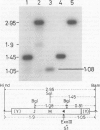
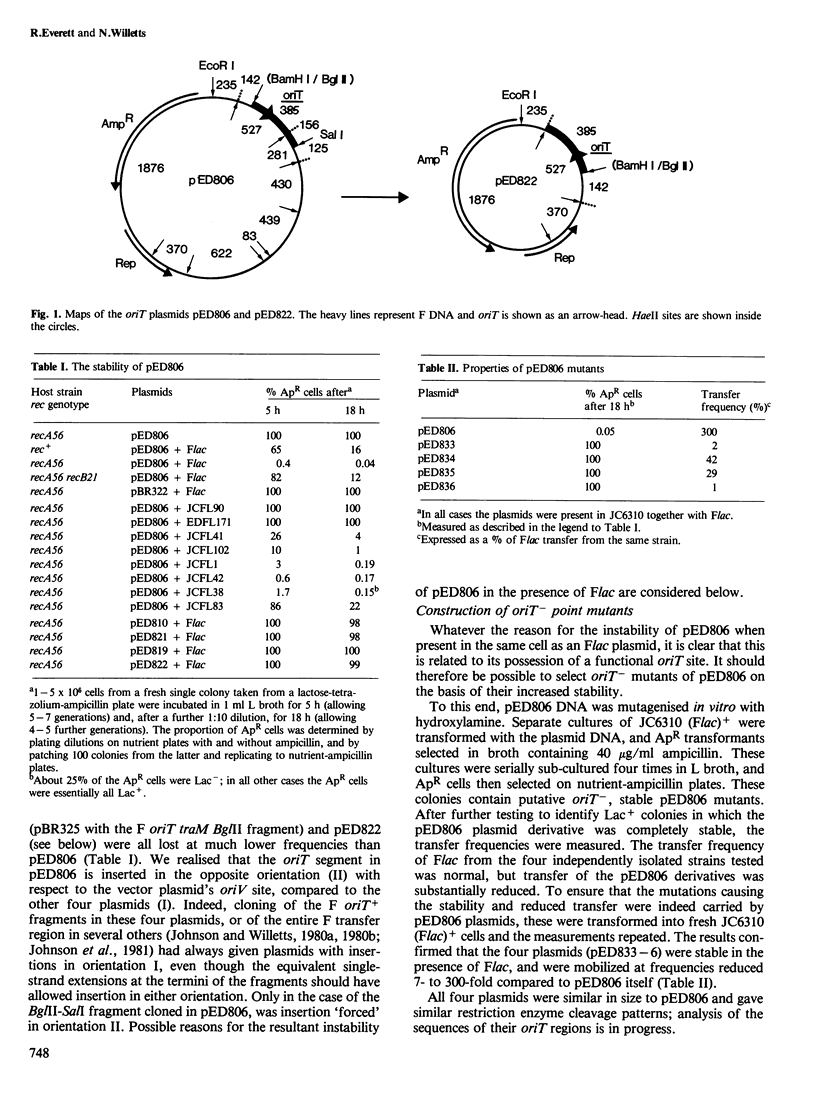
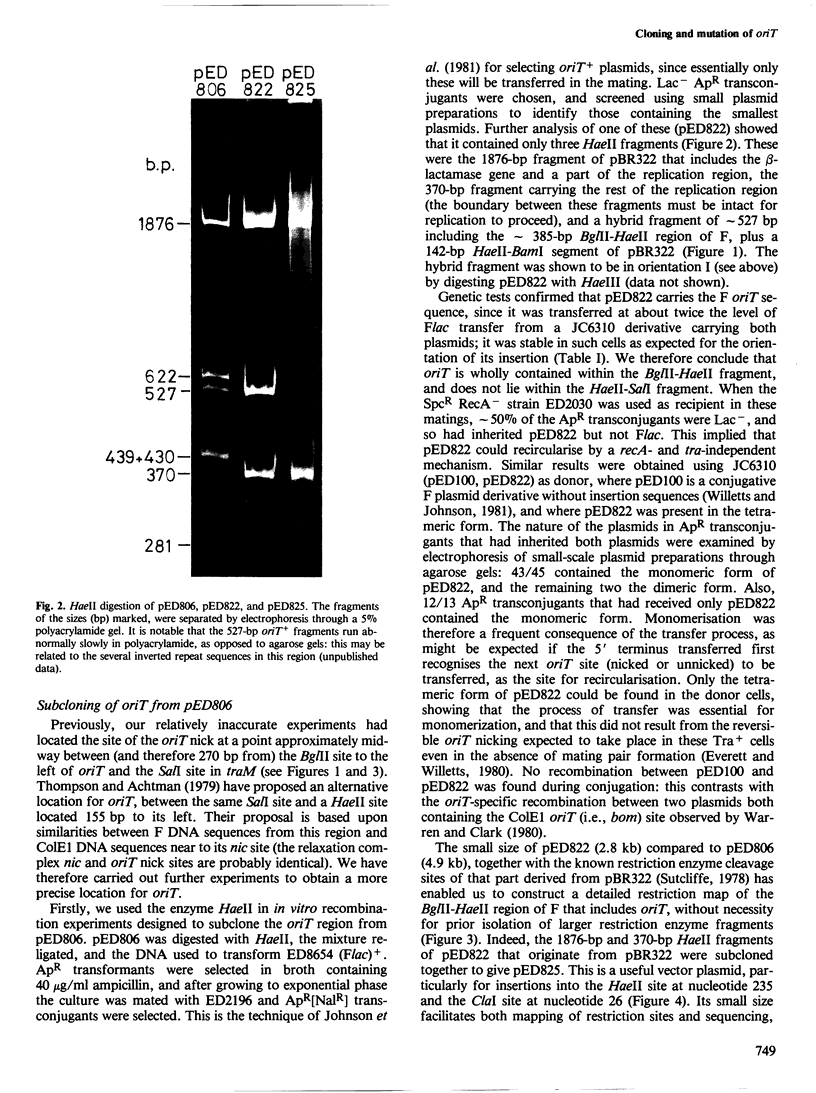
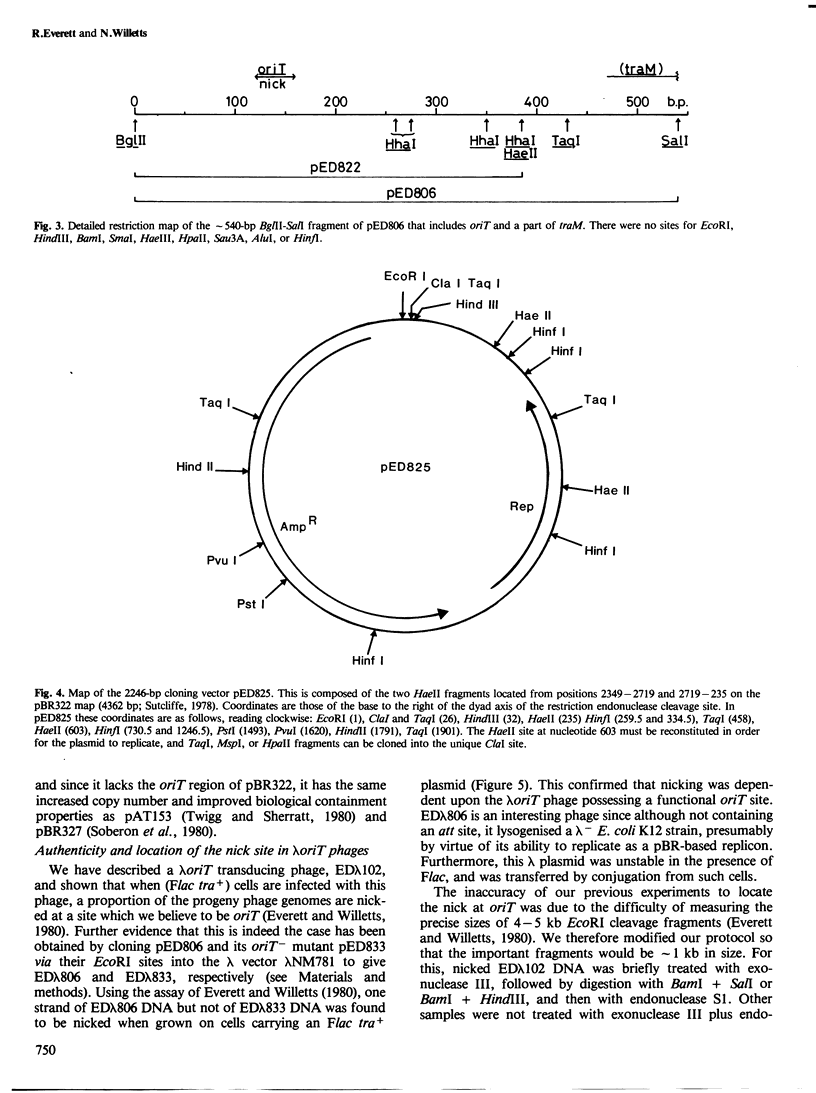
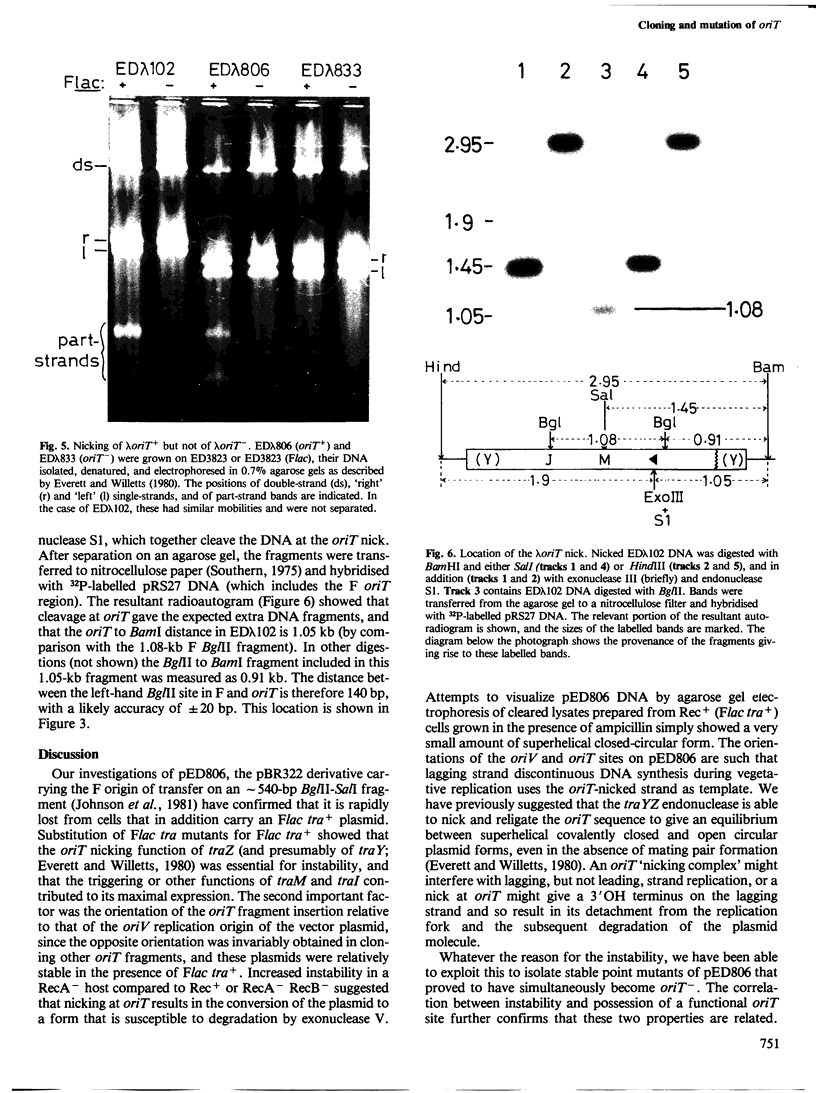
pED806 , a pBR322 derivative carrying the origin of transfer ( oriT ) of F, was rapidly lost from cells carrying an F tra+ plasmid. Instability was increased in a RecA- host, and depended in particular upon the Ftra YZ genes that produce the nick at oriT at which transfer is initiated. Instability was also correlated with the orientation of the oriT fragment in the vector plasmid. Mutants of pED806 selected as being stable in the presence of Flac proved to carry cis-dominant oriT mutations. The oriT site was subcloned from pED806 on a HaeII fragment including a HaeII-Bg/II segment of F DNA approximately 385 base pair (bp) long into the 2.25 kilobase (kb) vector plasmid pED825 , giving pED822 . pED822 was fully proficient for oriT function, and recircularised in recipient cells by a recA- and tra-independent oriT -specific ligation/recombination event. ' Phasmids ' constructed by cloning pED806 or an oriT - mutant into a lambda vector were used to confirm that the nick site in lambda oriT phages grown in the presence of Flac tra+ is indeed at oriT . The nick site in a further lambda oriT phage (ED lambda 102) was then located 140 +/- 20 bp from the Bg/II site forming one terminus of the F fragment cloned in pED806 and pED822 .
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achtman M., Skurray R. A., Thompson R., Helmuth R., Hall S., Beutin L., Clark A. J. Assignment of tra cistrons to EcoRI fragments of F sex factor DNA. J Bacteriol. 1978 Mar;133(3):1383–1392. doi: 10.1128/jb.133.3.1383-1392.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Beginning a genetic analysis of conjugational transfer determined by the F factor in Escherichia coli by isolation and characterization of transfer-deficient mutants. J Bacteriol. 1971 May;106(2):529–538. doi: 10.1128/jb.106.2.529-538.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Willetts N., Clark A. J. Conjugational complementation analysis of transfer-deficient mutants of Flac in Escherichia coli. J Bacteriol. 1972 Jun;110(3):831–842. doi: 10.1128/jb.110.3.831-842.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Brenner S., Cesareni G., Karn J. Phasmids: hybrids between ColE1 plasmids and E. coli bacteriophage lambda. Gene. 1982 Jan;17(1):27–44. doi: 10.1016/0378-1119(82)90098-1. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- Everett R., Willetts N. Characterisation of an in vivo system for nicking at the origin of conjugal DNA transfer of the sex factor F. J Mol Biol. 1980 Jan 15;136(2):129–150. doi: 10.1016/0022-2836(80)90309-5. [DOI] [PubMed] [Google Scholar]
- Finnegan D., Willetts N. The site of action of the F transfer inhibitor. Mol Gen Genet. 1973 Dec 31;127(4):307–316. doi: 10.1007/BF00267101. [DOI] [PubMed] [Google Scholar]
- Foster T. J., Willetts N. S. Genetic analysis of deletions of R100-1 that are both transfer-deficient and tetracycline-sensitive. J Gen Microbiol. 1976 Mar;93(1):133–140. doi: 10.1099/00221287-93-1-133. [DOI] [PubMed] [Google Scholar]
- Gasson M. J., Willetts N. S. Further characterization of the F fertility inhibition systems of "unusual" Fin+ plasmids. J Bacteriol. 1977 Aug;131(2):413–420. doi: 10.1128/jb.131.2.413-420.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer M. S., Davidson N., Clark A. J. Heteroduplex analysis of tra delta f' plasmids and the mechanism of their formation. J Bacteriol. 1977 Sep;131(3):970–980. doi: 10.1128/jb.131.3.970-980.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley R. G., Deonier R. C. Specificity in the formation of delta tra F-prime plasmids. J Bacteriol. 1980 Aug;143(2):680–692. doi: 10.1128/jb.143.2.680-692.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S., Saitoh T. F deoxyribonucleic acid transferred to recipient cells in the presence of rifampin. J Bacteriol. 1975 Mar;121(3):1000–1006. doi: 10.1128/jb.121.3.1000-1006.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Smith H. R., Anderson E. S. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol Gen Genet. 1976 Apr 23;145(1):101–108. doi: 10.1007/BF00331564. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Willetts N. S. Construction and characterization of multicopy plasmids containing the entire F transfer region. Plasmid. 1980 Nov;4(3):292–304. doi: 10.1016/0147-619x(80)90068-2. [DOI] [PubMed] [Google Scholar]
- Johnson D. A., Willetts N. S. Tn2301, a transposon construct carrying the entire transfer region of the F plasmid. J Bacteriol. 1980 Sep;143(3):1171–1178. doi: 10.1128/jb.143.3.1171-1178.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D., Everett R., Willetts N. Cloning of F DNA fragments carrying the origin of transfer oriT and the fertility inhibition gene finP. J Mol Biol. 1981 Dec 5;153(2):187–202. doi: 10.1016/0022-2836(81)90273-4. [DOI] [PubMed] [Google Scholar]
- Kennedy N., Beutin L., Achtman M., Skurray R., Rahmsdorf U., Herrlich P. Conjugation proteins encoded by the F sex factor. Nature. 1977 Dec 15;270(5638):580–585. doi: 10.1038/270580a0. [DOI] [PubMed] [Google Scholar]
- Kingsman A., Willetts N. The requirements for conjugal DNA synthesis in the donor strain during flac transfer. J Mol Biol. 1978 Jul 5;122(3):287–300. doi: 10.1016/0022-2836(78)90191-2. [DOI] [PubMed] [Google Scholar]
- McIntire S., Willetts N. Plasmid cointegrates of Flac and lambda prophage. J Bacteriol. 1978 Apr;134(1):184–192. doi: 10.1128/jb.134.1.184-192.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray N. E., Brammar W. J., Murray K. Lambdoid phages that simplify the recovery of in vitro recombinants. Mol Gen Genet. 1977 Jan 7;150(1):53–61. doi: 10.1007/BF02425325. [DOI] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R., Achtman M. The control region of the F sex factor DNA transfer cistrons: restriction mapping and DNA cloning. Mol Gen Genet. 1978 Oct 24;165(3):295–304. doi: 10.1007/BF00332530. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Warren G. J., Clark A. J. Sequence-specific recombination of plasmid ColE1. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6724–6728. doi: 10.1073/pnas.77.11.6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S., Crowther C., Holloway B. W. The insertion sequence IS21 of R68.45 and the molecular basis for mobilization of the bacterial chromosome. Plasmid. 1981 Jul;6(1):30–52. doi: 10.1016/0147-619x(81)90052-4. [DOI] [PubMed] [Google Scholar]
- Willetts N. S., Finnegan D. J. Characteristics of E. coli K12 strains carrying both an F prime and an R factor. Genet Res. 1970 Aug;16(1):113–122. doi: 10.1017/s0016672300002329. [DOI] [PubMed] [Google Scholar]
- Willetts N. S. Location of the origin of transfer of the sex factor F. J Bacteriol. 1972 Nov;112(2):773–778. doi: 10.1128/jb.112.2.773-778.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N. S. Recombination and the Escherichia coli K-12 sex factor F. J Bacteriol. 1975 Jan;121(1):36–43. doi: 10.1128/jb.121.1.36-43.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Johnson D. pED100, a conjugative F plasmid derivative without insertion sequences. Mol Gen Genet. 1981;182(3):520–522. doi: 10.1007/BF00293948. [DOI] [PubMed] [Google Scholar]
- Willetts N., Skurray R. The conjugation system of F-like plasmids. Annu Rev Genet. 1980;14:41–76. doi: 10.1146/annurev.ge.14.120180.000353. [DOI] [PubMed] [Google Scholar]
- Willetts N. The transcriptional control of fertility in F-like plasmids. J Mol Biol. 1977 May 5;112(1):141–148. doi: 10.1016/s0022-2836(77)80161-7. [DOI] [PubMed] [Google Scholar]