Abstract
Undernutrition during critical or sensitive prenatal periods may ‘program’ the fetus for increased chronic disease and mortality in later life. Using birth cohorts that were or were not exposed to severe food shortage in Utah in the mid-19th century, this study examines how in utero exposure to undernutrition is associated with mortality after age 50. The Utah Population Database is used to identify 1392 prenatally exposed individuals and 29,022 individuals from subsequent, unexposed birth cohorts. Gompertz hazards with parametric frailty show that males born between April and June of the famine period (and hence exposed during critical periods in utero during the winter months) have higher mortality risks compared with post-famine cohorts. Alternative Cox non-proportional hazard models suggest that females born during the same period have higher initial mortality risks (starting at age 50) that decline over time creating a mortality crossover with unexposed women at approximately age 70, a result not found for men. An ancillary sibling analysis that uses shared frailty survival models to compare individuals with prenatal exposure to undernutrition to their younger (post-famine) same-sex siblings finds no significant differences in adult mortality for males but the pattern for females support the findings from the previous analysis. Although findings are sensitive to model choice, this study presents evidence that is consistent with an association between undernutrition in utero and adult mortality, shows that effects may be sensitive to the duration and gestational period of exposure, and illustrates the differential exposure effects between genders.
Keywords: maternal undernutrition, longevity, early life conditions, selection
Introduction
Early life conditions have been shown to be significantly correlated with adult mortality for individuals and cohorts.1–4 Individuals exposed to adverse conditions in utero may have altered mortality trajectories due to altered development of key organ systems or epigenetic modifications during gestation. There continues to be debate over both the mechanisms and significance of early life conditions in determining later-life mortality.5–8
This study examines a cohort of individuals exposed to undernutrition in utero during a significant mid-19th century famine during the early settlement years of Utah to assess their long-term effects on mortality after age 50. Studying long-term health consequences of adverse prenatal and childhood events will generate new insights about adverse exposures in early life and whether they create delayed later-life mortality differentials.
The purpose of this study is to address two fundamental research questions.
Do later-life mortality risks differ between birth cohorts with in utero exposure to famine in Utah during the mid-19th century in relation to birth cohorts unexposed to famine and unexposed siblings?
Do later-life mortality effects associated with in utero famine exposure differ by gestational age and gender?
Utah’s early settlement and periods of famine
The first pioneers from The Church of Jesus Christ of Latter-day Saints settled in present day Utah in July of 1847. In 1855, a series of natural disasters created conditions leading to a widespread famine for the first settlers of Utah. Most significantly, swarms of grasshoppers had destroyed the previous year’s crops, leaving many settlers without adequate food in early 1855. Food supplies and agricultural productivity became dire as a hot dry summer and light runoff from the mountains caused a severe drought. That summer, grasshoppers again ‘devoured every living thing in sight’ leaving the pioneers with a depleted crop and no grass for cattle.9,10 According to the Daily National Intelligencer, ‘Mormons, in their colony far remote from the markets of the East and the West alike, may be reduced to a famine by the swarms of grasshoppers’.11 Most residents did not have sufficient grain to last the whole winter and many lacked enough grain to last just one month.12 An influx of immigrants in 1855 made a desperate situation worse.
The winter of 1855–1856 was the most severe winter experienced by these early settlers. In February of 1856, Heber C. Kimball, an official of the Church, wrote that there was ‘scarcely any grain in the country, and there are thousands that have none at all’ and that the food shortage was ‘universal through all the settlements’.12 The loss of an estimated 50% to 80% of the settlers’ cattle made the food shortage problem all the more catastrophic. Households were placed on a ration of one-half pound of breadstuff per day by the Church leadership, and any surplus was to be donated to individuals who were without any food.12 Journal excerpts detail ways in which mothers attempted to provide sustenance for their children. One mother tried to enhance her ‘meager supply of flour by mixing it with sawdust – a failure it turned out’.13 Individuals living near Utah Lake were able to supplement their diet with fish, but most settlers subsisted on a food supply so inadequate that they were driven to eat roots and thistles.10,12 Utilizing comprehensive genealogical and vital records for cohorts undernourished in utero in Utah and later unexposed cohorts creates an opportunity to study this period as a natural experiment in which adverse later life can be assessed.
Literature review
Several mechanisms linking adverse early life conditions, such as those described in pioneer Utah, to later-life mortality have been hypothesized. Two of the proposed mechanisms, intrauterine programming and mortality selection, are the most appropriate for explaining the effects of in utero exposure to undernutrition on adult mortality risks. Both hypotheses argue that intrauterine conditions can alter adult mortality trajectories, however, they make discrepant predictions.
The underlying hypothesis linking the prenatal nutritional environment to later-life health outcomes argues that there are critical periods of intrauterine development that when compromised by undernutrition, elevate later-life mortality. These changes can be mutagenic or adaptive;14–16 however, both responses may lead to higher adult mortality. Mutagenic changes can permanently impair genetic, cellular or whole organ functionality and lead to a shortened life span.14 Adaptive in utero changes are permanent changes to the physiology or structure of the fetus that confer an immediate survival advantage or a response that improves survival chances postnatally.14,15
The intrauterine response to an undernourished environment may also lead to lower adult mortality through mortality selection. Genetic heterogeneity in the population may lead to variation in the severity of the physiological outcomes and those at the highest risk, the frail, die first leaving a more robust population surviving to older ages.17–19 A mortality crossover can occur if mortality rates strike the frailest members of an exposed population with sufficient force that mortality rates for the surviving subset will be lower than those unexposed.
The combination of intrauterine programming and mortality selection may also lead to positive or negative mortality outcomes after age 50 depending on the timing, duration and severity of the undernutrition.14,20,21 Table 1 displays the hypothesized all-cause mortality outcome when both intrauterine programming and mortality selection are operating to alter the mortality trajectories after age 50. As previously discussed, when intrauterine programming and mortality selection have strong effects they predict opposing mortality outcomes. However, when they are operating simultaneously they may cancel each other out or lead to elevated mortality. Only the net effects of intrauterine programming and mortality selection are observable. However, the framework presented in Table 1 allows for better understanding of the various ways the intrauterine environment could shape adult mortality through the proposed mechanisms.
Table 1.
Hypothesized mortality outcomes for varying conditions of in utero deprivation
| Effects of mortality selection on mortality after age 50
|
||
| Effects of intrauterine programming on mortality after age 50 | Weak: nutritional depletion not severe enough to select out the weakest members of the population | Strong: nutritional depletion leads to physiological damage severe enough to select out the weakest members of the population |
|
| ||
| Weak: nutritional depletion leads to minor physiological changes | Timing and duration of the exposure do not lead to physiological differences between the exposed and unexposed. The surviving population at age 50 is a heterogeneous group with little or no physiological change Hypothesis: no mortality differences |
The exposure leads to selective attrition in utero and increased childhood and early adult mortality. The surviving population at age 50 is a robust group with little or no physiological change Hypothesis: lower mortality |
| Strong: nutritional depletion leads to physiological changes that alter the risk for mortality | The fetus adapts to an undernourished environment or is scarred by the exposure. The surviving population is a heterogeneous group with adaptive or mutagenic changes that decreases survival after age 50 Hypothesis: excess mortality |
The in utero exposure to undernutrition causes the frail to die and leads to adaptation or scarring that alters mortality for the remaining cohort. This leads to a population of robust survivors with adaptive or mutagenic changes that decrease survival after age 50 Hypothesis: null or excess mortality |
Sex differences may exist in the effects of early life conditions on later-life health. Male fetuses have higher mortality rates than female fetuses, a disadvantage that continues throughout the life course.22,23 This susceptibility may cause gender differences in both fetal programming and mortality selection. Lindeboom’s study of the Dutch Potato Famine of 1846–1847 found a significant adverse effect for exposure to undernutrition in utero for males, but not for females.24 However, van Abeelen et al.25 found increased mortality for females exposed to undernutrition during the 1944–1945 Dutch Famine in early gestation, but not for males. Differences in the strength, duration and timing of the famines may lead to variation in gender-specific findings.
The physiological changes associated with exposure to undernutrition in utero may be sensitive to the timing and duration of the insult. Different fetal organs grow at different rates, making the timing of the insult an important aspect to take into account. Lack of proper nutrition during the period around conception may adversely affect placental development.23 A recent study found that females exposed to famine in the first trimester had increased risk of adult mortality, cardiovascular mortality and cancer mortality compared with the unexposed. However, the adverse effects of early gestational exposure to undernutrition were not observed for men.25 Exposure to undernutrition in the second trimester may lead to decreased renal function in adulthood and an increased risk for obstructive airway disease.26,27 Late-gestational exposure has been linked to impaired glucose tolerance.28
Undernutrition in utero has not been definitively linked to differences in adult mortality. For example, some studies of the 1866–1868 Finnish Famine, the 1944–1945 Dutch Famine and the 1959–1961 Great Leap Forward Famine in China found no later-life mortality differences in adult mortality between cohorts exposed to famine and non-exposed cohorts.29–32 However, studies of the Great Leap Forward Famine and the Dutch Famine were only able to observe mortality up to ages 31 and 57, respectively.29,31 The null findings may also be the result of net effects of fetal programming combined with mortality selection, which may cancel each other out.
The estimated effects of undernutrition in utero on adult mortality using historical cohorts may not be independent of other early life exposures during critical periods. The inflammation hypothesis argues that exposures to infectious disease during infancy and childhood result in altered morbidity and mortality trajectories in adulthood. Competing theories have suggested that the direction of the association can be positive or negative. The ‘cohort morbidity phenotype’ hypothesis predicts a positive association between exposure to infectious agents and morbidity and mortality related to chronic conditions in old age.33,34 McDade et al.35 have proposed a second hypothesis predicting a negative relationship between exposure to infectious disease during infancy and childhood and inflammation in adulthood, arguing that exposure to infectious diseases are necessary for healthy development of the immune system. The infectious agents plaguing a population, such as influenza or pneumonia, are normally virulent during specific months or seasons and this, along with exposure to undernutrition in utero, may possibly contribute to observed variations in longevity by month of birth.34,36,37
In this analysis, we focus on these two proposed mechanisms linking early life environmental exposures to later-life health: intrauterine programming and mortality selection. On the basis of these mechanisms, exposure to undernutrition in utero is hypothesized to increase (if the intrauterine programming mechanisms are operating), decrease (if mortality selection is operating) or lead to no observable differences (if neither or both mechanisms are operating) in adult mortality. To test the theory that effects are sensitive to timing and duration of exposure we estimate the effects by birth quarter. In the northern hemisphere, individuals born between the months of October and December may confer a survival advantage36,37 and these observed effects remain after controlling for early shared environments and common genetic backgrounds.37 To rule out effects based on month of birth, possibly due to seasonal differences in nutrition or infection, we test for significant differences in mortality by quarter of birth for exposed to undernutrition in utero and the subsequent cohorts. Research has demonstrated sex differences in developmental programming in utero, separate analyses are conducted for males and females.
Methods
Data
This study relies on data drawn from the Utah Population Database (UPDB). The UPDB is a comprehensive health research database containing linked demographic, medical and genealogical data spanning the Utah population from the last two centuries.38 Although the data held within UPDB are extensive, given the historic period under investigation, prenatal and birth information was based on genealogical records. UPDB was used to identify 2069 individuals born in Utah between September 1855 and December 1856, all of whom are defined to have been exposed to undernutrition in utero.
Two control groups were identified with which to compare to the exposed subjects: a population-based group and a design based on siblings. For the population-based cohort analysis, three birth cohorts, all born during post-famine years, were selected as controls: birth cohorts 1857–1861, 1862–1866 and 1867–1871. This design yielded 2069 exposed to famine in utero and 46,147 post-famine controls. Six exposed and 41 post-famine individuals were removed from the study because of poor data quality. To keep the focus on adult mortality, individuals were further required to survive to at least age 50, thereby excluding 671 and 17,084 exposed and control individuals, respectively. The final sample comprised 715 and 677 exposed men and women, respectively, and 15,132 and 13,890 control men and women, respectively.
Using a separate strategy that controls for unobserved heterogeneity within sibships, exposed individuals were compared with their younger same-sex siblings. Individuals without a younger same-sex sibling were excluded from this analysis, decreasing the exposed sample size by ~32% for the men and 34% for the women. For men, the final sibling sample included 1537 individuals, including 484 exposed males and 1053 younger brothers. The final sibling sample size for women was 1411 individuals, with 444 exposed and 967 younger sisters.
Measures
The dependent variable, the all-cause mortality hazard rate, was measured starting at age 50. The sample comprises an extinct cohort with exact age at death known for ~99.8% of the sample. Individuals with an unknown death age were censored at their last known living date. Conditioning on survival to age 50, the average age at death for males was 73.05 and 75.12 for females. The key independent variable was exposure to undernutrition in utero. The period of the famine spanned from September 1855 to June 1856.10,12,13 Individuals were identified as exposed in utero if their birth occurred between September 1855 and December 1856. Separating this exposure period into quarters allowed for the test of differences between duration of exposure and trimesters exposed. Three post-famine birth cohorts were used as control populations. Birth year was grouped into 5-year periods, 1857–1861, 1862–1866 and 1867–1871, with the 1857–1861 cohort serving as the referent category. For the sibling analysis, the birth year of the younger sibling was used as a control variable.
A series of covariates were introduced to control for other factors associated with both mortality and the key exposure. Birth order has been shown to have life-course consequences for health and survival.39 A dummy variable was used to specify whether a person was first-born or not. Exact birth order was not used because it is highly correlated with the number of siblings. The death of a parent during childhood may have adverse effects later in life.40,41 Parental death during childhood was defined as a death of a parent prior to their 10th birthday. Parental death dates were not available for ~9% of men and women. A separate indicator variable was introduced to adjust for differences between individuals with and without known parental death dates. Active followers of the Church are more likely to abstain from alcohol and tobacco as well as participate in church activities and have been shown to have survival benefits.42 The UPDB contains information on baptism and endowment dates from family history records and this was used to classify individuals as active followers, inactive, or unaffiliated with the Church. Individuals with an endowment date have agreed to live their lives following the doctrine of the Church and were considered active church followers if endowed before age 40. Individuals with a baptism but no endowment date were considered inactive.
Marital status may be partly linked to health selection into marriage and may affect later mortality risk. If true, the never-married would be a frailer population;43,44 accordingly marital status is included as a dummy variable that specifies whether a person was ever married by age 50. An individual’s fertility has been shown to be a strong predictor of mortality,45 however the results are mixed. Smith et al.40 found that women with higher parity have an increased post-menopausal mortality risk; however, Gavrilov and Gavrilova46 showed evidence of a positive association between the number of children and longevity. A dummy variable was created to indicate whether the person had more than the average number of children for the sample (seven children was average for parous women). Individuals with no children were identified with a dummy variable. Only a portion of the individuals with no children are truly nulliparous (as distinct from lacking a fertility history in UPDB) and it is impossible to identify those individuals using information currently available. Women with no children are a mixture of nulliparous and individuals without complete fertility information in the UPDB. Socioeconomic status (SES) in adulthood is also inversely associated with mortality outcomes. Utah-issued death certificates from 1904 forward are linked to the UPDB, the source of information for SES. Usual occupation and industry information from death certificates have been converted to Nam-Powers socioeconomic index scores (NP SES).47 Scores range from 1 to 99, with higher values indicating higher SES. Women were assigned the SES reported on their first spouse’s death certificate or their own SES if they were not married. A large percentage of individuals have the occupation of ‘farmer’, resulting in a large heaping at the NP SES score of 40. Farming may also confer a survival advantage related to lifestyle factors.46 A separate category was created for the occupation of farmer to control for the occupational effects of being a farmer. Approximately 39% of the men and 50% of the women did not have an NP SES score based on death certificates. Missing SES was mean-imputed and an indicator variable was used to identify individuals with imputed SES.
All sibling models control for sibling birth year, marital status and fertility. Early childhood covariates were not added to the study because they would be highly correlated (if not identical) between siblings. To control for possible differences due to cohort effects, all models controlled for the siblings’ birth year centered on the mean.
Statistical methods
A set of alternative survival models were estimated to select the best fitting model specification for males and females beginning at age 50. Models considered included Cox Proportional Hazard (Cox PH) models, Cox non-Proportional Hazard models (Cox NPH), piecewise exponential models, Weibull and Gompertz models with and without frailty corrections. Final models were selected on the basis of the lowest Akaike Information Criterion (AIC). Cox NPH models were the best fitting models for females and Gompertz hazard models with gamma-distributed individual frailty were the best fitting models for males (all estimated models are available by request from the authors).
Cox NPH models were used to model all-cause mortality after age 50 for females. The Cox NPH model is an extension of the Cox PH model, but allows for non-proportional effects of covariates by interacting them with time. This model allows for testing for the presence of differential mortality effects of prenatal exposure to famine with time.
Gompertz hazards model with gamma-distributed individual frailty was the best fitting model for the males and was used to model all-cause mortality between the ages of 50 and 85. The Gompertz model is appropriate for modeling human mortality between the ages of 30 and 85.48 As such, individuals living past age 85 were censored at 85 for this model. Frailty is included in the Gompertz model as an individual risk factor and the measure of population heterogeneity, the theta parameter, was used to assess variability about the baseline risk.
The final model, a Cox Proportional Hazard Model that allows for gamma-distributed shared frailty, was used for the sibling models.49 This model differs from individual frailty effects model because it accounts for variation in risk of death, such as shared environment and genetic effects shared among relatives. A random effect that reflects sibship-specific frailty is therefore introduced into the model.
Results
Individuals exposed to undernutrition in utero were grouped by year and quarter of birth. A separate group was created for individuals with an unknown birth month (n = 7). This yielded six groups of individuals born between September of 1855 and December of 1856 and exposed to undernutrition in utero. The estimated time of conception and trimester of exposure to undernutrition in utero by birth quarter and sex is displayed in Table 2. Individuals born in 1855 would have been exposed to undernutrition in utero during their third trimester, as well being undernourished during a large portion of their first year of life. Individuals born during the last quarter of 1856 would have been exposed to undernutrition in utero during their first trimester and presumably none during their first year of life.
Table 2.
Number of individuals exposed to undernutrition in utero by birth quarter and gender
| Birth year | Birth month | Estimated time of conception | Trimester of exposure | Number of females surviving to age 50 | Number of males surviving to age 50 |
|---|---|---|---|---|---|
| 1855 | Sep–Dec | December 1854 to March 1855 | 3rd | 185 | 176 |
| Jan–Mar | April 1855 to June 1855 | 2nd and 3rd | 144 | 147 | |
| Apr–Jun | July 1855 to September 1855 | 1st, 2nd and 3rd | 94 | 108 | |
| 1856 | Jul–Sep | October 1855 to December 1855 | 1st, 2nd | 109 | 118 |
| Oct–Dec | January 1856 to March 1856 | 1st | 141 | 163 | |
| Unknown birth month | 4 | 3 | |||
| Total | 677 | 715 |
Table 3 shows descriptive statistics for the sample. Compared with individuals from the combined period of 1857 to 1871, exposed males had a 2% and females a 1% reduction in average years lived after age 50. Individuals in the cohorts exposed to famine are relatively similar on other measures to the post-famine cohorts. There are, however, differences between groups by fertility. Both men and women exposed to undernutrition in utero have more children than the post-famine cohorts. On average, the difference in the number of children is 1.3 and 1.5 for men and women, respectively. Both men and women in the post-famine cohorts have a slightly higher proportion of individuals who are nulliparous or lacking information on fertility. When these differences are accounted for, the differences in the number of children are slightly attenuated, but still remain.
Table 3.
Descriptive statistics by gender and exposure to undernutrition
| Exposed to famine in utero (winter of 1855–1856)
|
Post-famine birth (1857–1871)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Obs | Mean | S.D. | Min | Max | Obs | Mean | S.D. | Min | Max |
| Male | ||||||||||
| Birth year | 715 | 1855.75 | 0.43 | 1855 | 1856 | 15,132 | 1864.94 | 4.24 | 1857 | 1871 |
| Inactive church participation (= 1) | 715 | 0.16 | 0.37 | 0 | 1 | 15,132 | 0.22 | 0.42 | 0 | 1 |
| Active church participation (= 1) | 715 | 0.61 | 0.49 | 0 | 1 | 15,132 | 0.53 | 0.50 | 0 | 1 |
| Nam-Powers 1990 Scorea | 441 | 44.95 | 15.38 | 6 | 99 | 9354 | 45.33 | 16.84 | 3 | 99 |
| Missing SESa (= 1) | 715 | 0.38 | 0.49 | 0 | 1 | 15,132 | 0.38 | 0.49 | 0 | 1 |
| Farmera (= 1) | 715 | 0.38 | 0.49 | 0 | 1 | 15,132 | 0.35 | 0.48 | 0 | 1 |
| Married before age 50 (= 1) | 715 | 0.93 | 0.25 | 0 | 1 | 15,132 | 0.94 | 0.24 | 0 | 1 |
| Incomplete fertility or no children (= 1) | 715 | 0.22 | 0.42 | 0 | 1 | 15,132 | 0.24 | 0.43 | 0 | 1 |
| Number of children (= 1) | 715 | 6.90 | 4.93 | 0 | 35 | 15,132 | 5.68 | 4.53 | 0 | 30 |
| Death age | 711 | 72.70 | 10.54 | 50 | 99 | 15,099 | 73.07 | 10.61 | 50 | 104 |
| Missing either parent’s death age (= 1) | 715 | 0.09 | 0.28 | 0 | 1 | 15,132 | 0.09 | 0.24 | 0 | 1 |
| Mother died when child age 10 and under (= 1) | 715 | 0.06 | 0.24 | 0 | 1 | 15,132 | 0.06 | 0.24 | 0 | 1 |
| Father died when child age 10 and under (= 1) | 715 | 0.07 | 0.25 | 0 | 1 | 15,132 | 0.05 | 0.22 | 0 | 1 |
| Female | ||||||||||
| Birth year | 677 | 1855.73 | 0.45 | 1855 | 1856 | 13,890 | 1864.85 | 4.23 | 1857 | 1871 |
| Inactive church participation (= 1) | 677 | 0.13 | 0.33 | 0 | 1 | 13,890 | 0.20 | 0.40 | 0 | 1 |
| Active church participation (= 1) | 677 | 0.67 | 0.47 | 0 | 1 | 13,890 | 0.59 | 0.49 | 0 | 1 |
| Nam-Powers 1990 Scorea | 322 | 46.44 | 16.17 | 6 | 99 | 6914 | 46.08 | 16.50 | 2 | 99 |
| Missing SESa (= 1) | 677 | 0.54 | 0.50 | 0 | 1 | 13,890 | 0.51 | 0.50 | 0 | 1 |
| Farmera (= 1) | 677 | 0.29 | 0.45 | 0 | 1 | 13,890 | 0.29 | 0.45 | 0 | 1 |
| Married before age 50 (= 1) | 677 | 0.98 | 0.14 | 0 | 1 | 13,890 | 0.96 | 0.18 | 0 | 1 |
| Incomplete fertility or no children | 677 | 0.19 | 0.39 | 0 | 1 | 13,890 | 0.23 | 0.42 | 0 | 1 |
| Number of children | 677 | 7.22 | 4.65 | 0 | 18 | 13,890 | 5.74 | 4.31 | 0 | 21 |
| Death age | 677 | 74.84 | 11.15 | 50 | 102 | 13,875 | 75.13 | 11.14 | 50 | 108 |
| Missing either parent’s death age (= 1) | 677 | 0.10 | 0.29 | 0 | 1 | 13,890 | 0.09 | 0.28 | 0 | 1 |
| Mother died when child age 10 and under | 677 | 0.03 | 0.17 | 0 | 1 | 13,890 | 0.06 | 0.24 | 0 | 1 |
| Father died when child age 10 and under | 677 | 0.07 | 0.25 | 0 | 1 | 13,890 | 0.06 | 0.23 | 0 | 1 |
SES, socioeconomic status.
Source: Death certificate or spouse death certificate for married female.
Figure 1 shows the average age of death by birth quarter for individuals surviving to age 50 for men (panel a) and women (panel b), respectively. An additional point was added to the x-axis for the individuals in utero during the famine period so that individuals born during the last quarters of 1855 and 1856 could be displayed separately. Males exposed to undernutrition during all three trimesters (i.e., birth month April to June) and surviving to age 50 had the lowest average age of death (71.6) and live 1.6 years less than males born between April and June in the 1857–1871 post-famine cohorts (73.2). Females born between September and December of 1855 (the beginning of the famine period) and surviving to age 50 had the lowest average death age (74.1), living 1.2 years less than those born during the same months of 1857–1871 post-famine cohorts (75.3).
Fig. 1.
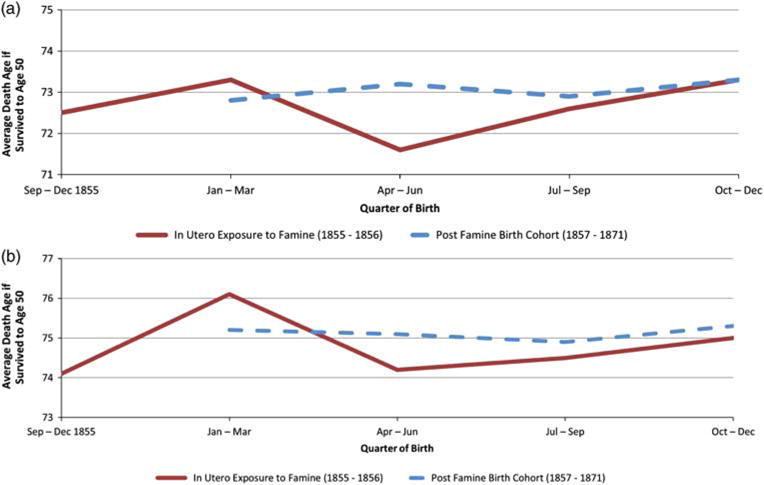
Average age of death for individuals born in Utah between 1855 and 1871 and surviving to age 50 stratified by exposure to undernutrition in utero. Panel (a) – Male and Panel (b) – Female.
Table 4 shows the results for the female Cox NPH models. Model 1 shows that for females born during the second quarter of 1856, the main effect is insignificant [hazard ratio (HR) = 1.35, 95% CI = 0.86, 2.14] and positively associated with mortality, but the time interaction is significantly and negatively associated with mortality (HR = 0.99, 95% CI = 0.97, 1.00). These results suggest that women born between April and June of 1856 experience higher rates of mortality at younger ages (past age 50) compared with women born in the post-famine cohort of 1857–1861 (though not significant), and this effect significantly decreases with age past 50. For women exposed in the second quarter of 1856, the mortality decrease over time results in a mortality crossover at approximately age 70. For the remaining exposure periods, both main effects and time interactions were insignificant.
Table 4.
Female all-cause mortality Cox non-proportional hazard rate ratios: exposed v. population-based controls.
| Model 1
|
Model 2
|
|||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Born 1856, unknown birth month | ± | ± | ||
| Born Sep–Dec 1855 | 1.146 | 0.82, 1.61 | 1.138 | 0.81, 1.6 |
| Born Jan–Mar 1856 | 0.782 | 0.51, 1.19 | 0.78 | 0.51, 1.19 |
| Born Apr–Jun 1856 | 1.352 | 0.86, 2.14 | 1.347 | 0.85, 2.13 |
| Born Jul–Sep 1856 | 0.823 | 0.51, 1.33 | 0.822 | 0.51, 1.33 |
| Born Oct–Dec 1856 | 0.844 | 0.55, 1.3 | 0.838 | 0.54, 1.29 |
| Born 1857–1861 (ref) | 1.00 | 1.00 | ||
| Born 1862–1866 | 0.932*** | 0.89, 0.97 | 0.934*** | 0.89, 0.98 |
| Born 1867–1871 | 0.911*** | 0.87, 0.95 | 0.914*** | 0.88, 0.95 |
| Mother died when child was age 10 and under | 1.021 | 0.95, 1.1 | ||
| Father died when child was age 10 and under | 1.079** | 1, 1.16 | ||
| Missing information on child’s age at either parent’s death | 1.008 | 0.95, 1.07 | ||
| Nam-Powers Score (Source: Spouse or Ego Death Certificate, Unit = 10) | 0.973 | 0.96, 0.99 | ||
| Nam-Powers Score missing (= 1) | 1.031 | 0.98, 1.08 | ||
| Spouse is a farmer (= 1) | 0.996 | 0.94, 1.05 | ||
| First-born child (= 1) | 1.072** | 1.03, 1.12 | ||
| No LDS church participation (ref) | 1.00 | |||
| Inactive LDS church participation | 0.965 | 0.91, 1.02 | ||
| Active LDS Church participation | 0.935** | 0.89, 0.98 | ||
| Married before age 50 (= 1) | 0.982 | 0.9, 1.08 | ||
| 1–6 children (ref) | 1.00 | |||
| 7 or more children | 1.028 | 0.99, 1.07 | ||
| Nulliparous | 1.017 | 0.97, 1.07 | ||
| Non-proportional effects (interaction with time) | ||||
| Born Sep–Dec 1855 | 0.995 | 0.98, 1.01 | 0.996 | 0.98, 1.01 |
| Born Jan–Mar 1856 | 1.005 | 0.99, 1.02 | 1.005 | 0.99, 1.02 |
| Born Apr–Jun 1856 | 0.985* | 0.97, 1.00 | 0.985* | 0.97, 1.00 |
| Born Jul–Sep 1856 | 1.007 | 0.99, 1.03 | 1.008 | 0.99, 1.03 |
| Born Oct–Dec 1856 | 1.007 | 0.99, 1.02 | 1.007 | 0.99, 1.02 |
| n | 14,567 | 14,567 | ||
| Likelihood ratio χ2 (df) | 31.99 (13) | 78.83 (25) | ||
| AIC | 251,191.4 | 251,168.6 | ||
HR, hazard ratio; CI, confidence interval; LDS, Latter-Day Saint; AIC, Akaike information criterion.
Significant differences for this group exist; however, the sample size is small (n = 4) and the estimates are not reliable.
Exponentiated coefficients; 95% CIs in second column.
P < 0.1,
P < 0.05,
P < 0.001.
Model 2 controls for additional covariates known to affect mortality. The results show that a father’s death during childhood and being the first-born child significantly increases the mortality hazard rate. Increasing spouse SES and church participation reduce the rate of mortality. For women born during the second quarter of 1856, the main effect remained positively associated with mortality (HR = 1.35, 95% CI = 0.85, 2.13) and insignificant, and the time interaction was negatively associated with mortality (HR = 0.99, 95% CI = 0.97, 1.00), thus suggesting that women born between April and June of 1856 experience higher rates of mortality after age 50 compared with women born in the post-famine cohort of 1857–1861 (though not significant), and this effect decreases with age past 50.
The Gompertz hazards model with individual-level gamma-distributed frailty was estimated for men. The outcome of interest for these Gompertz models was mortality between the ages of 50 and 85 and the results are displayed in Table 5. Model 1 shows that males born during the second quarter of 1856 had an elevated risk of mortality that was marginally significant (HR = 1.24, 95% CI = 1.00, 1.53) compared with those in the post-famine cohort of 1857–1861 when including frailty. Other factors contributing to survival differences are added to Model 2. The death of mother before age 10, missing information on child’s age at time of parent’s death and having no reported children in the database all increase the hazard rate. The significant protective effects include individual adult SES, being a farmer, active participation in the Church and ever been married. The magnitude of the effect of being born during the second quarter of 1856 is not reduced when measures of childhood and adult conditions are added to the model (HR = 1.24, 95% CI = 0.99, 1.55), however the statistical significance is. The theta parameter of 0.17 (P < 0.001) indicates that there is heterogeneity in the population, suggesting a population with a hazard that is ~51% higher or lower than the baseline risk (exp(0.171/2) − 1 = 0.510).
Table 5.
Male all-cause Gompertz with gamma-distributed individual-level frailty mortality hazard rate ratios: exposed v. population-based controls
| Model 1
|
Model 2
|
|||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Born 1856, unknown birth month | ± | ± | ||
| Born Sep–Dec 1855 | 1.055 | 0.89, 1.25 | 1.097 | 0.92, 1.31 |
| Born Jan–Mar 1856 | 1.019 | 0.85, 1.23 | 1.01 | 0.83, 1.23 |
| Born Apr–Jun 1856 | 1.239** | 1, 1.53 | 1.241* | 0.99, 1.55 |
| Born Jul–Sep 1856 | 1.034 | 0.84, 1.27 | 1.043 | 0.84, 1.30 |
| Born Oct–Dec 1856 | 0.966 | 0.81, 1.15 | 0.99 | 0.82, 1.20 |
| Born 1857–1861 (ref) | 1.00 | 1.00 | ||
| Born 1862–1866 | 1.001 | 0.96, 1.05 | 0.983 | 0.93, 1.03 |
| Born 1867–1871 | 1.001 | 0.96, 1.05 | 0.975 | 0.93, 1.02 |
| Mother died when child was age 10 and under | 1.069* | 0.99, 1.16 | ||
| Father died when child was age 10 and under | 1.059 | 0.97, 1.15 | ||
| Missing information on child’s age at either parent’s death | 1.072* | 1.00, 1.15 | ||
| Nam-Powers Score (Source: Death Certificate, Unit = 10) | 0.957*** | 0.94, 0.97 | ||
| Nam-Powers Score missing (= 1) | 0.852*** | 0.81, 0.9 | ||
| Farmer (= 1) | 0.823*** | 0.78, 0.87 | ||
| First-born child (= 1) | 1.019 | 0.97, 1.07 | ||
| No LDS church participation (ref) | 1.00 | |||
| Inactive church participation | 1.033 | 0.98, 1.09 | ||
| Active church participation | 0.821*** | 0.78, 0.87 | ||
| Married before age 50 (= 1) | 0.834*** | 0.77, 0.91 | ||
| 1–6 children (ref) | 1.00 | |||
| 7 or more children | 0.975 | 0.93, 1.02 | ||
| Nulliparous | 1.101*** | 1.04, 1.17 | ||
| Gamma | 0.0881*** | 0.083, 0.094 | 0.0950*** | 0.09, 0.1 |
| In theta | −2.819*** | −4.39, −1.25 | −1.799*** | −2.33, −1.27 |
| Theta (P-value) | 0.06 (0.105) | 0.17 (0.001) | ||
| n | 15,847 | 15,847 | ||
| Likelihood ratio χ2 (df) | 5.97 (8) | 377.40 (20) | ||
| AIC | 30,300.01 | 29,952.79 | ||
HR, hazard ratio; CI, confidence interval; LDS, Latter-Day Saint; AIC, Akaike information criterion.
Estimates for this group have been suppressed because the sample size is small (n = 3) and estimates are not reliable.
Exponentiated coefficients; 95% CIs in second column.
P < 0.1,
P < 0.05,
P < 0.001.
Studies have shown that birth month affects longevity with second quarter births having a 0.19 year shorter lifespan compared with fourth quarter births.3 To rule out the possibility that observed outcome for individuals born in the second quarter of 1856 are related to birth month or exposure to infectious disease during infancy rather than timing of in utero exposure to undernutrition, two separate additional analyses (results not shown) for each gender included quarter of and the interaction between exposed and birth quarter. The magnitude and significance of the effect of being born in the second quarter of 1856 for men and women were similar to the results presented in Tables 4 and 5, which suggests that our findings are not related to month of birth effects.
Finally, Cox NPH and Cox PH models with shared frailty effects were estimated for individuals with at least one younger same-sex sibling that survived to age 50. This design is potentially very powerful because it allows for the control of unobserved shared environment and genetic effects within sibships. Cox NPH results are not displayed for males because the time interactions are insignificant. For females born during the second quarter of 1856, Table 6 shows that the main effect of prenatal exposure to undernutrition is negatively related with survival (HR = 1.43, 95% CI = 0.79, 2.59) and the time interaction is significant and positively related to survival (HR = 0.98, 95% CI = 0.96, 1.00). This excess mortality risk compared with younger sisters diminishes through time and is also observed in the population cohort models; however, the results are marginally significant in both models. There was no significant difference in survival between males born in the second quarter of 1856 and their younger brothers (HR = 1.14, 95% CI = 0.87, 1.49). The shared frailty results show that there are significant shared frailty effects for men (theta = 0.07), but not for women (theta = 0.02). This suggests that there are shared familial components that affect the frailty of an individual, with the hazard varying by ~30% from the baseline hazard for groups of brothers. However, controlling for shared frailty does not change the null finding.
Table 6.
Female and male all-cause mortality hazard rate ratios: exposed to undernutrition v. younger same-sex siblings
| Female
|
Male
|
|||
|---|---|---|---|---|
| HR | 95% CI | HR | 95% CI | |
| Born Sep–Dec 1855 | 1.298 | 0.81, 2.07 | 1.048 | 0.83, 1.32 |
| Born Jan–Mar 1856 | 0.851 | 0.49, 1.48 | 0.983 | 0.78, 1.24 |
| Born Apr–Jun 1856 | 1.431 | 0.79, 2.6 | 1.138 | 0.87, 1.49 |
| Born Jul–Sep 1856 | 0.867 | 0.46, 1.62 | 1.075 | 0.83, 1.39 |
| Born Oct–Dec 1856 | 0.765 | 0.42, 1.4 | 1.069 | 0.85, 1.34 |
| Younger same-sex siblings (ref) | 1.00 | 1.00 | ||
| Birth year | 0.995 | 0.98, 1.01 | 1.002 | 0.99, 1.01 |
| Nam-Powers Score (Source: Death Certificate, Unit = 10) | 0.988 | 0.94, 1.04 | 0.956* | 0.91, 1.00 |
| Nam-Powers Score missing (= 1) | 1.106 | 0.95, 1.28 | 0.890 | 0.77, 1.03 |
| Farmer (= 1) | 1.083 | 0.91, 1.28 | 0.914 | 0.79, 1.06 |
| Married before age 50 (= 1) | 0.839** | 0.71, 0.99 | 0.882* | 0.77, 1.01 |
| 1–6 children (ref) | 1.00 | 1.00 | ||
| 7 or more children | 0.998 | 0.87, 1.14 | 1.075 | 0.94, 1.23 |
| Nulliparous | 0.932 | 0.79, 1.1 | 1.15* | 0.98, 1.35 |
| No LDS church participation (ref) | 1.00 | 1.00 | ||
| Active church participation | 0.840** | 0.72, 0.98 | 0.898 | 0.77, 1.04 |
| Inactive church participation | 0.929 | 0.77, 1.12 | 1.154* | 0.98, 1.36 |
| Non-proportional effects (interaction with time) | ||||
| Born Sep–Dec 1855 | 0.99 | 0.97, 1.01 | ||
| Born Jan–Mar 1856 | 1.005 | 0.99, 1.02 | ||
| Born Apr–Jun 1856 | 0.981* | 0.96, 1.00 | ||
| Born Jul–Sep 1856 | 1.005 | 0.98, 1.03 | ||
| Born Oct–Dec 1856 | 1.014 | 0.99, 1.04 | ||
| Theta | 0.02 | 0.067*** | ||
| n | 1411 | 1537 | ||
| Likelihood ratio χ2 (df) | 22.78 (19) | 30.86 (14) | ||
HR, hazard ratio; CI, confidence interval; LDS, Latter-Day Saint.
Exponentiated coefficients; 95% CIs in second column.
P < 0.1,
P < 0.05,
P < 0.001.
Discussion
We found evidence supporting both the intrauterine programming and mortality selection hypotheses and our results suggest that these effects differ by gender, duration or timing of exposure and model selection. We find that men born during the months of April and June of the famine period had higher mortality rates than individuals born in the post-famine periods, consistent with the intrauterine programming hypothesis. The results for females born between the months of April and June were suggestive of a declined mortality risk over time in relation to females born in the post-famine periods and their younger sisters after age 70, supporting the mortality selection hypothesis. Our results suggest that both strong intrauterine programming and selection mechanisms may affect adult mortality, with effects differing by gender. Overall, these results support a mounting body of literature showing the association between early life conditions and adult mortality.
Studies of the 1944–1945 Dutch Famine and the 1959–1961 Great Leap Forward Famine did not find evidence of mortality differentials between famine and post-famine cohorts.29–31 However, these studies used cohorts exposed to famine during the mid-20th century and did not observe mortality past the age of 57. Our study used a distinct set of cohorts to show that there are differences in mortality for men and suggest differences for women starting at age 50. Mortality differentials existed for individuals exposed to undernutrition during all three trimesters, with perhaps the harshest conditions during the end of their first trimester. Prenatal exposure to undernutrition during these trimesters has been associated with reduced glucose tolerance and raised insulin concentrations, decreased renal function, increased risk for obstructive airway disease, slower rates of fetal growth and decreased life expectancy.29,50
The timing and duration of the prenatal exposure to undernutrition should be considered. Shorter lifespans for individuals exposed to undernutrition in utero during the months of April and June of the famine period may be the result of several factors. Individuals born during these months have the longest exposure to nutrient depletion. This is consistent with the birth month literature showing that individuals born during these months have shorter life expectancies when compared with individuals born during the months of October to December.3,37 Individuals born during April and June may have also experienced ‘catch-up growth’ due to an improved nutritional environment during the summer months. Animal models have shown that compensatory growth may confer immediate benefits at the cost of a reduced life span.51 The effect of exposure to infectious diseases should also be considered when interpreting these results.33–35 Individuals born before May 1856 would have also been exposed to both undernutrition and infectious diseases during the first few months of life. However, the lack of mortality differences between those born from September 1855 to March 1856 and individuals in the post-famine cohort suggests that this study’s findings were not the result of increased exposure to infectious disease. Other studies have also suggested that there may be an increase in infectious diseases during periods of famine.52 We do not have historical evidence of this occurrence; however, if these findings were driven by the role of infectious disease one would expect to find similar adverse results across all affected birth quarters, which we do not. More research is needed to assess how the timing and duration of the prenatal exposure to under-nutrition affect later-life mortality.
Seemingly contrary to other findings of sex differences in the effect of undernutrition in utero and adult mortality,24,25,53 we find that both sexes experience altered mortality trajectories, but they are not equivalent. Disparate sex-specific findings of the effects of undernutrition in utero may be due to variation in the severity of famines. It is estimated that mortality during the Dutch Potato Famine of 1846–1847 increased by 0.5% compared with an almost doubling of mortality rates during the Dutch Famine of 1944–1945. A reanalysis of the Dutch Potato Famine of 1846–1847 found that men exposed to undernutrition in utero were a more heterogeneous than the unexposed cohorts and there was little difference between female cohorts.53 This finding suggests that for this famine exposure there were strong effects of intrauterine programming and weak effects of mortality selection for males and little difference for females. In contrast, the mortality during the Dutch Famine of 1944–1945 almost doubled. It is possible that this famine led to a situation where high levels of intrauterine programming and mortality selection created a null effect or possibly elevated mortality for the males and high levels of intrauterine programming combined with weak mortality selection led to excess mortality for the females. The suboptimal intrauterine environment in our study may have led to a situation of high mortality selection and intrauterine programming for the male population while only increasing mortality for the frailer sub-population of females.
The sibling models further support the mortality selection argument, providing stronger evidence that the mortality crossover is not merely a data artifact. The insignificant findings for the males when compared with their younger brothers may be the result of relying on a smaller, more select sample. The sibling sample is a select subset of individuals. For the exposed individuals to be included in the sample, the mother must have been robust and young enough to have gone on to have at least one more child of the same sex as the exposed child and both must have survived to age 50. As such, this sample may be a more homogeneous, robust group of survivors making it difficult to detect survival differences between siblings.
Several other findings of this study require further explanation. The descriptive statistics show that both men and women exposed to undernutrition in utero have higher fertility than the post-famine cohorts. This may be partially explained by the transition in fertility from high to low during these women’s childbearing years.54 These individuals also survived the initial shock of undernutrition and lived to age 50, and therefore may be a more robust, fecund group of individuals. Missing SES information is positively associated with survival. Utah death certificates were used to assign SES information to individuals in the study. Individuals that migrated out of the state and have death certificates issued elsewhere are missing SES information. Although speculative, the protective effect of missing SES information is consistent with the ‘Healthy Migrant’ hypothesis, which argues that migrants are more likely to be a select group of healthy individuals.55 We also found that marriage before age 50 was beneficial for men but not women and this sex-specific finding is consistent with other studies considering the effects of marital status on mortality.56,57
Some limitations to this study are worth noting. This study may be limited by our definition of the famine period. Although there is ample literature describing the period, it is impossible to know the exact start and end dates of the food shortages and pinpoint the most severe exposure times and exact locations. Conception time is estimated by birth date and we have introduced some error by assuming full-term births; however, it is reasonable to assume that on average the dates assigned are reasonable. Requiring individuals to survive to age 50 has potential to bias the results, although this would result in a conservative bias.
The strengths of this study include the reliance on a large sample of individuals exposed to famine, the ability to compare individuals exposed to undernutrition in utero to their younger siblings to net out genetic and childhood environmental effects, ability to control for several known confounders at different stages of the life course, and nearly complete information on death dates. This is the first study to test the effects of undernutrition in utero on adult mortality in a North American population. We also utilize a range of statistical models to control for individual and shared frailty and allow for the effects to vary with time.
This study supports mounting evidence of the adverse long-term effects of undernutrition in utero as well selective forces of mortality and suggests that the effects may differ by gender and the timing and duration of exposure. Future research should be aimed at determining how the timing and severity of nutritional shock to the fetus may alter adult mortality trajectories. Public health policies aimed at ensuring a nutrient rich diet for pregnant women during the entirety of the pregnancy are essential to diminishing health disparities.
Acknowledgments
This work was supported by NIA grant ‘Early Life Conditions, Survival, and Health’ (R01AG022095, Smith PI). The authors thank the Huntsman Cancer Foundation for database support provided to the Pedigree and Population Resource of the Huntsman Cancer Institute, University of Utah. They also thank Alison Fraser and Diana Lane Reed at the Pedigree and Population Resource of the Huntsman Cancer Institute for valuable assistance in managing and providing the data.
References
- 1.Barker D. Fetal origins of coronary heart disease. BMJ. 1995;311:171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eriksson J, Forsén T, Tuomilehto J, et al. Early growth and coronary heart disease in later life: longitudinal study. BMJ. 2001;322:949–953. doi: 10.1136/bmj.322.7292.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proc Natl Acad Sci U S A. 2001;98:2934–2939. doi: 10.1073/pnas.041431898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abel EL, Kruger ML. Birth month affects longevity. Death Studies. 2010;34:757–763. doi: 10.1080/07481181003772499. [DOI] [PubMed] [Google Scholar]
- 5.Barbi E, Vaupel JW. Comment on “inflammatory exposure and historical changes in human life-spans”. Science. 2005;308:1743a. doi: 10.1126/science.1108707. [DOI] [PubMed] [Google Scholar]
- 6.Bengtsson T, Lindstrom M. Childhood misery and disease in later life: the effects on mortality in old age of hazards experienced in early life, southern Sweden, 1760–1894. Popul Stud (Camb) 2000;54:263–277. doi: 10.1080/713779096. [DOI] [PubMed] [Google Scholar]
- 7.Bengtsson T, Mineau GP. Early-life effects on socio-economic performance and mortality in later life: a full life-course approach using contemporary and historical sources. Soc Sci Med. 2009;68:1561–1564. doi: 10.1016/j.socscimed.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Gagnon A, Mazan R. Does exposure to infectious diseases in infancy affect old-age mortality? Evidence from a pre-industrial population. Soc Sci Med. 2009;68:1609–1616. doi: 10.1016/j.socscimed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Arrington LJ. Great Basin Kingdom: An Economic History of the Latter-Day Saints, 1830–1900. University of Illinois Press; Urbana and Chicago, United States of America: 2004. [Google Scholar]
- 10.Whitney OF. Popular History of Utah. Vol. 1. The Deseret News; Salt Lake City, Utah: 1916. [Google Scholar]
- 11.Daily National Intelligencer. Gales & Seaton; Washington, DC: Aug 11, 1855. A Prospect of Famine at Salt Lake; p. 3. Print. [Google Scholar]
- 12.Arrington LJ. Great Basin Kingdom. Harvard University Press; Cambridge: 1958. p. 534. [Google Scholar]
- 13.Walker RW, Dant DR. Nearly Everything Imaginable: The Everyday Life of Utah’s Mormon Pioneers. Brigham Young University Press; Provo, Utah: 1999. [Google Scholar]
- 14.Fowden AL, Giussani DA, Forhead AJ. Intrauterine programming of physiological systems: causes and consequences. Physiology. 2006;21:29–37. doi: 10.1152/physiol.00050.2005. [DOI] [PubMed] [Google Scholar]
- 15.Gluckman P, Hanson M, Buklijas T. A conceptual framework for the developmental origins of health and disease. J Dev Orig Health Dis. 2010;1:6–18. doi: 10.1017/S2040174409990171. [DOI] [PubMed] [Google Scholar]
- 16.Barker DJP, Gluckman PD, Godfrey KM, et al. Fetal nutrition and cardiovascular disease in adult life. Lancet. 1993;341:938–941. doi: 10.1016/0140-6736(93)91224-a. [DOI] [PubMed] [Google Scholar]
- 17.Elo IT, Preston SH. Effects of early-life conditions on adult mortality: a review. Popul Index. 1992;58:186–212. [PubMed] [Google Scholar]
- 18.Preston SH, Hill ME, Drevenstedt GL. Childhood conditions that predict survival to advanced ages among African–Americans. Soc Sci Med. 1998;47:1231–1246. doi: 10.1016/s0277-9536(98)00180-4. [DOI] [PubMed] [Google Scholar]
- 19.Hawkes K, Smith KR, Blevins JK. Human actuarial aging increases faster when background death rates are lower: a consequence of differential heterogeneity? Evolution. 2012;66:103–114. doi: 10.1111/j.1558-5646.2011.01414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gluckman PD, Hanson M. The Fetal Matrix: Evolution, Development, and Disease. Cambridge University Press; Cambridge, United Kingdom: 2005. [Google Scholar]
- 21.Mcmillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 22.Kraemer S. Clinical Medicine NetPrints. 2000. The fragile male; p. 1. [Google Scholar]
- 23.Barker DJP, Thornburg KL, Osmond C, et al. Beyond birthweight: the maternal and placental origins of chronic disease. J Dev Orig Health Dis. 2010;1:360–364. doi: 10.1017/S2040174410000280. [DOI] [PubMed] [Google Scholar]
- 24.Lindeboom M, Portrait F, van den Berg GJ. Long-run effects on longevity of a nutritional shock early in life: the Dutch Potato famine of 1846–1847. J Health Econ. 2010;95:617–629. doi: 10.1016/j.jhealeco.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 25.van Abeelen AF, Veenendaal MVE, Painter RC, et al. Survival effects of prenatal famine exposure. Am J Clin Nutr. 2012;95:179–183. doi: 10.3945/ajcn.111.022038. [DOI] [PubMed] [Google Scholar]
- 26.Lopuhaa CE, Roseboom TJ, Osmond C, et al. Atopy, lung function, and obstructive airways disease after prenatal exposure to famine. Thorax. 2000;55:555–561. doi: 10.1136/thorax.55.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82:485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Ravelli ACJ, van der Meulen JHP, Michels RPJ, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/s0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- 29.Painter RC, Roseboom TJ, Bossuyt PMM, et al. Adult mortality at age 57 after prenatal exposure to the Dutch famine. Eur J Epidemiol. 2005;20:673–676. doi: 10.1007/s10654-005-7921-0. [DOI] [PubMed] [Google Scholar]
- 30.Roseboom TJ, van der Meulen JHP, Osmond C, et al. Adult survival after prenatal exposure to the Dutch famine 1944–45. Paediatr Perinat Epidemiol. 2001;15:220–225. doi: 10.1046/j.1365-3016.2001.00336.x. [DOI] [PubMed] [Google Scholar]
- 31.Song S. Does famine have a long-term effect on cohort mortality? Evidence from the 1959-1961 great leap forward famine in China. J Biosoc Sci. 2009;41:469–491. doi: 10.1017/S0021932009003332. [DOI] [PubMed] [Google Scholar]
- 32.Kannisto V, Christensen K, Vaupel JW. No increased mortality in later life for cohorts born during famine. Am J Epidemiol. 1997;145:987–994. doi: 10.1093/oxfordjournals.aje.a009067. [DOI] [PubMed] [Google Scholar]
- 33.Crimmins EM, Finch CE. Infection, inflammation, height, and longevity. Proc Natl Acad Sci U S A. 2006;103:498–503. doi: 10.1073/pnas.0501470103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Finch CE, Crimmins EM. Inflammatory exposure and historical changes in human life-spans. Science. 2004;305:1736–1739. doi: 10.1126/science.1092556. [DOI] [PubMed] [Google Scholar]
- 35.McDade TW, Rutherford J, Adair L, et al. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proc R Soc B Biol Sci. 2010;277:1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Doblhammer G, Vaupel JW. Lifespan depends on month of birth. Proc Natl Acad Sci. 2001;98:2934–2939. doi: 10.1073/pnas.041431898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gavrilov LA, Gavrilova NS. Season of birth and exceptional longevity: comparative study of American centenarians, their siblings, and spouses. J Aging Res. 2011:11. doi: 10.4061/2011/104616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bean LL, Mineau GP, Anderton DL. Fertility Change on the American Frontier: Adaptation and Innovation. University of California Press; Berkley and Los Angeles, California: 1990. [Google Scholar]
- 39.Modin B. Birth order and mortality: a life-long follow-up of 14,200 boys and girls born in early 20th century Sweden. Soc Sci Med. 2002;54:1051–1064. doi: 10.1016/s0277-9536(01)00080-6. [DOI] [PubMed] [Google Scholar]
- 40.Smith KR, Mineau GP, Garibotti G, et al. Effects of childhood and middle-adulthood family conditions on later-life mortality: evidence from the Utah Population Database, 1850–2002. Soc Sci Med. 2009;68:1649–1658. doi: 10.1016/j.socscimed.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Poppel F. Children in one-parent families: survival as an indicator of the role of the parents. J Fam Hist. 2000;25:269. [Google Scholar]
- 42.Mineau GP, Smith KR, Bean LL. Historical trends of survival among widows and widowers. Soc Sci Med. 2002;54:245–254. doi: 10.1016/s0277-9536(01)00024-7. [DOI] [PubMed] [Google Scholar]
- 43.Goldman N. Marriage selection and mortality patterns: inferences and fallacies. Demography. 1993;30:189–208. [PubMed] [Google Scholar]
- 44.Goldman N, Hu Y. Excess mortality among the unmarried: a case study of Japan. Soc Sci Med. 1993;36:533–546. doi: 10.1016/0277-9536(93)90414-y. [DOI] [PubMed] [Google Scholar]
- 45.Smith KR, Mineau GP, Bean LL. Fertility and post-reproductive longevity. Biodemography Soc Biol. 2002;49:185–205. [PubMed] [Google Scholar]
- 46.Gavrilov LA, Gavrilova NS. Biodemography of exceptional longevity: early-life and mid-life predictors of human longevity. Biodemography Soc Biol. 2012;58:14–39. doi: 10.1080/19485565.2012.666121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nam CB, Powers MG. The Socioeconomic Approach to Status Measurement: With a guide to Occupational and Socioeconomic Status Scores. Cap and Gown Press; Houston: 1983. [Google Scholar]
- 48.Olshansky SJ, Carnes BA. Ever since Gompertz. Demography. 1997;34:1–15. [PubMed] [Google Scholar]
- 49.Therneau TM, Grambsch PM, Pankratz VS. Penalized survival models and frailty. J Comput Graph Stat. 2003;12:156–175. [Google Scholar]
- 50.Roseboom TJ, van der Meulen JHP, Osmond C, et al. Coronary heart disease after prenatal exposure to the Dutch famine, 1944–45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- 52.Gagnon A, Mazan R. Does exposure to infectious diseases in infancy affect old-age mortality? Evidence from a pre-industrial population. Soc Sci Med. 2009;68:1609–1616. doi: 10.1016/j.socscimed.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Doblhammer-Reiter G, Van den Berg GJ, Lumey L. Long-term effects of famine on life expectancy: a re-analysis of the great Finnish famine of 1866–1868. IZA Discussion Papers. 2011 doi: 10.1080/00324728.2013.809140. 5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skolnick M, Bean L, May D, et al. Mormon demographic history. I. Nuptiality and fertility of once-married couples. Popul Stud. 1978;32:5–19. [PubMed] [Google Scholar]
- 55.Jasso G, Massey DS, Rosenzweig MR, Smith JP. Immigrant health: selectivity and acculturation. In: Anderson NB, Bulatao RA, Cohen B, editors. Critical Perspectives on Racial and Ethnic Differences in Health in Late Life. National Academies Press; Washington, DC: 2004. pp. 227–266. [PubMed] [Google Scholar]
- 56.Berkman LF, Syme SL. Social networks, host resistance, and mortality: a nine-year follow-up study of Alameda county residents. Am J Epidemiol. 1979;109:186–204. doi: 10.1093/oxfordjournals.aje.a112674. [DOI] [PubMed] [Google Scholar]
- 57.Hu Y, Goldman N. Mortality differentials by marital status: an international comparison. Demography. 1990;27:233–250. [PubMed] [Google Scholar]


