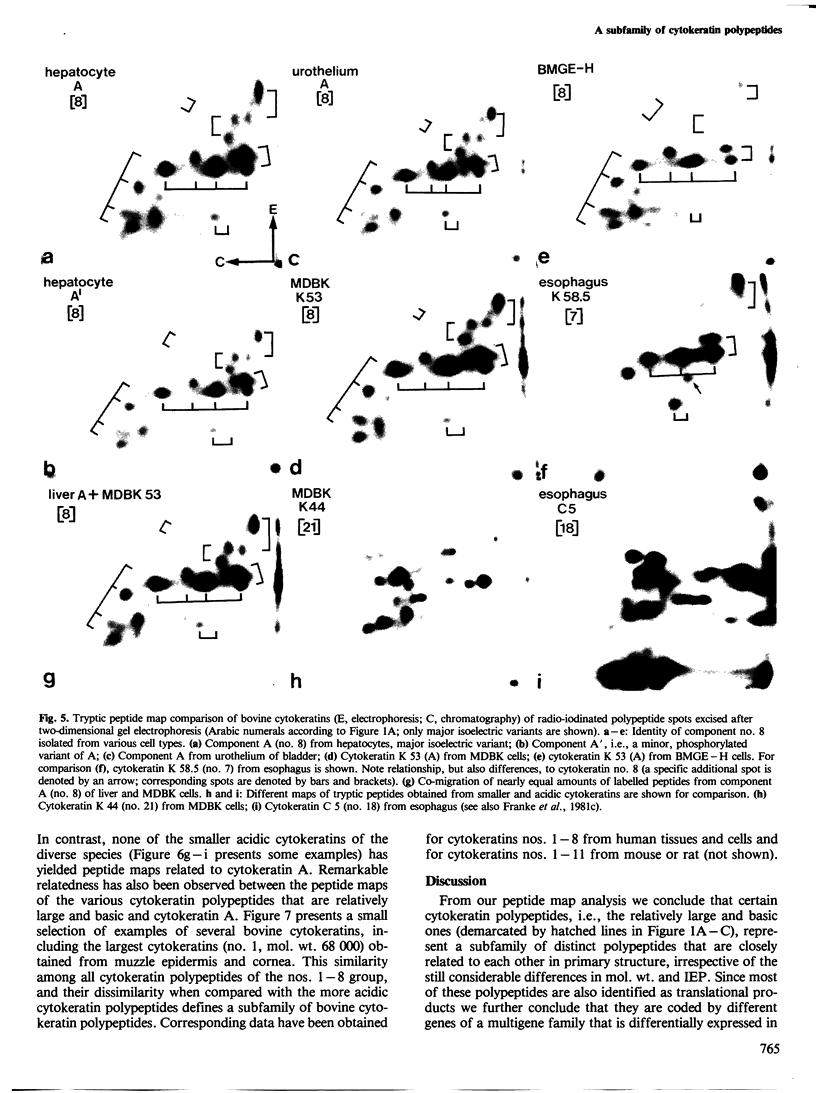
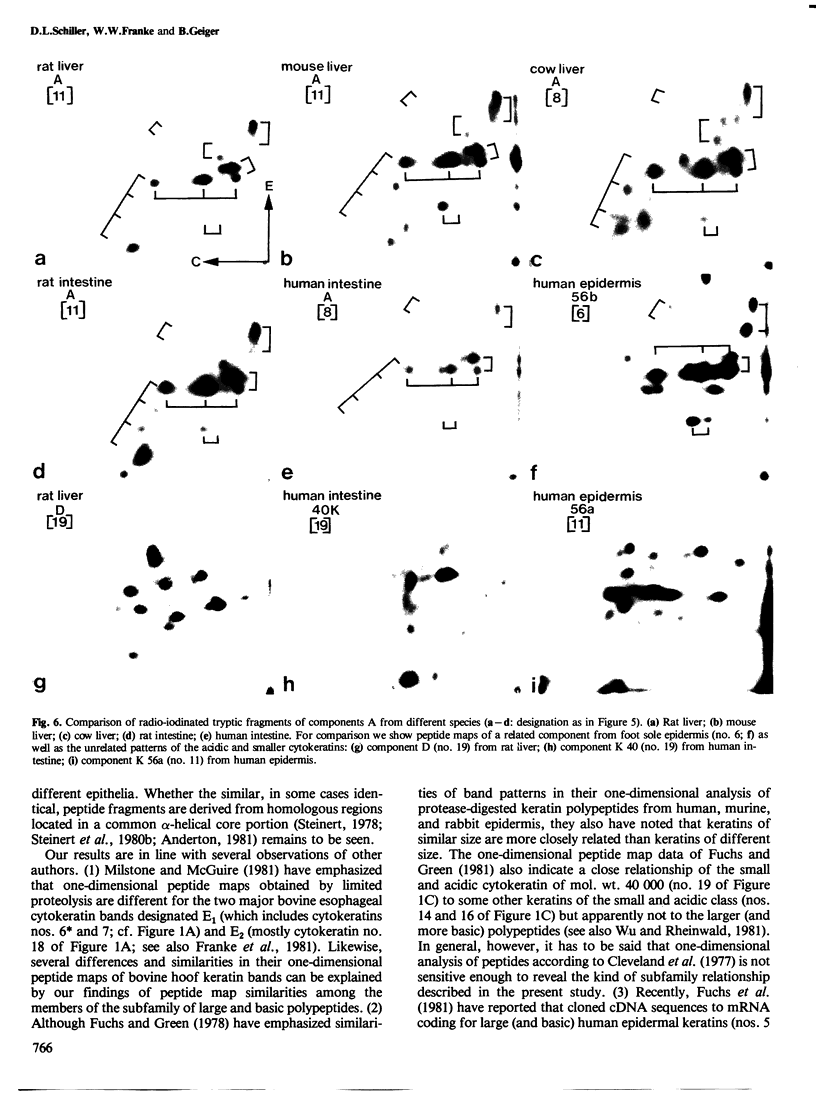
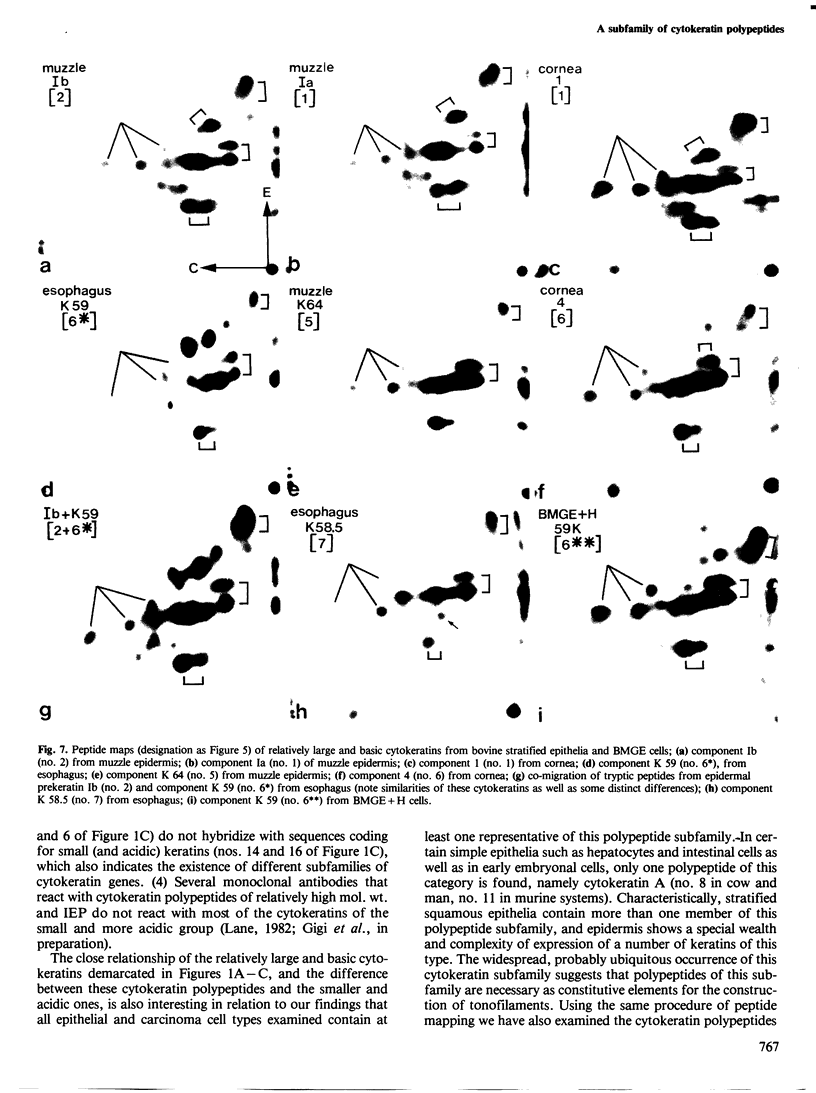
Abstract
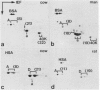
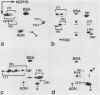
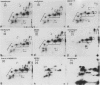
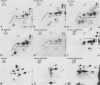
Epithelial cells contain a class of intermediate-sized filaments formed by proteins related to epidermal alpha-keratins ('cytokeratins'). Different epithelia can express different combinations of cytokeratin polypeptides widely varying in apparent mol. wt. (40 000-68 000) and isoelectric pH (5.0-8.5). We have separated, by two-dimensional gel electrophoresis, cytokeratin polypeptides from various tissues and cultured cells of man, cow, and rodents and examined their relatedness by tryptic peptide mapping. By this method, a subfamily of closely related cytokeratin polypeptides has been identified which comprises the relatively large (greater than or equal to mol. wt. 52 500 in human cells) and basic (pH greater than or equal to 6.0) polypeptides but not the smaller and acidic cytokeratins. In all species examined, the smallest polypeptide of this subfamily is cytokeratin A, which is widespread in many simple epithelia and is the first cytokeratin expressed during embryogenesis. This cytokeratin polypeptide subfamily is represented by at least one member in all epithelial and carcinoma cells examined, indicating that polypeptides of this subfamily serve an important role as tonofilament constitutents . Diverse stratified epithelia and tumours derived therefrom contain two or more polypeptides of this subfamily, and the patterns of expression in different cell types suggest that some polypeptides of this subfamily are specific for certain routes of epithelial differentiation.
Full text
PDF
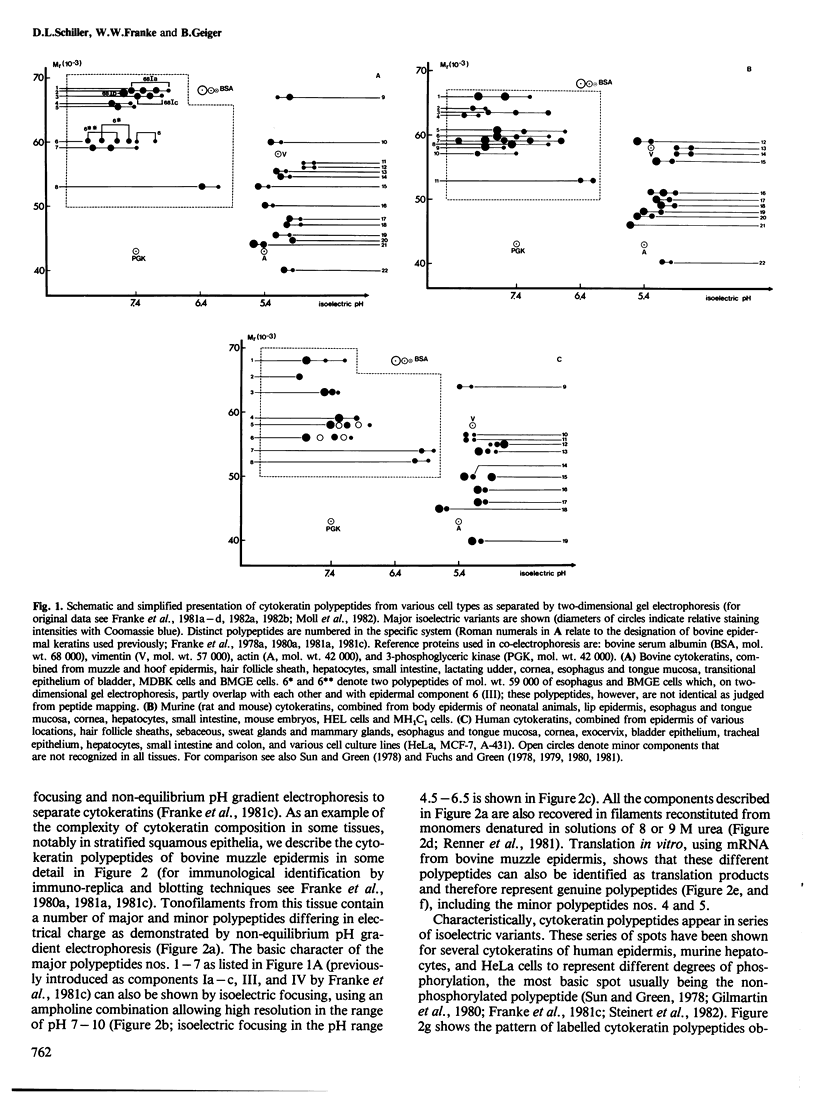
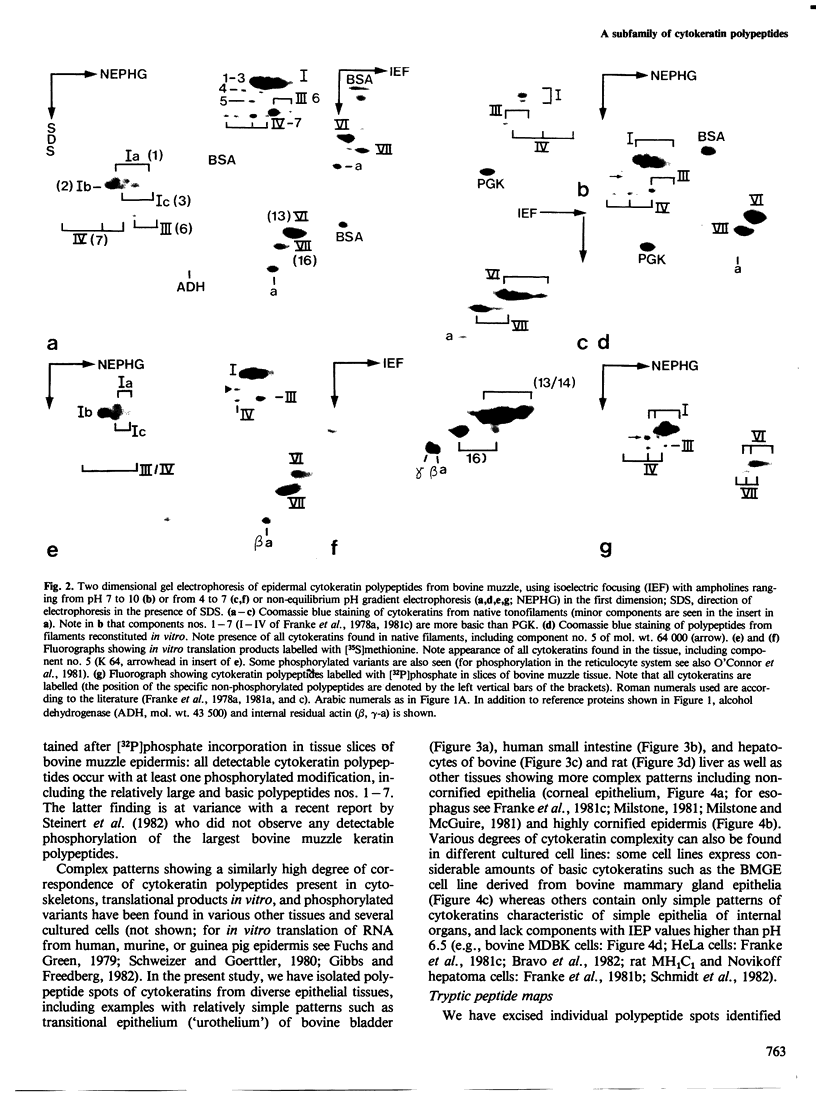
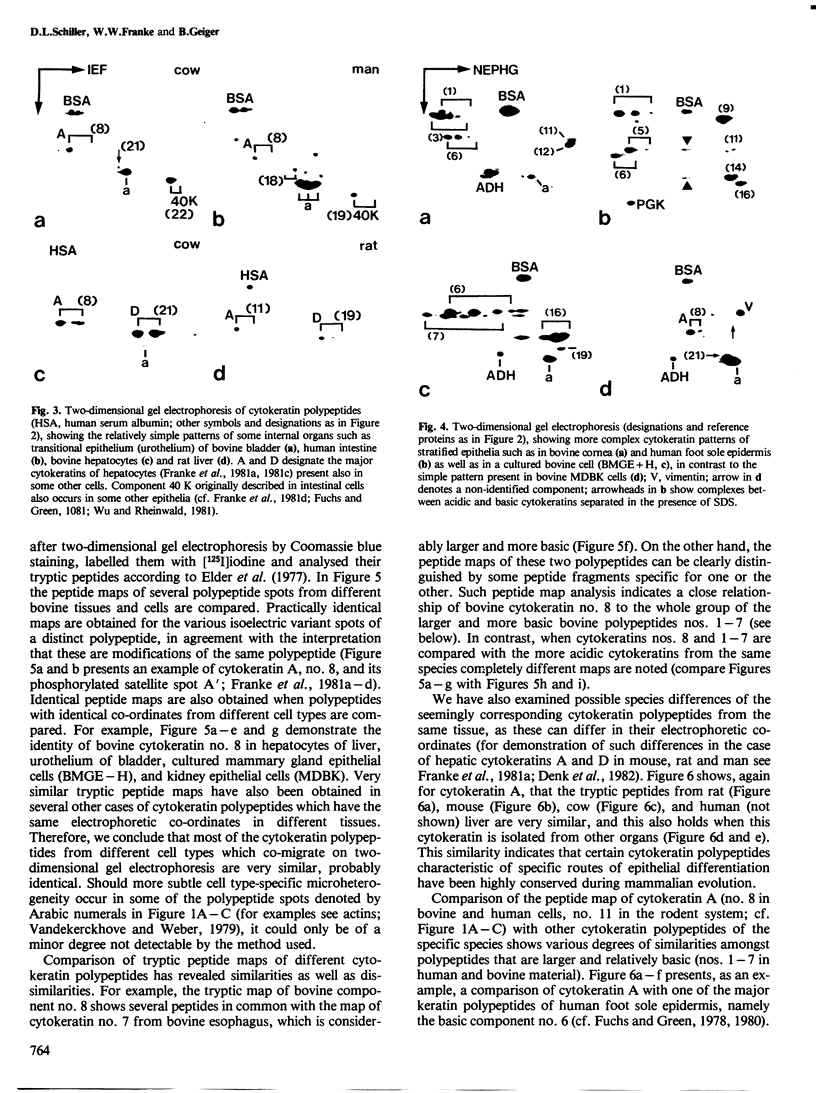


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderton B. H. Intermediate filaments: a family of homologous structures. J Muscle Res Cell Motil. 1981 Jun;2(2):141–166. doi: 10.1007/BF00711866. [DOI] [PubMed] [Google Scholar]
- Bowden P. E., Cunliffe W. J. Modification of human prekeratin during epidermal differentiation. Biochem J. 1981 Oct 1;199(1):145–154. doi: 10.1042/bj1990145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Doran T. I., Vidrich A., Sun T. T. Intrinsic and extrinsic regulation of the differentiation of skin, corneal and esophageal epithelial cells. Cell. 1980 Nov;22(1 Pt 1):17–25. doi: 10.1016/0092-8674(80)90150-6. [DOI] [PubMed] [Google Scholar]
- Drochmans P., Freudenstein C., Wanson J. C., Laurent L., Keenan T. W., Stadler J., Leloup R., Franke W. W. Structure and biochemical composition of desmosomes and tonofilaments isolated from calf muzzle epidermis. J Cell Biol. 1978 Nov;79(2 Pt 1):427–443. doi: 10.1083/jcb.79.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Franke W. W., Denk H., Kalt R., Schmid E. Biochemical and immunological identification of cytokeratin proteins present in hepatocytes of mammalian liver tissue. Exp Cell Res. 1981 Feb;131(2):299–318. doi: 10.1016/0014-4827(81)90234-2. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Mayer D., Schmid E., Denk H., Borenfreund E. Differences of expression of cytoskeletal proteins in cultured rat hepatocytes and hepatoma cells. Exp Cell Res. 1981 Aug;134(2):345–365. doi: 10.1016/0014-4827(81)90435-3. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Freudenstein C., Appelhans B., Osborn M., Weber K., Keenan T. W. Intermediate-sized filaments of the prekeratin type in myoepithelial cells. J Cell Biol. 1980 Mar;84(3):633–654. doi: 10.1083/jcb.84.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Osborn M., Weber K. Different intermediate-sized filaments distinguished by immunofluorescence microscopy. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5034–5038. doi: 10.1073/pnas.75.10.5034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Schiller D. L., Winter S., Jarasch E. D., Moll R., Denk H., Jackson B. W., Illmensee K. Differentiation-related patterns of expression of proteins of intermediate-size filaments in tissues and cultured cells. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):431–453. doi: 10.1101/sqb.1982.046.01.041. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Vandekerckhove J., Weber K. Permanently proliferating rat vascular smooth muscle cell with maintained expression of smooth muscle characteristics, including actin of the vascular smooth muscle type. J Cell Biol. 1980 Dec;87(3 Pt 1):594–600. doi: 10.1083/jcb.87.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Winter S., Grund C., Schmid E., Schiller D. L., Jarasch E. D. Isolation and characterization of desmosome-associated tonofilaments from rat intestinal brush border. J Cell Biol. 1981 Jul;90(1):116–127. doi: 10.1083/jcb.90.1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. V., Coppock S. M., Green H., Cleveland D. W. Two distinct classes of keratin genes and their evolutionary significance. Cell. 1981 Nov;27(1 Pt 2):75–84. doi: 10.1016/0092-8674(81)90362-7. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Changes in keratin gene expression during terminal differentiation of the keratinocyte. Cell. 1980 Apr;19(4):1033–1042. doi: 10.1016/0092-8674(80)90094-x. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Multiple keratins of cultured human epidermal cells are translated from different mRNA molecules. Cell. 1979 Jul;17(3):573–582. doi: 10.1016/0092-8674(79)90265-4. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978 Nov;15(3):887–897. doi: 10.1016/0092-8674(78)90273-8. [DOI] [PubMed] [Google Scholar]
- Gibbs P. E., Freedberg I. M. Epidermal keratin messenger RNAs: a heterogeneous family. Biochim Biophys Acta. 1982 Feb 26;696(2):124–133. doi: 10.1016/0167-4781(82)90019-7. [DOI] [PubMed] [Google Scholar]
- Gilmartin M. E., Culbertson V. B., Freedberg I. M. Phosphorylation of epidermal keratins. J Invest Dermatol. 1980 Sep;75(3):211–216. doi: 10.1111/1523-1747.ep12522887. [DOI] [PubMed] [Google Scholar]
- Jackson B. W., Grund C., Schmid E., Bürki K., Franke W. W., Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. Intermediate filaments of the cytokeratin type and desmosomes in preimplantation embryos. Differentiation. 1980;17(3):161–179. doi: 10.1111/j.1432-0436.1980.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Jackson B. W., Grund C., Winter S., Franke W. W., Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. II. Epithelial differentiation and intermediate-sized filaments in early postimplantation embryos. Differentiation. 1981;20(3):203–216. doi: 10.1111/j.1432-0436.1981.tb01177.x. [DOI] [PubMed] [Google Scholar]
- Kubilus J., MacDonald M. J., Baden H. P. Epidermal proteins of cultured human and bovine keratinocytes. Biochim Biophys Acta. 1979 Jun 19;578(2):484–492. doi: 10.1016/0005-2795(79)90178-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane E. B. Monoclonal antibodies provide specific intramolecular markers for the study of epithelial tonofilament organization. J Cell Biol. 1982 Mar;92(3):665–673. doi: 10.1083/jcb.92.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Intermediate filaments as mechanical integrators of cellular space. Nature. 1980 Jan 17;283(5744):249–256. doi: 10.1038/283249a0. [DOI] [PubMed] [Google Scholar]
- Lee L. D., Baden H. P. Organisation of the polypeptide chains in mammalian keratin. Nature. 1976 Nov 25;264(5584):377–379. doi: 10.1038/264377a0. [DOI] [PubMed] [Google Scholar]
- Lee L. D., Fleming B. C., Waitkus R. F., Baden H. P. Isolation of the polypeptide chains of prekeratin. Biochim Biophys Acta. 1975 Nov 18;412(1):82–90. doi: 10.1016/0005-2795(75)90341-4. [DOI] [PubMed] [Google Scholar]
- Lee L. D., Kubilus J., Baden H. P. Intraspecies heterogeneity of epidermal keratins isolated from bovine hoof and snout. Biochem J. 1979 Jan 1;177(1):187–196. doi: 10.1042/bj1770187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstone L. M. Isolation and characterization of two polypeptides that form intermediate filaments in bovine esophageal epithelium. J Cell Biol. 1981 Feb;88(2):317–322. doi: 10.1083/jcb.88.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstone L. M., McGuire J. Different polypeptides form the intermediate filaments in bovine hoof and esophageal epithelium and in aortic endothelium. J Cell Biol. 1981 Feb;88(2):312–316. doi: 10.1083/jcb.88.2.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C. M., Asai D. J., Flytzanis C. N., Lazarides E. In vitro translation of the intermediate filament proteins desmin and vimentin. Mol Cell Biol. 1981 Apr;1(4):303–309. doi: 10.1128/mcb.1.4.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Renner W., Franke W. W., Schmid E., Geisler N., Weber K., Mandelkow E. Reconstitution of intermediate-sized filaments from denatured monomeric vimentin. J Mol Biol. 1981 Jun 25;149(2):285–306. doi: 10.1016/0022-2836(81)90303-x. [DOI] [PubMed] [Google Scholar]
- Schmidt W. N., Pardue R. L., Tutt M. C., Briggs R. C., Brinkley B. R., Hnilica L. S. Identification of cytokeratin antigens in Novikoff ascites hepatoma. Proc Natl Acad Sci U S A. 1982 May;79(10):3138–3142. doi: 10.1073/pnas.79.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer J., Goerttler K. Synthesis in vitro of keratin polypeptides directed by mRNA isolated from newborn and adult mouse epidermis. Eur J Biochem. 1980 Nov;112(2):243–249. doi: 10.1111/j.1432-1033.1980.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Goldman R. D. Intermediate filaments of baby hamster kidney (BHK-21) cells and bovine epidermal keratinocytes have similar ultrastructures and subunit domain structures. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4534–4538. doi: 10.1073/pnas.77.8.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Wantz M. L. Characterization of the keratin filament subunits unique to bovine snout epidermis. Biochem J. 1980 Jun 1;187(3):913–916. doi: 10.1042/bj1870913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert P. M., Idler W. W., Zimmerman S. B. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976 Dec 15;108(3):547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Steinert P. M. Structure of the three-chain unit of the bovine epidermal keratin filament. J Mol Biol. 1978 Jul 25;123(1):49–70. doi: 10.1016/0022-2836(78)90376-5. [DOI] [PubMed] [Google Scholar]
- Steinert P. M., Wantz M. L., Idler W. W. O-phosphoserine content of intermediate filament subunits. Biochemistry. 1982 Jan 5;21(1):177–183. doi: 10.1021/bi00530a030. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Green H. Keratin filaments of cultured human epidermal cells. Formation of intermolecular disulfide bonds during terminal differentiation. J Biol Chem. 1978 Mar 25;253(6):2053–2060. [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandekerckhove J., Weber K. The complete amino acid sequence of actins from bovine aorta, bovine heart, bovine fast skeletal muscle, and rabbit slow skeletal muscle. A protein-chemical analysis of muscle actin differentiation. Differentiation. 1979;14(3):123–133. doi: 10.1111/j.1432-0436.1979.tb01021.x. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]