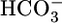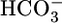Abstract
Respiratory acidosis, a decrease in blood pH caused by a rise in [CO2], rapidly triggers a compensatory response in which the kidney markedly increases its secretion of H+ from blood to urine. However, in this and other acid-base disturbances, the equilibrium CO2 + H2O ⇄ 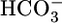 + H+ makes it impossible to determine whether the critical parameter is [CO2],
+ H+ makes it impossible to determine whether the critical parameter is [CO2], 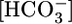 , and/or pH. Here, we used out-of-equilibrium
, and/or pH. Here, we used out-of-equilibrium 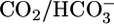 solutions to alter basolateral (BL)
solutions to alter basolateral (BL) 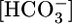 , [CO2], or pH, systematically and one at a time, on isolated perfused S2 rabbit proximal tubules. We found that increasing
, [CO2], or pH, systematically and one at a time, on isolated perfused S2 rabbit proximal tubules. We found that increasing 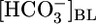 from 0 to 44 mM, at a fixed [CO2]BL of 5% and a fixed pHBL of 7.40, caused
from 0 to 44 mM, at a fixed [CO2]BL of 5% and a fixed pHBL of 7.40, caused 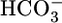 reabsorption (JHCO3) to fall by half but did not significantly affect volume reabsorption (JV). Increasing [CO2]BL from 0% to 20%, at a fixed
reabsorption (JHCO3) to fall by half but did not significantly affect volume reabsorption (JV). Increasing [CO2]BL from 0% to 20%, at a fixed 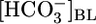 of 22 mM and pHBL of 7.40, caused JHCO3 to rise 2.5-fold but did not significantly affect JV. Finally, increasing pHBL from 6.80 to 8.00, at a fixed
of 22 mM and pHBL of 7.40, caused JHCO3 to rise 2.5-fold but did not significantly affect JV. Finally, increasing pHBL from 6.80 to 8.00, at a fixed 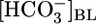 of 22 mM and [CO2]BL of 5%, did not affect either JHCO3 or JV. Analysis of the JHCO3 and JV data implies that, as the tubule alters JHCO3, it compensates the reabsorption of other solutes to keep JV approximately constant. Because the cells cannot respond acutely to pH changes, we propose that the responses of JHCO3 and the reabsorption of other solutes to changes in
of 22 mM and [CO2]BL of 5%, did not affect either JHCO3 or JV. Analysis of the JHCO3 and JV data implies that, as the tubule alters JHCO3, it compensates the reabsorption of other solutes to keep JV approximately constant. Because the cells cannot respond acutely to pH changes, we propose that the responses of JHCO3 and the reabsorption of other solutes to changes in 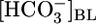 or [CO2]BL involve sensors for basolateral
or [CO2]BL involve sensors for basolateral 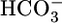 and CO2.
and CO2.
Keywords: kidney, out-of-equilibrium solution, acid-base disturbances, volume reabsorption
A major task of the kidney is to secrete H+ into the urine. Inadequate renal H+ secretion caused, for example, by mutations to acid-base transporters (1–4) or carbonic anhydrases (5), or by renal failure (6, 7), can lead to a life-threatening decrease in blood pH. Moreover, to maintain a stable blood pH, the kidney must appropriately increase H+ secretion in response to metabolic acidosis (a decrease in blood pH caused by a decrease in 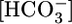 at a fixed CO2) or to respiratory acidosis. However, a half century after the classical observation that respiratory acidosis rapidly stimulates renal H+ secretion (8, 9), we still have little insight into how the kidney senses acute acid-base disturbances.
at a fixed CO2) or to respiratory acidosis. However, a half century after the classical observation that respiratory acidosis rapidly stimulates renal H+ secretion (8, 9), we still have little insight into how the kidney senses acute acid-base disturbances.
The renal proximal tubule (PT) reabsorbs (from lumen to blood) a liquid that contains ≈80% of the 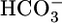 filtered by the glomerulus. The PT cell does this reabsorbtion by secreting H+ into the PT lumen and using this H+ to titrate luminal
filtered by the glomerulus. The PT cell does this reabsorbtion by secreting H+ into the PT lumen and using this H+ to titrate luminal 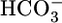 to CO2 and H2O. After entering the cell across the apical membrane, the CO2 and H2O recombine to produce H+ and
to CO2 and H2O. After entering the cell across the apical membrane, the CO2 and H2O recombine to produce H+ and 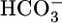 . The cell extrudes the H+ into the lumen across the apical membrane through Na-H exchangers (10–12) and H+ pumps (13) and moves the
. The cell extrudes the H+ into the lumen across the apical membrane through Na-H exchangers (10–12) and H+ pumps (13) and moves the 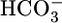 out across the basolateral membrane via the electrogenic Na/HCO3 cotransporter NBCe1-A (14, 15). Carbonic anhydrases catalyze the interconversions between CO2 and H2O on the one hand and
out across the basolateral membrane via the electrogenic Na/HCO3 cotransporter NBCe1-A (14, 15). Carbonic anhydrases catalyze the interconversions between CO2 and H2O on the one hand and 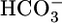 and H+ on the other (5). Because blood pH is the parameter regulated by these acid-base transport processes, blood pH or, more likely, intracellular pH (pHi), has been thought to be the parameter sensed by renal cells. However, because of the interconversion CO2 + H2O ⇄
and H+ on the other (5). Because blood pH is the parameter regulated by these acid-base transport processes, blood pH or, more likely, intracellular pH (pHi), has been thought to be the parameter sensed by renal cells. However, because of the interconversion CO2 + H2O ⇄ 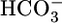 + H+, it had been impossible to distinguish pH unambiguously from
+ H+, it had been impossible to distinguish pH unambiguously from 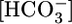 and CO2 as potential signals. Using the method that our laboratory developed for generating out-of-equilibrium (OOE)
and CO2 as potential signals. Using the method that our laboratory developed for generating out-of-equilibrium (OOE) 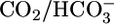 solutions (16), we can now approach the problem by independently varying basolateral
solutions (16), we can now approach the problem by independently varying basolateral 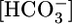 , [CO2], and pH.
, [CO2], and pH.
In the present study, we perfused single, isolated S2 segments of rabbit PTs and collected the fluid that had passed along the PT lumen. Analysis of this collected fluid allowed us to compute volume reabsorption (JV); 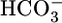 reabsorption (JHCO3), which is virtually the same as the H+-secretion rate under the conditions of our experiments; and the reabsorption of solutes other than NaHCO3 (JOther). We made the surprising observation that, at least in the short term, JHCO3 and JOther do not respond to changes in basolateral or intracellular pH. The most straightforward hypothesis is that PT cells have sensors for basolateral
reabsorption (JHCO3), which is virtually the same as the H+-secretion rate under the conditions of our experiments; and the reabsorption of solutes other than NaHCO3 (JOther). We made the surprising observation that, at least in the short term, JHCO3 and JOther do not respond to changes in basolateral or intracellular pH. The most straightforward hypothesis is that PT cells have sensors for basolateral 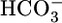 and a parameter related to CO2.
and a parameter related to CO2.
Materials and Methods
Except for the compositions of most of the OOE 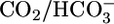 solutions, our methods are described in detail in ref. 17.
solutions, our methods are described in detail in ref. 17.
Tubule Perfusion. We hand dissected kidneys from “pathogen-free” female New Zealand White rabbits (1.4–2.0 kg) to yield individual segments of midcortical S2 PTs in accordance with an approved animal protocol. We used two assemblies of concentric glass pipettes to perfuse lumens of single isolated PTs at 37°C, as described by Burg and coworkers (18) and modified by Quigley and Baum (19). We used a calibrated collection pipette (volume ≅ 55 nl) to collect samples of fluid that had flowed down the PT lumen; the luminal collection rate was 12.6 ± 0.3 nl/min (n = 50 JV/JHCO3 measurements). We superfused the basolateral surface of the PT at 7 ml/min.
Solutions. Table 1 lists the compositions of the solutions. We dissected PTs in Hanks' solution (solution 1) at 4°C (20). During tubule perfusion, the luminal perfusate always was solution 2, which contained [3H]methoxyinulin (molecular mass ≈ 7,146 Da; catalog no. NET-086L, PerkinElmer). We dialyzed the [3H]methoxyinulin for 72 h by using a membrane with a molecular mass cutoff of 3,500 Da. After establishing luminal perfusion, we allowed a 20- to 30-min warmup period in which solution 3 flowed through the bath (i.e., basolateral solution) at 37°C. We then changed the bath to either solution 4 (Fig. 1A) or to solution 7 (Fig. 1 B and C), which differed only in [Cl-], achieved by replacing NaCl with Na gluconate. In control experiments (data not shown), we found that JV and JHCO3 were indistinguishable in solutions 4 and 7. All solutions had osmolalities of 300 ± 2 mosM. Note that solution 7 was identical to luminal solution 2 except for the addition of 32.5 mM Hepes (titrated with NaOH) in solution 7, which replaced an osmotically equivalent amount of NaCl in solution 2.
Table 1. Physiological solutions.
| Solution
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Component | 1 | 2 | 3 | 4 | 5A | 5B | 6A | 6B | 7 | 8A | 8B | 9A | 9B | 10A | 10B | 11A | 11B | 12A | 12B | 13A | 13B | 14 |
| NaCl | 137 | 124.7 | 115 | 79 | 137.3 | 20.7 | 137.3 | 20.7 | 101 | 91 | 113 | 113 | 91 | 113 | 91 | 113 | 91 | 54.7 | 142.1 | 54.7 | 132 | 130.7 |
| KCl | 5 | 5 | 5 | 5 | 0 | 10 | 0 | 10 | 5 | 0 | 10 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 10 | 0 | 5 |
| Na2HPO4 | 0.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| NaH2PO4 | 0 | 2 | 2.3 | 2 | 4 | 0 | 0 | 0 | 2 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 2 |
| CaCl2 | 0.2 | 1 | 3.6 | 1 | 2 | 0 | 2 | 0 | 1 | 2 | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 | 2 | 1 |
| MgSO4 | 0.8 | 1.2 | 1 | 1.2 | 2.4 | 0 | 2.4 | 0 | 1.2 | 2.4 | 0 | 0 | 2.4 | 0 | 2.4 | 0 | 2.4 | 0 | 2.4 | 0 | 2.4 | 1.2 |
| MgCl2 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Glucose | 0 | 10.5 | 8.3 | 10.5 | 21 | 0 | 21 | 0 | 10.5 | 21 | 0 | 0 | 21 | 0 | 21 | 0 | 21 | 0 | 21 | 0 | 21 | 10.5 |
| Glutamine | 2 | 2 | 0 | 2 | 4 | 0 | 4 | 0 | 2 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 2 |
| l-alanine | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| l-lactic acid | 2 | 2 | 2 | 2 | 4 | 0 | 4 | 0 | 2 | 4 | 0 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 0 | 4 | 2 |
| CO2, mM (%) | 0 | 1.2 (5) | 1.2 (5) | 1.2 (5) | 2.4 (10) | 0 | 0 | 2.4 (10) | 1.2 (5) | 0 | 0 | 1.2 (5) | 0 | 4.8 (20) | 0 | 9.6 (40) | 0 | 2.4 (10) | 0 | 2.4 (10) | 0 | 0 |
| NaHCO3 | 0 | 22 | 22 | 22 | 0 | 0 | 0 | 88 | 22 | 0 | 44 | 44 | 0 | 44 | 0 | 44 | 0 | 44 | 0 | 44 | 0 | 0 |
| Na gluconate | 0 | 0 | 0 | 22 | 0 | 88 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Tris·HCl | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hepes | 0 | 0 | 0 | 32.5 | 0 | 65 | 65 | 0 | 32.5 | 65 | 0 | 0 | 65 | 0 | 65 | 0 | 65 | 0 | 65 | 0 | 65 | 32.5 |
| Albumin, g/liter | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| pH | 7.40 | 7.40 | 7.40 | 7.40 | 5.40 | 7.55 | 7.29 | 7.70 | 7.40 | 6.99 | 9.40 | 7.70 | 7.34 | 7.10 | 7.46 | 6.80 | 7.52 | 7.40 | 6.64 | 7.40 | 8.11 | 7.40 |
The concentrations are in mM except for CO2 (given both in mM and %) and albumin (g/liter). Except for solution 1 (4°C), all solutions were titrated to the indicated pH at 37°C. Tris·HCl and Hepes were titrated with NaOH. Solutions: 1, Hanks' dissection; 2, lumen, equilibriated 5% CO2, 22 mM HCO2-; 3, warmup bath, equilibrated 5% CO2 22 mM HCO2; 4, 86 mM Cl- bath, equilibrated 5% CO2 22 mM HCO3-; 5, 86 mM Cl- bath, OOE solution 5% CO2 0 mM HCO3-; 6, 86 mM Cl- bath, OOE solution 5% CO2 44 mM HCO3-; 7, standard bath, equilibrated 5% CO2 22 mM HCO3-; 8, bath, OOE solution 0% CO2 22 mM HCO3-; 9, bath, OOE solution 2.5% CO2 22 mM HCO3-; 10, bath, OOE solution 10% CO2 22 mM HCO3-; 11, bath, OOE solution 20% CO2 22 mM HCO3-; 12, pH 6.8 bath, OOE solution 5% CO2 22 mM HCO3-; 13, pH 8.0 bath, OOE solution 5% CO2 22 mM HCO3-; 14, Hepes lumen/bath. Solution 2 was used as a luminal perfusate. Solution 3 was used as a basolateral perfusate. OOE solutions were generated by rapidly mixing their respective A and B components in 1:1 ratio. Solutions 5B, 6A, 8A, 8B, 9B, 10B, 11B, 12B, and 13B were vigorously gassed with 100% O2 to render them free of CO2. Mixed pH was 7.40 for solutions 5, 6, 8—11, 6.80 for solution 12, and 8.00 for solution 13.
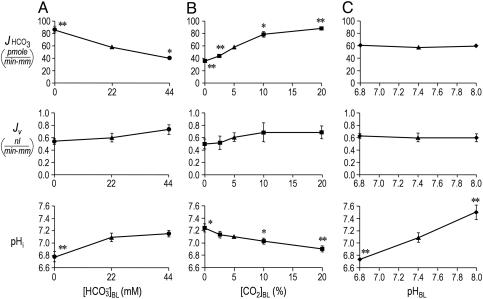
Fig. 1.
Effect of isolated changes in basolateral acid-base parameters on JHCO3, JV, and pHi. (A) Effect of changing 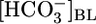 at a fixed [CO2]BL of 5% and a fixed pHBL of 7.40. Data represent mean ± SEM; error bars are omitted when they are smaller than the size of the symbol. For Top and Middle, in each of the 13 experiments we paired an equilibrated bath
at a fixed [CO2]BL of 5% and a fixed pHBL of 7.40. Data represent mean ± SEM; error bars are omitted when they are smaller than the size of the symbol. For Top and Middle, in each of the 13 experiments we paired an equilibrated bath 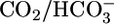 solution (▴) with one of two OOE solutions (circles; see Materials and Methods). n = 7,
solution (▴) with one of two OOE solutions (circles; see Materials and Methods). n = 7, 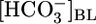 = 0; n = 6,
= 0; n = 6, 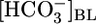 = 44 mM. For Bottom, each pHi experiment (n = 6) included bath exposures to each of the three solutions in Top.(B) Effect of changing [CO2]BL at a fixed
= 44 mM. For Bottom, each pHi experiment (n = 6) included bath exposures to each of the three solutions in Top.(B) Effect of changing [CO2]BL at a fixed 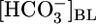 of 22 mM and a fixed pHBL of 7.40. For Top and Middle, each of the 25 experiments paired an equilibrated bath
of 22 mM and a fixed pHBL of 7.40. For Top and Middle, each of the 25 experiments paired an equilibrated bath 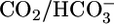 solution (▴) with one of four OOE solutions (▪). n = 6, [CO2]BL values of 0, 2.5, and 10%; n = 7, [CO2]BL = 20%. For Bottom, each of the 20 pHi experiments included bath exposures to the equilibrated and one or two OOE solutions. n = 12, [CO2]BL = 0; n = 6, [CO2]BL = 2.5%; n = 8, [CO2]BL values of 10% and 20%. (C) Effect of changing pHBL at a fixed [CO2]BL of 5% and a fixed
solution (▴) with one of four OOE solutions (▪). n = 6, [CO2]BL values of 0, 2.5, and 10%; n = 7, [CO2]BL = 20%. For Bottom, each of the 20 pHi experiments included bath exposures to the equilibrated and one or two OOE solutions. n = 12, [CO2]BL = 0; n = 6, [CO2]BL = 2.5%; n = 8, [CO2]BL values of 10% and 20%. (C) Effect of changing pHBL at a fixed [CO2]BL of 5% and a fixed 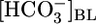 of 22 mM. For Top and Middle, each of the 12 experiments paired an equilibrated bath
of 22 mM. For Top and Middle, each of the 12 experiments paired an equilibrated bath 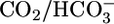 solution (▴) with one of two OOE solutions (♦). n = 6, pHBL values of 6.80 and 8.00. For Bottom, each pHi experiment included bath exposures to each of the three solutions in Top (n = 6). *, P < 0.01; **, P < 0.001; absence of * or ** indicates P > 0.05 in paired two-tailed t tests comparing OOE data with equilibrated data.
solution (▴) with one of two OOE solutions (♦). n = 6, pHBL values of 6.80 and 8.00. For Bottom, each pHi experiment included bath exposures to each of the three solutions in Top (n = 6). *, P < 0.01; **, P < 0.001; absence of * or ** indicates P > 0.05 in paired two-tailed t tests comparing OOE data with equilibrated data.
We generated OOE 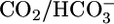 solutions by rapidly mixing streams of two dissimilar solutions (17), delivering the newly mixed solution to the PT within ≈200 ms. Fig. 1C in ref. 17 shows the detailed compositions of two newly mixed OOE solutions and the minute degree of equilibration that occurs during the ≈200-ms interval. The final compositions of OOE solutions in Fig. 1A (solutions 5 and 6) were the same as for solution 4 except that we replaced
solutions by rapidly mixing streams of two dissimilar solutions (17), delivering the newly mixed solution to the PT within ≈200 ms. Fig. 1C in ref. 17 shows the detailed compositions of two newly mixed OOE solutions and the minute degree of equilibration that occurs during the ≈200-ms interval. The final compositions of OOE solutions in Fig. 1A (solutions 5 and 6) were the same as for solution 4 except that we replaced 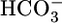 with gluconate or vice versa to keep [Cl-] constant. The final compositions of the OOE solutions in Fig. 1B (solutions 7–11) were the same as for solution 7 except for [CO2]. The final compositions of the OOE solutions in Fig. 1C (solutions 12 and 13) were the same as for solution 7 except for pH, the concentrations of neutral and anionic Hepes, and the [NaCl] (which we adjusted to keep osmolality constant).
with gluconate or vice versa to keep [Cl-] constant. The final compositions of the OOE solutions in Fig. 1B (solutions 7–11) were the same as for solution 7 except for [CO2]. The final compositions of the OOE solutions in Fig. 1C (solutions 12 and 13) were the same as for solution 7 except for pH, the concentrations of neutral and anionic Hepes, and the [NaCl] (which we adjusted to keep osmolality constant).
We used solution 14 only in experiments in which we measured pHi. Here, solution 14 was present in the lumen before we switched to solution 2. In addition, solution 14 was present in the bath when a 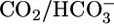 -containing solution (i.e., solutions 4–13) was not present.
-containing solution (i.e., solutions 4–13) was not present.
Measurement of JV and JHCO3. We measured JHCO3 (pmol/min per mm tubule length) and JV (nl·min-1·mm-1) by using an approach similar to that of McKinney and Burg (21). We assayed total CO2 in aliquots of the perfusate and collected fluid by using a NanoFlo microfluorometer (World Precision Instruments, Sarasota, FL) and reagents (Diagnostic Kit 132-A) from Sigma-Aldrich (22, 23). Luminal [3H]methoxyinulin served as a volume marker for calculating JV.
Each experiment consisted of two data collection periods. The bath contained equilibrated 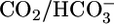 (solutions 4 or 7) during the first period and an OOE solution (solutions 5, 6, 8, 9, 10, 11, 12, or 13) during the second. In control experiments in which identical equilibrated
(solutions 4 or 7) during the first period and an OOE solution (solutions 5, 6, 8, 9, 10, 11, 12, or 13) during the second. In control experiments in which identical equilibrated 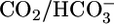 solutions (solution 7) were present during both periods, JHCO3 values were identical, as were JV values (17). In each experiment, we divided the JHCO3 (and JV) value obtained during the second (OOE) collection period by the comparable values obtained during the first (control) period to generate OOE/control ratios for JHCO3 (and JV). In Fig. 1, JHCO3 (and JV) values for control conditions (triangles) are raw mean values; each OOE value (circles, squares, and diamonds) is the product of the raw mean JHCO3 (or JV) value and the average OOE/control ratio for JHCO3 (or JV).
solutions (solution 7) were present during both periods, JHCO3 values were identical, as were JV values (17). In each experiment, we divided the JHCO3 (and JV) value obtained during the second (OOE) collection period by the comparable values obtained during the first (control) period to generate OOE/control ratios for JHCO3 (and JV). In Fig. 1, JHCO3 (and JV) values for control conditions (triangles) are raw mean values; each OOE value (circles, squares, and diamonds) is the product of the raw mean JHCO3 (or JV) value and the average OOE/control ratio for JHCO3 (or JV).
Calculation of 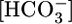 in the Fluid Reabsorbed by the PT. In experiments in which we varied
in the Fluid Reabsorbed by the PT. In experiments in which we varied 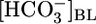 (Fig. 1A) or [CO2]BL (Fig. 1B), we computed JHCO3 (Fig. 1 A and B Top) and JV (Fig. 1 A and B Middle). From these values, we calculated the
(Fig. 1A) or [CO2]BL (Fig. 1B), we computed JHCO3 (Fig. 1 A and B Top) and JV (Fig. 1 A and B Middle). From these values, we calculated the 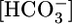 in the reabsorbate as the ratio JHCO3/JV for each
in the reabsorbate as the ratio JHCO3/JV for each 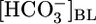 (Fig. 2A Lower) and each [CO2]BL (Fig. 2B Lower). Assuming that the PT reabsorbs fluid isosmotically (300 mosM),
(Fig. 2A Lower) and each [CO2]BL (Fig. 2B Lower). Assuming that the PT reabsorbs fluid isosmotically (300 mosM),
 |
[1] |
Here, 2 × JHCO3 is the reabsorption of NaHCO3, and JOther is the reabsorption of solutes other than NaHCO3. We used this equation to compute JOther for each value of 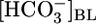 and [CO2]BL (Fig. 2 Upper). Finally, knowing JOther and JV, we computed the concentration of all Other solutes in the reabsorbate as the ratio JOther/JV (Fig. 2 Lower).
and [CO2]BL (Fig. 2 Upper). Finally, knowing JOther and JV, we computed the concentration of all Other solutes in the reabsorbate as the ratio JOther/JV (Fig. 2 Lower).
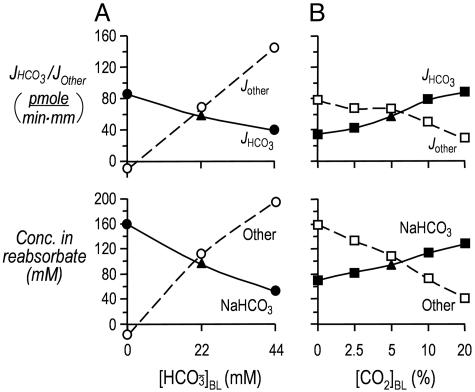
Fig. 2.
Effect of changes in basolateral 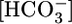 or [CO2] on the calculated reabsorption of solutes other than NaHCO3. (A) Changes in
or [CO2] on the calculated reabsorption of solutes other than NaHCO3. (A) Changes in 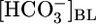 . (A Upper) the JHCO3 data are replotted from Fig. 1 A Top. The JOther values were calculated as described in Materials and Methods, assuming (JHCO3 + JOther)/JV = 300 mosM. (A Lower) the calculated reabsorbate [NaHCO3] values were obtained by dividing JHCO3 values in A by the corresponding JV values in B. The calculated concentrations of all other solutes (Other) was obtained by dividing JOther in A by the corresponding JV in B.(B) Isolated changes in [CO2]BL. The JHCO3 data in Top are replotted from Fig. 1B Top. We computed the other values as described for Fig. 2 A.
. (A Upper) the JHCO3 data are replotted from Fig. 1 A Top. The JOther values were calculated as described in Materials and Methods, assuming (JHCO3 + JOther)/JV = 300 mosM. (A Lower) the calculated reabsorbate [NaHCO3] values were obtained by dividing JHCO3 values in A by the corresponding JV values in B. The calculated concentrations of all other solutes (Other) was obtained by dividing JOther in A by the corresponding JV in B.(B) Isolated changes in [CO2]BL. The JHCO3 data in Top are replotted from Fig. 1B Top. We computed the other values as described for Fig. 2 A.
Measurement of pHi. We calculated pHi from the fluorescence excitation ratio of 2′,7′-bis-(2-carboxyethyl)-5(and-6)carboxyfluorescein (24), loaded into cells as the acetoxymethyl ester (no. B-1170, Molecular Probes) (17). The inverted microscope was a Zeiss IM-35, equipped with apparatus for epiillumination, a 40×/NA 0.85 objective, dual filter wheels (Ludl Electronic Products, Hawthorne, NY) for alternating between 495 ± 5 nm and 440 ± 5 nm excitation filters (Thermo Oriel, Stratford, CT), a 510-nm long-pass dichroic mirror, a 530-nm long-pass filter, an image intensifier (KS-1381 intensifier, Videoscope, Dulles, VA), and a camera (CCD 72, Dage–M.T.I., Michigan City, IN). We converted the I490/I440 ratios to pHi values by using the high-K+/nigericin technique (25), as modified for one-point calibrations (26).
Supporting Information. For additional information on results and discussion, see Figs. 5 and 6, Table 2, and Supporting Text, which are published as supporting information on the PNAS web site.
Results
Effect of Variations in 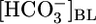 . In the Fig. 1 A Top, the triangle (
. In the Fig. 1 A Top, the triangle (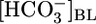 = 22 mM) represents JHCO3 with all three basolateral acid-base parameters at their equilibrated, physiological values. Switching to an OOE basolateral (i.e., bath) solution that was nominally
= 22 mM) represents JHCO3 with all three basolateral acid-base parameters at their equilibrated, physiological values. Switching to an OOE basolateral (i.e., bath) solution that was nominally 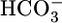 -free, while holding [CO2]BL and pHBL at their physiological values, caused JHCO3 to increase by ≈50%. This maneuver approximates the “metabolic” half of metabolic acidosis. Conversely, switching from the equilibrated 5% CO2/22 mM
-free, while holding [CO2]BL and pHBL at their physiological values, caused JHCO3 to increase by ≈50%. This maneuver approximates the “metabolic” half of metabolic acidosis. Conversely, switching from the equilibrated 5% CO2/22 mM 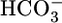 at pH 7.40 solution to an OOE bath solution with twice the normal
at pH 7.40 solution to an OOE bath solution with twice the normal 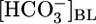 , but with [CO2]BL and pHBL at their physiological values, caused JHCO3 to fall by ≈30%. Thus, changes in
, but with [CO2]BL and pHBL at their physiological values, caused JHCO3 to fall by ≈30%. Thus, changes in 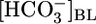 cause JHCO3 to change in a direction that would help the PT to respond appropriately to stabilize blood pH.
cause JHCO3 to change in a direction that would help the PT to respond appropriately to stabilize blood pH.
The fluid that the PT reabsorbs is nearly isosmotic, and the calculated 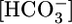 in this reabsorbed fluid is the ratio JHCO3/JV. Fig. 1A Middle shows that changes in
in this reabsorbed fluid is the ratio JHCO3/JV. Fig. 1A Middle shows that changes in 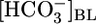 do not cause significant changes in JV. Thus, JHCO3/JV (i.e.,
do not cause significant changes in JV. Thus, JHCO3/JV (i.e., 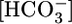 in the reabsorbate) must vary considerably. In Fig. 1A, this calculated
in the reabsorbate) must vary considerably. In Fig. 1A, this calculated 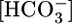 was 159 mM at a
was 159 mM at a 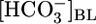 of 0 mM (i.e., the reabsorbate was isotonic NaHCO3). In Fig. 2A Upper, we replot the JHCO3 data from Fig. 1A Top and also plot the flux of all Other solutes, as described in Materials and Methods. Fig. 2A Lower summarizes the computed reabsorbate values of [NaHCO3] and all Other solutes. The analysis in Fig. 2A illustrates that increasing
of 0 mM (i.e., the reabsorbate was isotonic NaHCO3). In Fig. 2A Upper, we replot the JHCO3 data from Fig. 1A Top and also plot the flux of all Other solutes, as described in Materials and Methods. Fig. 2A Lower summarizes the computed reabsorbate values of [NaHCO3] and all Other solutes. The analysis in Fig. 2A illustrates that increasing 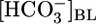 not only lowers JHCO3 but reciprocally raises the reabsorption of other solutes (JOther), the appropriate response for maintaining a constant JV and, thus, a constant blood pressure.
not only lowers JHCO3 but reciprocally raises the reabsorption of other solutes (JOther), the appropriate response for maintaining a constant JV and, thus, a constant blood pressure.
Because extracellular acid-base disturbances generally cause pHi to change (27), we examined how changes in 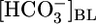 affect the steady-state pHi of PT cells under the same conditions that prevailed for Fig. 1A Top and Middle. As shown in the Fig. 1A Bottom, an increase in
affect the steady-state pHi of PT cells under the same conditions that prevailed for Fig. 1A Top and Middle. As shown in the Fig. 1A Bottom, an increase in 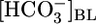 from 0 to 22 mM caused steady-state pHi to increase by 0.32. However, further increasing
from 0 to 22 mM caused steady-state pHi to increase by 0.32. However, further increasing 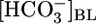 to 44 mM did not cause a statistically significant increase in steady-state pHi.
to 44 mM did not cause a statistically significant increase in steady-state pHi.
Effect of Isolated Variations in [CO2]BL. In Fig. 1B Top, the triangle ([CO2]BL = 5%) represents virtually the same conditions as the triangle in Fig. 1A Top. Switching from this equilibrated, physiological solution to one in which we increased [CO2]BL to four times its physiological value, while holding 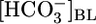 and pHBL at their physiological values, caused JHCO3 to rise by ≈50% This maneuver approximates the “respiratory” half of respiratory acidosis. If we instead exposed the PT to a nominally CO2-free OOE bath solution, while holding
and pHBL at their physiological values, caused JHCO3 to rise by ≈50% This maneuver approximates the “respiratory” half of respiratory acidosis. If we instead exposed the PT to a nominally CO2-free OOE bath solution, while holding 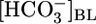 and pHBL at their physiological values, JHCO3 fell by 40% compared with normal. The small JHCO3 observed in the nominal absence of basolateral CO2 may be due, in part, to CO2 from the lumen reaching the basolateral membrane. The midpoint of the JHCO3 response to CO2 is at a [CO2]BL of ≈6%, which is somewhat above the physiological [CO2] of arterial blood and somewhat below that of the renal cortex (28, 29). Thus, isolated changes in [CO2]BL cause JHCO3 to change in a direction that would help the PT to respond appropriately to stabilize blood pH.
and pHBL at their physiological values, JHCO3 fell by 40% compared with normal. The small JHCO3 observed in the nominal absence of basolateral CO2 may be due, in part, to CO2 from the lumen reaching the basolateral membrane. The midpoint of the JHCO3 response to CO2 is at a [CO2]BL of ≈6%, which is somewhat above the physiological [CO2] of arterial blood and somewhat below that of the renal cortex (28, 29). Thus, isolated changes in [CO2]BL cause JHCO3 to change in a direction that would help the PT to respond appropriately to stabilize blood pH.
Fig. 1B Middle shows that isolated changes in [CO2]BL tended to cause JV to increase. However, none of the differences between the JV values obtained in OOE solutions and the JV value obtained in the equilibrated solution (solution 7) were statistically significant. As [CO2]BL rose from 0% to 20%, the calculated reabsorbate [NaHCO3] rose from 70 mM (i.e., the isosmotic reabsorbate was ≈50% NaHCO3) to 129 mM (i.e., the reabsorbate was mainly isotonic NaHCO3). As summarized in Fig. 2B, increases in [CO2]BL not only raise JHCO3 and reabsorbate [NaHCO3] but also reciprocally lower JOther and the total concentration of other solutes in the reabsorbate.
We also measured pHi of the PT cells under the conditions of Fig. 1B Top and Middle. Fig. 1B Bottom shows that increases in [CO2]BL cause graded decreases in steady-state pHi. Thus, the data in Fig. 1B are consistent with the hypothesis that elevations in [CO2]BL increase JHCO3 indirectly by lowering pHi. According to this hypothesis, the increased JHCO3 that we observed when decreasing 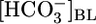 (Fig. 1A Top) also would have been caused by the attendant decrease in pHi (Fig. 1A Bottom).
(Fig. 1A Top) also would have been caused by the attendant decrease in pHi (Fig. 1A Bottom).
Effect of Variations in pHBL. If pHi changes determine JHCO3, we should be able to increase JHCO3 by lowering pHi, even without changing 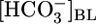 or [CO2]BL. In Fig. 1C Top, the triangle (pHBL = 7.40) represents the same conditions as the triangle in Fig. 1B Top. Surprisingly, switching to either a pH-6.80 or pH-8.00 OOE bath solution, while holding
or [CO2]BL. In Fig. 1C Top, the triangle (pHBL = 7.40) represents the same conditions as the triangle in Fig. 1B Top. Surprisingly, switching to either a pH-6.80 or pH-8.00 OOE bath solution, while holding 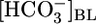 and [CO2]BL at their physiological values, caused no change in either JHCO3 (Top) or JV (Middle). As pHBL rose from 6.80 to 8.00, the calculated
and [CO2]BL at their physiological values, caused no change in either JHCO3 (Top) or JV (Middle). As pHBL rose from 6.80 to 8.00, the calculated 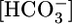 in the reabsorbate ranged between 96 mM and 99 mM. On the other hand, Fig. 1C Middle shows that raising pHBL from 6.80 to 8.00 caused substantial, graded increases in steady-state pHi. In fact, the ΔpHi/ΔpHo ratio for these PT cells (≈60%) is among the highest recorded for any cell (30–33).
in the reabsorbate ranged between 96 mM and 99 mM. On the other hand, Fig. 1C Middle shows that raising pHBL from 6.80 to 8.00 caused substantial, graded increases in steady-state pHi. In fact, the ΔpHi/ΔpHo ratio for these PT cells (≈60%) is among the highest recorded for any cell (30–33).
Discussion
What Contributes to JOther? One of our most striking observations is that JHCO3 and JOther (i.e., reabsorption rate of solutes other than 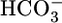 and its obligated Na+) change reciprocally to keep JV relatively constant. The Other solutes include (i) Cl-, (ii) organic solutes, and (iii) Na+ in excess of that accompanying
and its obligated Na+) change reciprocally to keep JV relatively constant. The Other solutes include (i) Cl-, (ii) organic solutes, and (iii) Na+ in excess of that accompanying 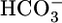 . Diffusion and solvent drag through tight junctions, possibly augmented by apical Cl-base exchange (34), could contribute to Cl- reabsorption (Fig. 3). Apical cotransport of Na+ with glucose, lactate, and glutamine could contribute to the reabsorption of organics. The near constancy of JV implies that JNa and, thus, the Na-K pump rate, must also be relatively stable.
. Diffusion and solvent drag through tight junctions, possibly augmented by apical Cl-base exchange (34), could contribute to Cl- reabsorption (Fig. 3). Apical cotransport of Na+ with glucose, lactate, and glutamine could contribute to the reabsorption of organics. The near constancy of JV implies that JNa and, thus, the Na-K pump rate, must also be relatively stable.
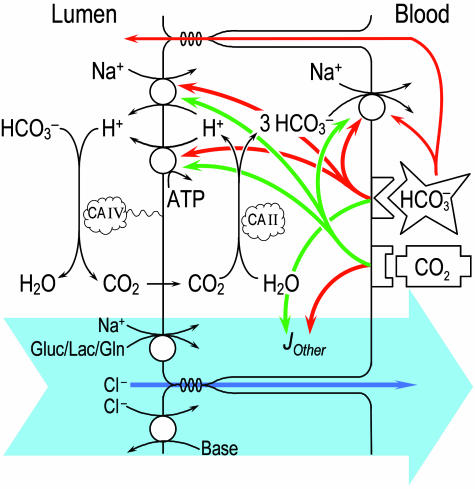
Fig. 3.
Model of 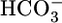 reabsorption by the proximal tubule. Changes in pHBL cause large changes in pHi in the same direction but have no short-term effect on JHCO3. Increases in
reabsorption by the proximal tubule. Changes in pHBL cause large changes in pHi in the same direction but have no short-term effect on JHCO3. Increases in 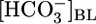 could moderate JHCO3 (red arrows) by increasing
could moderate JHCO3 (red arrows) by increasing 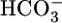 backleak into the lumen and/or by directly inhibiting the electrogenic Na/HCO3 cotransporter NBCe1-A. A
backleak into the lumen and/or by directly inhibiting the electrogenic Na/HCO3 cotransporter NBCe1-A. A 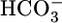 sensor would contribute to the decrease in JHCO3 (red arrows) and is necessary to account for the increased reabsorption (green arrow) of solutes other than NaHCO3 (JOther). At the apical membrane, this reabsorption of other solutes (enclosed by the large blue arrow) would be mediated by cotransporters mediating the uptake of Na/glucose, Na/lactate, and Na/glutamine; obligated Cl- would pass through tight junctions by diffusion and solvent drag. Apical Cl-base exchange (in parallel with Na-H exchange) could also contribute to JOther. Increases in CO2 would increase JHCO3 by stimulating a CO2 sensor at or near the basolateral membrane. This sensor would, in turn, stimulate three acid-base transporters (green arrows) but reduce JOther (red arrow). If the CO2 sensor is a membrane protein, the CO2 binding site is not necessarily facing outward as shown.
sensor would contribute to the decrease in JHCO3 (red arrows) and is necessary to account for the increased reabsorption (green arrow) of solutes other than NaHCO3 (JOther). At the apical membrane, this reabsorption of other solutes (enclosed by the large blue arrow) would be mediated by cotransporters mediating the uptake of Na/glucose, Na/lactate, and Na/glutamine; obligated Cl- would pass through tight junctions by diffusion and solvent drag. Apical Cl-base exchange (in parallel with Na-H exchange) could also contribute to JOther. Increases in CO2 would increase JHCO3 by stimulating a CO2 sensor at or near the basolateral membrane. This sensor would, in turn, stimulate three acid-base transporters (green arrows) but reduce JOther (red arrow). If the CO2 sensor is a membrane protein, the CO2 binding site is not necessarily facing outward as shown.
The PT could produce the highest JOther observed in the present study (≈143 pmol·min-1·mm-1 in Fig. 2A) by reabsorbing ≈4.25 mM or ≈30% of the 14.5 mM of organic solutes initially present in the lumen (i.e., 10.5 mM glucose, 2 mM lactate, and 2 mM glutamine), along with the coupled Na+ and obligated Cl-. To the extent that organic-solute transport contributes to JOther, PT cells could reciprocally alter JHCO3 and JOther by changing the rate of the apical Na-H exchanger, predominantly NHE3 (35). To the extent that apical Cl-base exchange in parallel with Na-H exchange contributes to JOther, the cell could reciprocally alter JHCO3 and JOther by changing JCl-base while keeping JNa-H constant.
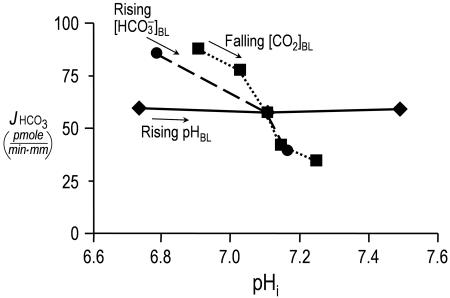
Do Proximal Tubules Sense Acute pHi Changes? The conventional wisdom is that acid-base chemosensitive cells, including central chemoreceptor neurons, peripheral chemoreceptor glomus cells, and certain epithelial cells (e.g., PT cells), sense acute acid-base disturbances through pHi changes (36). Although the evidence is consistent with a major role for pHi in neurons and glomus cells, our data suggest that this conclusion is not the case in the proximal tubule. The circles in Fig. 4 are replots of the JHCO3 data from Fig. 1A Top vs. the corresponding pHi data from Fig. 1A Bottom. Similarly, the squares are replots of the JHCO3 vs. the pHi data from Fig. 1B. For both data sets, increases in pHi, whether caused by a rising 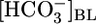 or a falling [CO2]BL, are associated with decreases in JHCO3. However, the replot of the JHCO3 vs. pHi data from Fig. 1C, plotted as diamonds in Fig. 4, shows that even large pHi changes are not sufficient to alter JHCO3, provided both
or a falling [CO2]BL, are associated with decreases in JHCO3. However, the replot of the JHCO3 vs. pHi data from Fig. 1C, plotted as diamonds in Fig. 4, shows that even large pHi changes are not sufficient to alter JHCO3, provided both 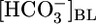 and [CO2]BL are constant. Supporting Text shows that large pHi changes are likewise insufficient to alter JOther (Fig. 5). Thus, pHi cannot be the parameter triggering reciprocal changes in JHCO3 and JOther in Fig. 2 A and B.
and [CO2]BL are constant. Supporting Text shows that large pHi changes are likewise insufficient to alter JOther (Fig. 5). Thus, pHi cannot be the parameter triggering reciprocal changes in JHCO3 and JOther in Fig. 2 A and B.
Fig. 4.
Relationship between JHCO3 and pHi. The circles are replots of the JHCO3 and pHi data from Fig. 1 A Top and Bottom, 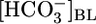 values ranging from 0 mM on the left to 44 mM on the right (fixed [CO2]BL = 5%, fixed pHBL = 7.40). The squares are replots of data from Fig. 1 B, [CO2]BL values ranging from 20% on the left to 0% on the right (fixed
values ranging from 0 mM on the left to 44 mM on the right (fixed [CO2]BL = 5%, fixed pHBL = 7.40). The squares are replots of data from Fig. 1 B, [CO2]BL values ranging from 20% on the left to 0% on the right (fixed 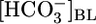 = 22 mM, fixed pHBL = 7.40). The diamonds are replots of data from Fig. 1 C, pHBL values ranging from 6.80 on the left to 8.00 on the right (fixed [CO2]BL = 5%, fixed
= 22 mM, fixed pHBL = 7.40). The diamonds are replots of data from Fig. 1 C, pHBL values ranging from 6.80 on the left to 8.00 on the right (fixed [CO2]BL = 5%, fixed 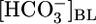 = 22 mM).
= 22 mM).
In retrospect, the insensitivity of JHCO3 to acute pHi changes could have been anticipated for both theoretical and experimental reasons. First, theory predicts that a fall in pHi could trigger an increase in JHCO3 only by stimulating both H+ extrusion across the apical membrane and 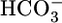 exit across the basolateral membrane. However, a fall in pHi ought to inhibit NBCe1-A (Fig. 3), either directly or indirectly by lowering
exit across the basolateral membrane. However, a fall in pHi ought to inhibit NBCe1-A (Fig. 3), either directly or indirectly by lowering 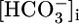 and
and 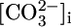 , just as a fall in pHi inhibits Cl-HCO3 exchange in other cell types (37–40).
, just as a fall in pHi inhibits Cl-HCO3 exchange in other cell types (37–40).
Second, previous experiments showed that, with 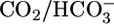 absent from both lumen and bath or present only in the lumen, pHi recovers (i.e., increases) slowly from acute intracellular acid loads (41). Just as important, the pHi recovery rates are only modestly pHi sensitive. On the other hand, with
absent from both lumen and bath or present only in the lumen, pHi recovers (i.e., increases) slowly from acute intracellular acid loads (41). Just as important, the pHi recovery rates are only modestly pHi sensitive. On the other hand, with 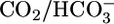 present both in lumen and bath or only in the bath, pHi recovers far more rapidly but still with only modest pHi sensitivity (see fig. 4 in ref. 41). In other experiments (42), in which
present both in lumen and bath or only in the bath, pHi recovers far more rapidly but still with only modest pHi sensitivity (see fig. 4 in ref. 41). In other experiments (42), in which 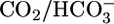 was either absent from both lumen and bath (bilateral Hepes) or present in both lumen and bath (bilateral
was either absent from both lumen and bath (bilateral Hepes) or present in both lumen and bath (bilateral 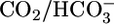 ), a more quantitative analysis of acid-extrusion rates (i.e., the sum of apical Na-H exchange and H+ pumping) was possible. In bilateral Hepes, acid extrusion was low and only modestly pHi dependent. In bilateral
), a more quantitative analysis of acid-extrusion rates (i.e., the sum of apical Na-H exchange and H+ pumping) was possible. In bilateral Hepes, acid extrusion was low and only modestly pHi dependent. In bilateral 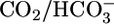 , acid extrusion was again only modestly pHi dependent but ≈8-fold higher at identical pHi values (see fig. 5 in ref. 42). Thus, the main determinant of acid extrusion in intact PTs is not pHi but parameters related to
, acid extrusion was again only modestly pHi dependent but ≈8-fold higher at identical pHi values (see fig. 5 in ref. 42). Thus, the main determinant of acid extrusion in intact PTs is not pHi but parameters related to 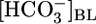 and/or [CO2]BL.
and/or [CO2]BL.
What, then, is the primary trigger for reciprocal changes in JHCO3 and JOther? A fall in 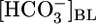 could lower
could lower 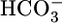 backleak through tight junctions (43, 44) and, thereby, raise JHCO3. NBCe1-A (Fig. 3) appears to transport
backleak through tight junctions (43, 44) and, thereby, raise JHCO3. NBCe1-A (Fig. 3) appears to transport 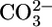 when operating with a
when operating with a 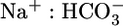 stoichiometry of 1:2 (I. Grichtchenko and W.F.B., unpublished data) and may well transport both
stoichiometry of 1:2 (I. Grichtchenko and W.F.B., unpublished data) and may well transport both 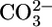 and
and 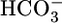 when operating with a 1:3 stoichiometry in the PT. Thus, increases in
when operating with a 1:3 stoichiometry in the PT. Thus, increases in 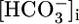 and
and 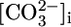 or decreases in
or decreases in 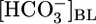 and
and 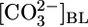 could stimulate NBCe1-A and thereby raise JHCO3. However, although effects on
could stimulate NBCe1-A and thereby raise JHCO3. However, although effects on 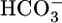 backleak or NBCe1-A might serve as secondary modulators of JHCO3, it is unclear how these effects could serve as primary triggers for changes in JOther, let alone closely coordinated, reciprocal changes in JHCO3 and JOther (Fig. 2 Upper).
backleak or NBCe1-A might serve as secondary modulators of JHCO3, it is unclear how these effects could serve as primary triggers for changes in JOther, let alone closely coordinated, reciprocal changes in JHCO3 and JOther (Fig. 2 Upper).
Even though our data indicate that PT cells do not respond to acute pHi changes by modulating JHCO3 or JOther, our data do not address the issue of how prolonged pHi changes might affect cells. In opossum-kidney cells, chronic metabolic or respiratory acidosis (pHo 7.0 vs. 7.4) over a period of 2 days increases Na-H exchange by ≈30% (45). This stimulation is accompanied by increased levels of mRNA encoding the Na-H exchanger NHE3 and abrogated either by herbimycin A (inhibitor of certain soluble tyrosine kinases) or by overexpressing csk (46), a physiological inhibitor of c-src.
What Does the PT Sense? Supporting Text includes an analysis of how the experimental maneuvers in Fig. 1 influence the intracellular and basolateral values of [CO2], pH, [H2CO3], 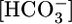 , and
, and 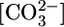 (Fig. 6). The analysis reveals that no single parameter exhibits a pattern that consistently correlates, positively or negatively, with the patterns of JHCO3 and JOther in Fig. 2 Upper. Moreover, the analysis reveals that the next most parsimonious hypothesis, that the patterns of JHCO3 and JOther in Fig. 2 Upper reflect the sensing of two parameters, is straightforward for only three of 45 parameter pairs: (i)
(Fig. 6). The analysis reveals that no single parameter exhibits a pattern that consistently correlates, positively or negatively, with the patterns of JHCO3 and JOther in Fig. 2 Upper. Moreover, the analysis reveals that the next most parsimonious hypothesis, that the patterns of JHCO3 and JOther in Fig. 2 Upper reflect the sensing of two parameters, is straightforward for only three of 45 parameter pairs: (i) 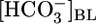 and [CO2]BL, (ii)
and [CO2]BL, (ii) 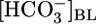 and [CO2]i, and (iii)
and [CO2]i, and (iii) 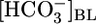 and [H2CO3]i (Table 2). As summarized in Fig. 3, we propose that PT cells respond to increases in
and [H2CO3]i (Table 2). As summarized in Fig. 3, we propose that PT cells respond to increases in 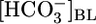 by reducing JHCO3 and raising JOther and respond to increases in [CO2]BL (or a related parameter) by raising JHCO3 and reducing JOther.
by reducing JHCO3 and raising JOther and respond to increases in [CO2]BL (or a related parameter) by raising JHCO3 and reducing JOther.
We already noted that adding 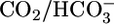 to the basolateral, but not the luminal, side of the PT triggers a large increase in H+ extrusion (41, 42). The simplest explanation for these earlier data are that the basolateral CO2 strongly enhanced acid-base transporters, overwhelming the inhibition imposed by the basolateral
to the basolateral, but not the luminal, side of the PT triggers a large increase in H+ extrusion (41, 42). The simplest explanation for these earlier data are that the basolateral CO2 strongly enhanced acid-base transporters, overwhelming the inhibition imposed by the basolateral 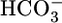 . Because adding
. Because adding 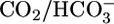 to the lumen fails to stimulate acid extrusion (41), we propose that CO2 stimulates a sensor at or near the basolateral membrane. In principle, the sensor could face the basolateral fluid and sense [CO2]BL per se, or the sensor could be inside the cell near its basolateral membrane and sense [CO2]i or [H2CO3]i.
to the lumen fails to stimulate acid extrusion (41), we propose that CO2 stimulates a sensor at or near the basolateral membrane. In principle, the sensor could face the basolateral fluid and sense [CO2]BL per se, or the sensor could be inside the cell near its basolateral membrane and sense [CO2]i or [H2CO3]i.
In summary, at least during the acute response to acid-base disturbances, the kidney appears to regulate whole-body acid-base balance not by monitoring blood pH per se but by monitoring the levels of the two major buffer components that determine pH: 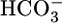 and CO2. Moreover, it appears that the
and CO2. Moreover, it appears that the 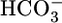 and CO2 sensors operate in a push–pull fashion to diminish JHCO3 and augment JOther in the case of
and CO2 sensors operate in a push–pull fashion to diminish JHCO3 and augment JOther in the case of 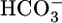 and to augment JHCO3 but diminish JOther in the case of CO2. The net effects are not only to trigger changes in JHCO3 that tend to restore blood pH but also to trigger compensatory alterations in the reabsorption of other solutes, thereby stabilizing JV. Understanding how the PT transduces the
and to augment JHCO3 but diminish JOther in the case of CO2. The net effects are not only to trigger changes in JHCO3 that tend to restore blood pH but also to trigger compensatory alterations in the reabsorption of other solutes, thereby stabilizing JV. Understanding how the PT transduces the 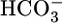 and CO2 signals may provide important clues for treating both acid-base disturbances and hypertension.
and CO2 signals may provide important clues for treating both acid-base disturbances and hypertension.
Supplementary Material
Acknowledgments
We thank Profs. E. Boulpaep and P. Aronson for valuable discussions and D. Wong for computer support. This work was funded by National Institutes of Health Grant PO1-DK17433. J.Z. was supported by a American Lung Association fellowship, and P.B. was supported by a National Kidney Foundation fellowship.
Author contributions: Y.Z., J.Z., P.B., and W.F.B. designed research; Y.Z. and J.Z. performed research; P.B. contributed new reagents/analytic tools; Y.Z., J.Z., P.B., and W.F.B. analyzed data; and Y.Z., P.B., and W.F.B. wrote the paper.
Abbreviations: BL, basolateral; JHCO3, rate of 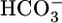 reabsorption; JV, rate of volume reabsorption; JOther, rate of reabsorption of other solutes; OOE, out-of-equilibrium; pHi, intracellular pH; PT, proximal tubule.
reabsorption; JV, rate of volume reabsorption; JOther, rate of reabsorption of other solutes; OOE, out-of-equilibrium; pHi, intracellular pH; PT, proximal tubule.
References
- 1.Karet, F. E., Gainza, F. J., Gyory, A. Z., Unwin, R. J., Wrong, O., Tanner, M. J. A., Nayir, A., Alpay, H., Santos, F., Hulton, S. A., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 6337-6342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karet, F. E., Finberg, K. E., Nelson, R. D., Nayir, A., Mocan, H., Sanjad, S. A., Rodriguez-Soriano, J., Santos, F., Cremers, C. W., di Pietro, A., et al. (1999) Nat. Genet. 21, 84-90. [DOI] [PubMed] [Google Scholar]
- 3.Alper, S. L. (2002) Annu. Rev. Physiol. 64, 899-923. [DOI] [PubMed] [Google Scholar]
- 4.Romero, M. F., Fulton, C. M. & Boron, W. F. (2004) Pflügers Arch. 447, 495-509. [DOI] [PubMed] [Google Scholar]
- 5.Sly, W. S. & Hu, P. Y. (1995) Annu. Rev. Biochem. 64, 375-401. [DOI] [PubMed] [Google Scholar]
- 6.Rocktaeschel, J., Morimatsu, H., Uchino, S., Goldsmith, D., Poustie, S., Story, D., Gutteridge, G. & Bellomo, R. (2003) Crit. Care 7, R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan, J. C. (1983) Adv. Pediatr. 30, 401-471. [PubMed] [Google Scholar]
- 8.Brazeau, P. & Gilman, A. (1953) Am. J. Physiol. 175, 33-38. [DOI] [PubMed] [Google Scholar]
- 9.Dorman, P. J., Sullivan, W. J. & Pitts, R. F. (1954) J. Clin. Invest. 33, 82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murer, H., Hopfer, U. & Kinne, R. (1976) Biochem. J. 154, 597-604. [PMC free article] [PubMed] [Google Scholar]
- 11.Bichara, M., Paillard, M., Leviel, F. & Gardin, J. (1980) Am. J. Physiol. 238, F445-F451. [DOI] [PubMed] [Google Scholar]
- 12.Bichara, M., Paillard, M., Leviel, F., Pringent, A. & Gardin, J. P. (1983) Am. J. Physiol. 244, F165-F171. [DOI] [PubMed] [Google Scholar]
- 13.Gluck, S. L., Underhill, D. M., Iyori, M., Holliday, L. S., Kostrominova, T. Y. & Lee, B. S. (1996) Annu. Rev. Physiol 58, 427-445. [DOI] [PubMed] [Google Scholar]
- 14.Boron, W. F. & Boulpaep, E. L. (1983) J. Gen. Physiol. 81, 53-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Romero, M. F., Hediger, M. A., Boulpaep, E. L. & Boron, W. F. (1997) Nature 387, 409-413. [DOI] [PubMed] [Google Scholar]
- 16.Zhao, J., Hogan, E. M., Bevensee, M. O. & Boron, W. F. (1995) Nature 374, 636-639. [DOI] [PubMed] [Google Scholar]
- 17.Zhao, J., Zhou, Y. & Boron, W. F. (2003) Am. J. Physiol. 285, F359-F369. [DOI] [PubMed] [Google Scholar]
- 18.Burg, M., Grantham, J., Abramow, M. & Orloff, J. (1966) Am. J. Physiol. 210, 1293-1298. [DOI] [PubMed] [Google Scholar]
- 19.Quigley, R. & Baum, M. (1991) J. Clin. Invest. 88, 368-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baum, M., Quigley, R. & Quan, A. (1997) Am. J. Physiol. 273, F595-F600. [DOI] [PubMed] [Google Scholar]
- 21.McKinney, T. D. & Burg, M. B. (1977) Kidney Int. 12, 1-8. [DOI] [PubMed] [Google Scholar]
- 22.Forrester, R. L., Wataji, L. J., Silverman, D. A. & Pierre, K. J. (1976) Clin. Chem. 22, 243-245. [PubMed] [Google Scholar]
- 23.Star, R. A. (1990) Am. J. Physiol. 258, F429-F432. [DOI] [PubMed] [Google Scholar]
- 24.Rink, T. J., Tsien, R. Y. & Pozzan, T. (1982) J. Cell Biol. 95, 189-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, J. A., Buchsbaum, R. N., Zimniak, A. & Racker, E. (1979) Biochemistry 18, 2210-2218. [DOI] [PubMed] [Google Scholar]
- 26.Boyarsky, G., Ganz, M. B., Sterzel, B. & Boron, W. F. (1988) Am. J. Physiol. 255, C844-C856. [DOI] [PubMed] [Google Scholar]
- 27.Roos, A. & Boron, W. F. (1981) Physiol. Rev. 61, 296-434. [DOI] [PubMed] [Google Scholar]
- 28.Lucci, M. S., Pucacco, L. R., Carter, N. W. & DuBose, T. D. J. (1982) Am. J. Physiol. 243, F335-F341. [DOI] [PubMed] [Google Scholar]
- 29.Malnic, G. (1980) Am. J. Physiol. 239, F307-F318. [DOI] [PubMed] [Google Scholar]
- 30.Buckler, K. J., Vaughan-Jones, R. D., Peers, C., Lagadic-Gossmann, D. & Nye, P. C. G. (1991) J. Physiol. (London) 444, 703-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Putnam, R. W. & Grubbs, R. D. (1990) Am. J. Physiol. 258, C461-C469. [DOI] [PubMed] [Google Scholar]
- 32.Ritucci, N. A., Chambers-Kersh, L., Dean, J. B. & Putnam, R. W. (1998) Am. J. Physiol. 275, R1152-R1163. [DOI] [PubMed] [Google Scholar]
- 33.Bouyer, P., Bradley, S. R., Zhao, J., Wang, W., Richerson, G. B. & Boron, W. F. (2004) J. Physiol. (London) 559, 85-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah, M., Quigley, R. & Baum, M. (1998) Am. J. Physiol. 43, F883-F888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schultheis, P. J., Clarke, L. L., Meneton, P., Miller, M. L., Soleimani, M., Gawenis, L. R., Riddle, T. M., Duffy, J. J., Doetschman, T., Wang, T., et al. (1998) Nat. Genet. 19, 282-285. [DOI] [PubMed] [Google Scholar]
- 36.Putnam, R. W., Filosa, J. A. & Ritucci, N. A. (2004) Am. J. Physiol. 287, C1493-C1526. [DOI] [PubMed] [Google Scholar]
- 37.Olsnes, S., Tonnessen, T. I. & Sandvig, K. (1986) J. Cell Biol. 102, 967-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simchowitz, L. & Roos, A. (1985) J. Gen. Physiol. 85, 443-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaughan-Jones, R. D. (1982) in Intracellular pH: Its Measurement, Regulation and Utilization in Cellular Functions, ed. Nuccitelli, R. (Liss, New York), pp. 239-252.
- 40.Boyarsky, G., Ganz, M. B., Sterzel, B. & Boron, W. F. (1988) Am. J. Physiol. 255, C857-C869. [DOI] [PubMed] [Google Scholar]
- 41.Chen, L. K. & Boron, W. F. (1995) Am. J. Physiol. 268, F193-F203. [DOI] [PubMed] [Google Scholar]
- 42.Chen, L. K. & Boron, W. F. (1995) Am. J. Physiol. 268, F179-F192. [DOI] [PubMed] [Google Scholar]
- 43.Sasaki, S., Berry, C. A. & Rector, F. C., Jr. (1982) J. Clin. Invest. 70, 639-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu, F. Y. & Cogan, M. G. (1987) Am. J. Physiol. 253, F89-F94. [DOI] [PubMed] [Google Scholar]
- 45.Horie, S., Moe, O., Tejedor, A. & Alpern, R. J. (1990) Proc. Natl. Acad. Sci. USA 87, 4742-4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamaji, Y., Amemiya, M., Cano, A., Preisig, P. A., Miller, R. T., Moe, O. W. & Alpern, R. J. (1995) Proc. Natl. Acad. Sci. USA 92, 6274-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.