Abstract
Congenital anomalies of the kidney and urinary tract (CAKUT) are the most common cause of CKD in the first three decades of life. However, for most patients with CAKUT, the causative mutation remains unknown. We identified a kindred with an autosomal dominant form of CAKUT. By whole-exome sequencing, we identified a heterozygous truncating mutation (c.279delG, p.Trp93fs*) of the nuclear receptor interacting protein 1 gene (NRIP1) in all seven affected members. NRIP1 encodes a nuclear receptor transcriptional cofactor that directly interacts with the retinoic acid receptors (RARs) to modulate retinoic acid transcriptional activity. Unlike wild-type NRIP1, the altered NRIP1 protein did not translocate to the nucleus, did not interact with RARα, and failed to inhibit retinoic acid–dependent transcriptional activity upon expression in HEK293 cells. Notably, we also showed that treatment with retinoic acid enhanced NRIP1 binding to RARα. RNA in situ hybridization confirmed Nrip1 expression in the developing urogenital system of the mouse. In explant cultures of embryonic kidney rudiments, retinoic acid stimulated Nrip1 expression, whereas a pan-RAR antagonist strongly reduced it. Furthermore, mice heterozygous for a null allele of Nrip1 showed a CAKUT-spectrum phenotype. Finally, expression and knockdown experiments in Xenopus laevis confirmed an evolutionarily conserved role for NRIP1 in renal development. These data indicate that dominant NRIP1 mutations can cause CAKUT by interference with retinoic acid transcriptional signaling, shedding light on the well documented association between abnormal vitamin A levels and renal malformations in humans, and suggest a possible gene-environment pathomechanism in this disease.
Keywords: CAKUT, NRIP1, retinoic acid
Congenital anomalies of the kidney and urinary tract (CAKUT) constitute the most frequent cause of CKD in the first three decades of life, accounting for approximately 50% of all patients.1,2 CAKUT comprises a wide range of structural malformations that result from defects in the morphogenesis of the kidneys and/or the urinary tract.3–6 The pathologic basis of CAKUT lies in the disturbance of normal kidney development, primarily resulting from mutations in genes that direct this process. Most gene products that cause CAKUT in humans or mice if altered are transcription factors involved in protein-protein interactions that form large transcription complexes.7–13 These monogenic forms of CAKUT are often inherited in an autosomal dominant manner and exhibit the clinical features of incomplete penetrance and variable expressivity. Loose genotype/phenotype correlations, as well as extensive genetic locus heterogeneity, have rendered gene discovery in CAKUT very difficult. Although approximately 30 CAKUT genes have been identified,1,2,14–16 approximately 85% of patients with CAKUT do not have mutations in any known genes.17,18 Hence, the remaining challenge is to identify the missing components of the pathogenesis of CAKUT, in order to better understand how these proteins and transcription factor complexes exert their developmental and tissue-specific functions.
In addition to the regulatory roles of genetic factors for the proper development of the kidney and urinary tract, numerous studies also support the influence of environmental factors on normal and abnormal kidney development.4,19 This suggests that the pathogenesis of CAKUT can be multifactorial, likely due to a complex interplay between genes and the environment. The most prominent example of this is the effect of retinoic acid (RA), the active form of vitamin A, on the kidneys and urinary tract during development. During fetal life, nephrogenesis is influenced by RA levels, such that even modest fluctuations in maternal vitamin A levels in either direction can cause CAKUT in humans and rodents.20,21 In addition, elegant studies of murine kidney development by Mendelsohn et al. have shown that inactivation of genes in the RA pathway causes CAKUT in mice.22–24
To gain further insight into the pathogenesis of CAKUT, we investigated a three-generation family with renal malformations by using whole-exome sequencing (WES). Here we identify a dominant mutation in the transcriptional cofactor NRIP1 (nuclear receptor interacting protein 1) gene as causing human autosomal dominant CAKUT by interference with RA transcriptional signaling, thereby shedding light on the well documented association between RA and renal malformations.
Results
Mutations in NRIP1 Can Cause CAKUT
To identify a causative mutated gene for CAKUT, we investigated a three-generation Yemenite Jewish family with seven individuals who have CAKUT. Renal hypo/dysplasia was the predominant phenotype (six individuals), together with vesicoureteral reflux (VUR) (four individuals) and/or ectopia (two individuals) (Figure 1, A and B, Supplemental Figure 1, Table 1). The age at diagnosis ranged from the prenatal period to late adulthood (Table 1). Two of the seven affected individuals underwent unilateral nephrectomy for their malformations. None of the affected individuals had extrarenal malformations or syndromic features. The pedigree structure was compatible with an autosomal dominant mode of inheritance with variable expressivity and incomplete penetrance (Figure 1A). Individual II:1 had a renal ultrasound showing bilateral small renal cysts (Supplemental Figure 1), which can be a common finding in his age group and is not necessarily related to the CAKUT phenotype in this family. Therefore, individual II:1 was not included in the initial WES analysis, which considered only affected family members.
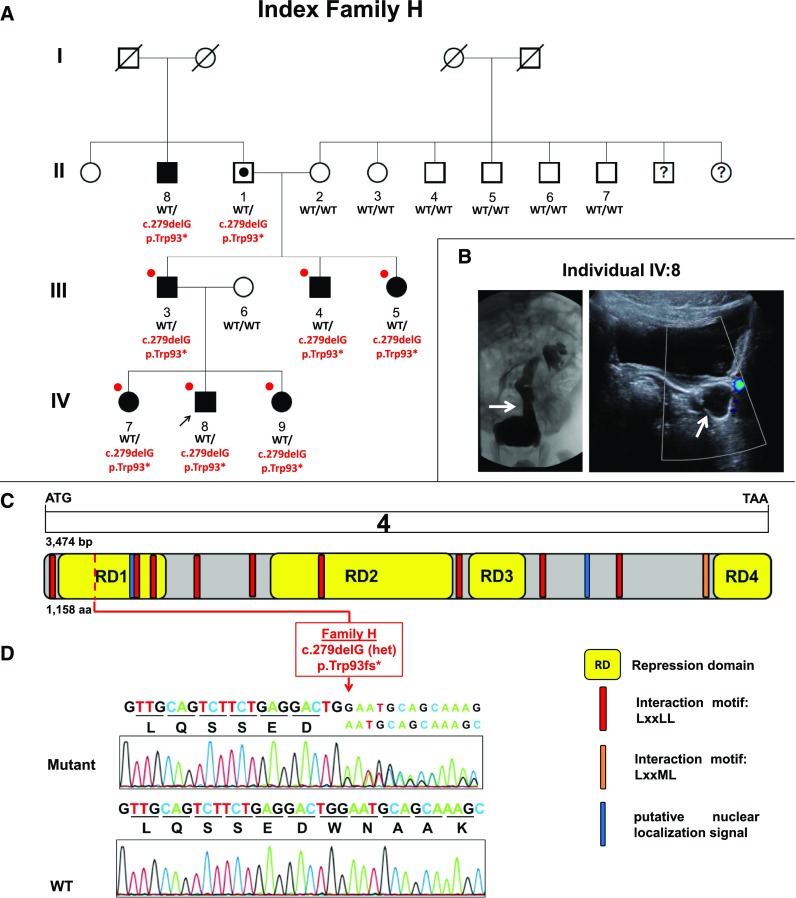
Figure 1.
Identification of a heterozygous NRIP1 truncating mutation in index family H with CAKUT. (A) Shows the pedigree of index family H. Squares represent males, circles females, black symbols affected persons, white symbols unaffected persons, and white symbol with black dot denotes an obligatory mutation carrier. White symbols with a black question mark indicate individuals with an unknown phenotype. Pedigree is compatible with an autosomal dominant mode of inheritance with incomplete penetrance and variable expressivity. Roman numerals denote generations. Individuals are numbered with Arabic numerals if DNA was tested in the study. The arrow points to the proband IV:8. WT denotes the wild-type allele. p.Trp93* indicates the mutation c.279 deletion of G in NRIP1, leading to a frame-shift mutation and introducing a premature stop codon. The mutation fully segregated heterozygously (WT/p.Trp93*) across all seven affected individuals examined and was absent from seven unaffected family members (WT/WT). Individual II:1 is an obligatory mutation carrier given the pedigree structure and segregation analysis. This patient was considered as having an inconclusive phenotype with only two renal cysts. Red circles indicate the persons selected for WES analysis. (B) Shows voiding cystourethrogram (left panel) and renal ultrasound (right panel) of the index individual IV:8, revealing severe grade five left VUR and hydroureter (white arrows, respectively). He presented during infancy after positive prenatal ultrasound screening. He is managed expectantly and has no extrarenal manifestations. (C) Shows exon structure of human NRIP1 cDNA and domain structure of the NRIP1 protein. NRIP1 contains two putative nuclear localization signals (blue), four transcriptional repression domains (RD, yellow), and 10 interaction motifs LxxLL/LxxML (red and orange). Start codon (ATG) and stop codon (TAA) are indicated. (D) Shows chromatograms of the heterozygous mutation and WT sequence detected in NRIP1 (in relation to exons and protein domains in [C] in index family H [red] with CAKUT). The index family’s heterozygous NRIP1 mutation c.279delG leads to a frameshift and premature stop codon resulting in p.Trp93fs*.
Table 1.
Dominant NRIP1 mutation detected in individuals with CAKUTa
| Family – Individuala,b,c | Presenting Symptoms or Diagnostic Test (at Age in yr) | Kidney Phenotype | Treatment | Serum Creatinine, mg/dl (at Age in yr) |
|---|---|---|---|---|
| H | ||||
| II:8 | Renal US (69) | R small pelvic kidney with hydronephrosis | Conservative | 0.93 (69) |
| III:3 | Abdominal mass (newborn) | L MCDK; R VUR, dilated ureter, and dysplasia | L nephrectomy | 1.5 (35) |
| R nephrostomy | ||||
| III:4 | Abdominal pain (7) | L dysplastic kidney and VUR | L nephrectomy | 0.75 (27) |
| III:5 | Renal US and VCUG (3) | Bilateral grade 2 VUR | Conservative | 0.64 (28) |
| IV:7 | Renal US (2) | Small right kidney | Conservative | 0.23 (5) |
| IV:8 | Hydronephrosis on renal US (prenatal) | L VUR grade 5 and dysplasia; R VUR grade 2 | Conservative | 0.45 (8) |
| IV:9 | Renal US (prenatal) | L ectopic dysplastic kidney | Conservative | 0.23 (3) |
Co-IP, coimmunoprecipitation; k.d., knock-down; US, ultrasound; R, right; L, left; MCDK, multicystic dysplastic kidney; VCUG, voiding cystourethrogram.
All individuals were found to harbor a c.279delG mutation in NRIP1. The cDNA mutation is numbered according to human cDNA reference sequence NM_003489.3, isoform (NRIP1), where +1 corresponds to the A of ATG start translation.
The c.279delG NRIP1 mutation corresponds to the following alteration in the coding sequence: p.Trp93fs*. This mutation was absent from 200 control individuals with renal ciliopathies, 429 individuals with steroid-resistant nephrotic syndrome, from approximately 13,000 healthy control alleles of the EVS (http://evs.gs.washington.edu/EVS), from 2577 control individuals of the “1000 genomes project” (http://www.1000genomes.org), and from approximately 100,000 control chromosomes of the ExAC server (http://exac.broadinstitute.org). In addition, NRIP1 had an ExAC: pLI (probability of being loss-of-function intolerant) score of 0.99. The closer the pLI is to 1, the more loss-of-function-intolerant the gene appears to be. Genes with pLI≥0.9 are considered as an extremely loss-of-function-intolerant set of genes.
Loss of function of the NRIP1 c.279delG allele was demonstrated in four assays: nuclear localization (Figures 2A and 2B), luciferase assay (Figure 2C), co-IP with RARα (Figure 3A), or Xenopus knockdown and rescue experiments (Figure 5, D–K).
Under the hypothesis that a mutation of an autosomal dominant gene causes CAKUT in this family, we selected six affected family members (individuals III:3, III:4, III:5, IV:7, IV:8, and IV:9) for WES. Given the pedigree structure (Figure 1A), the six affected individuals are expected to share about 3.125% of all alleles by descent, allowing a 32-fold reduction in the thousands of variants from the normal reference sequence that are expected to result from WES (Supplemental Table 1). We identified a novel heterozygous truncating mutation (c.279delG, p.Trp93fs*) in the gene encoding NRIP1 (MIM 602490, RefSeq accession number NM_003489.3) (Figure 1, C and D, Table 1). Segregation analysis revealed that this mutation was shared by all seven available affected family members as well as by individual II:1, who, as expected from the pedigree structure, is an obligatory mutation carrier given that his brother (individual II:8) was found to have CAKUT on renal ultrasound and to harbor the NRIP1 mutation (Figure 1A, Supplemental Figure 1, Table 1). In addition, the mutation was absent from seven unaffected family members, from all available databases of healthy controls including approximately 100,000 alleles in the ExAC database (Table 1), as well as from two in-house control cohorts of 200 individuals with nephronophthisis and 429 individuals with nephrotic syndrome. Finally, we showed by copy number variant (CNV) analysis that the affected index patient (IV:8) lacked any heterozygous deletions or duplications that have previously been associated with CAKUT, including the HNF1B locus and the DiGeorge/velocardiofacial syndrome locus.25 Because Yemenite Jews are a genetically distinct Jewish subcluster,26 we aimed to determine the frequency of the NRIP1 mutation in 236 anonymized samples of ethnically matched Yemenite Jews whose disease status is unknown. The NRIP1 c.279delG mutation was found in two individuals out of 236 screened (472 alleles) yielding a minor allele frequency (MAF) of 0.004. We next performed highly parallel exon sequencing of NRIP1 in 155 sporadic cases and 253 familial cases of CAKUT (total of 724 affected individuals), but did not identify additional families with CAKUT and novel NRIP1 variants.
The NRIP1 Mutation Interferes with Nuclear Translocation, Transcriptional Repression, and NRIP1 Interaction with Retinoic Acid Receptor α
NRIP1 is a nuclear receptor transcriptional cofactor.27 Previous biochemical analyses showed that the murine NRIP1 protein harbors two putative nuclear localization signals and four repression domains that mediate its transcriptional repression27 (Figure 1C). In addition, NRIP1 has been shown to directly interact with RA receptors (RARs) and retinoid receptors (RXRs) to suppress their RA-mediated transcriptional activity.28 The NRIP1 protein contains nine nuclear receptor interacting motifs (LxxLL) spread throughout the molecule (Figure 1C).27 Interestingly, the binding of NRIP1 to RARs and RXRs was suggested to also require a slightly different sequence, an LxxML motif, located at the protein’s C-terminus.29
To determine how the c.279delG (p.Trp93fs*) mutation, which segregated with the CAKUT phenotype in the large family H, conveys NRIP1 loss of function, we evaluated NRIP1 for its known functional features. These include: (1) nuclear localization, (2) transcriptional repression, and (3) interaction with retinoic acid receptor α (RARα). Transfection of expression constructs in HEK293 cells revealed that, whereas the wild-type NRIP1 protein translocated into the nucleus, the NRIP1-altered protein (p.Trp93fs*) remained localized in the cytoplasm (Figure 2, A and B). In target cells, RA, the active form of vitamin A, acts as a ligand for nuclear RARs and RXRs. The complex binds to a regulatory DNA segment, the RA response element (RARE), to control transcription of RA target genes.30 Accordingly, we next determined whether the NRIP1 mutation affects RA-dependent transcriptional activity using NRIP1 expression plasmids and a reporter plasmid harboring an RARE. Although the wild-type NRIP1 expression construct acted as a transcriptional repressor, and completely suppressed RA-mediated transcriptional activity, the mutant construct (p.Trp93fs*) showed lack of transcriptional repressor activity (Figure 2C).
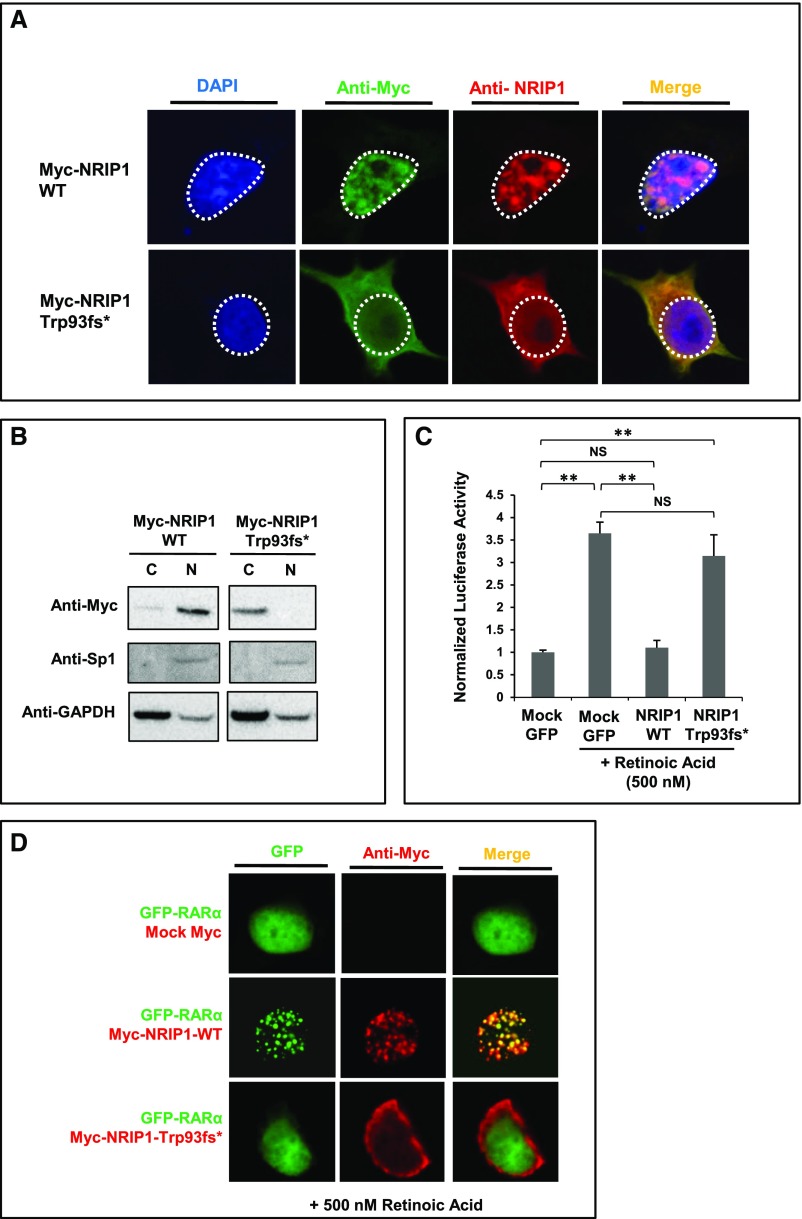
Figure 2.
NRIP1 p.Trp93fs* fails to translocate to the nucleus, does not suppress RA-induced transcriptional activation, and fails to interact with RARα. (A) Immunofluorescence staining of HEK293 cells transfected with Myc-tagged wild-type (WT) NRIP1 cDNA and Myc-tagged NRIP1 c.279delG cDNA. WT NRIP1 localizes to the nucleus, whereas the altered NRIP1 protein (p.Trp93fs*) predominantly localizes to the cytoplasm. (B) Western blot of cytoplasmic (C) and nuclear (N) extracts from transfected HEK293 cells shows localization of WT NRIP1 predominantly in the nucleus and of NRIP1 p.Trp93fs* in the cytoplasm. Sp1 and GAPDH were used as nuclear and cytoplasmic markers, respectively. (C) Luciferase assay of HEK293 cells transfected with Mock GFP, GFP-tagged WT NRIP1 cDNA, and GFP-tagged NRIP1 c.279delG mutant cDNA and subsequently treated with RA. WT NRIP1 suppresses RA-induced transcriptional activity, whereas the mutant form fails to do so. (D) Immunofluorescence staining of HEK293 cells cotransfected with GFP-tagged RARα and either Mock Myc, Myc-tagged WT NRIP1, or Myc-tagged NRIP1 c.279delG. RARα and WT NRIP1 colocalize in the nucleus (speckled pattern), whereas the Trp93fs* altered protein remains in the cytoplasm and fails to produce a speckled nuclear pattern together with RARα. **P<0.01.
We previously showed that monogenic CAKUT can be secondary to a dominant-negative pathomechanism.14 To test this possibility, we cotransfected the NRIP1 wild-type construct with increasing amounts of mutant NRIP1, and subsequently performed luciferase reporter assays. This did not yield a dose-dependent lack of repression, suggesting that the loss of function caused by the mutation is not mediated via a dominant-negative mechanism (Supplemental Figure 2A). To further support this, we showed that wild-type and mutant NRIP1 proteins did not colocalize when cotransfected, suggesting that they do not dimerize (Supplemental Figure 2B). Furthermore, because proteins that hetero-dimerize are particularly prone, when altered, to exerting dominant-negative effects by sequestering functioning molecules into inactive dimers, we tested the interaction between the altered NRIP1 protein and RARα. Notably, the altered NRIP1 protein still contains one intact interaction motif (Figure 1C), which may theoretically be sufficient to interact with the nuclear receptor ligand–binding domain of RARα. We performed overexpression experiments of RARα with either wild-type or altered NRIP1 protein. Interestingly, RARα, when overexpressed with the wild-type NRIP1 protein, colocalized in a speckled nuclear pattern. When overexpressed with the altered NRIP1 protein (p.Trp93fs*), however, RARα localized diffusely in the nucleus whereas the altered NRIP1 remained in the cytoplasm (Figure 2D). Furthermore, coimmunoprecipitation experiments showed that the NRIP1 mutation abrogates NRIP1-RARα interaction and that binding of NRIP1 to RARα is enhanced by RA treatment (Figure 3, A and B). Taken together, these data argue against a dominant-negative pathomechanism and render a dominant mechanism of haploinsufficiency more likely.
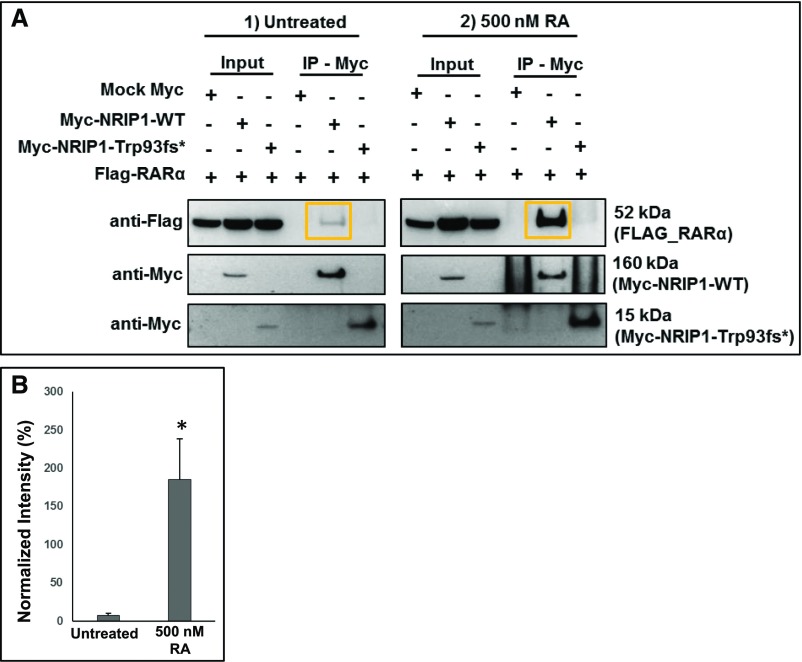
Figure 3.
RA increases NRIP1 binding to RARα in vitro. (A) Coimmunoprecipitation of protein lysates of wild type (WT) NRIP1 and RARα overexpressed in HEK293 cells. Note that the Trp93fs* mutation abrogates binding to RARα. Treatment with RA increases WT NRIP1 binding to RARα (orange boxes). (B) Quantification of data shown in (A). RARα coimmunoprecipitation band intensity was normalized to WT NRIP1 immunoprecipitation band intensity in the absence and presence of RA. *P<0.05, average of three separate experiments.
Nrip1 mRNA Expression in the Nephric Duct and Ureteric Epithelium Depends on RA
Formation of the ureter and kidney begins with ureter budding, during which the epithelial tube sprouts from the base of the Wolffian ducts immediately above the urogenital sinus—an embryonic structure that eventually gives rise to the urinary bladder. Distally, the ureteric bud invades the renal metanephric mesenchyme and, after successive branching, gives rise to the renal collecting-duct system. The ureteric bud stalk differentiates into the ureter, and its proximal part is transposed to the primitive bladder via the common nephric duct. Elegant studies have shown that the common nephric duct undergoes apoptosis, thereby severing connections with the Wolffian duct as the ureter orifice is transposed to its final insertion site in the bladder ureter.24 This crucial step is controlled by RA-induced signals, which govern ureter maturation and formation of proper connections between the bladder and ureter.24
To further characterize the role of NRIP1 in RA signaling, we determined whether the mouse ortholog Nrip1 is expressed in RA-dependent tissues in the development of the murine urogenital system. For this, we performed RNA in situ hybridization on whole-mount preparations of urogenital systems of E11.5–E18.5 mouse embryos and on sections of the urogenital system at the same stages (Figure 4A). Elevated levels of Nrip1 transcripts were found in the nephric duct and ureter from E11.5 to E14.5, as well as in the collecting ducts at E14.5 (Figure 4A). Expression in the kidney and ureter was abolished at E18.5 (Supplemental Figure 3). To test whether Nrip1 expression in the nephric duct and its derivatives depends on RA, we explanted E11.5 kidney rudiments and cultured them in the presence of 1 µM RA or 1 µM of the pan-RAR antagonist BMS493. Although BMS493 treatment strongly reduced Nrip1 expression in the nephric duct and ureter epithelium, RA increased Nrip1 expression in these tissues slightly (Figure 4B). Thus, Nrip1 represents a novel RA-responsive gene in the genitourinary system.
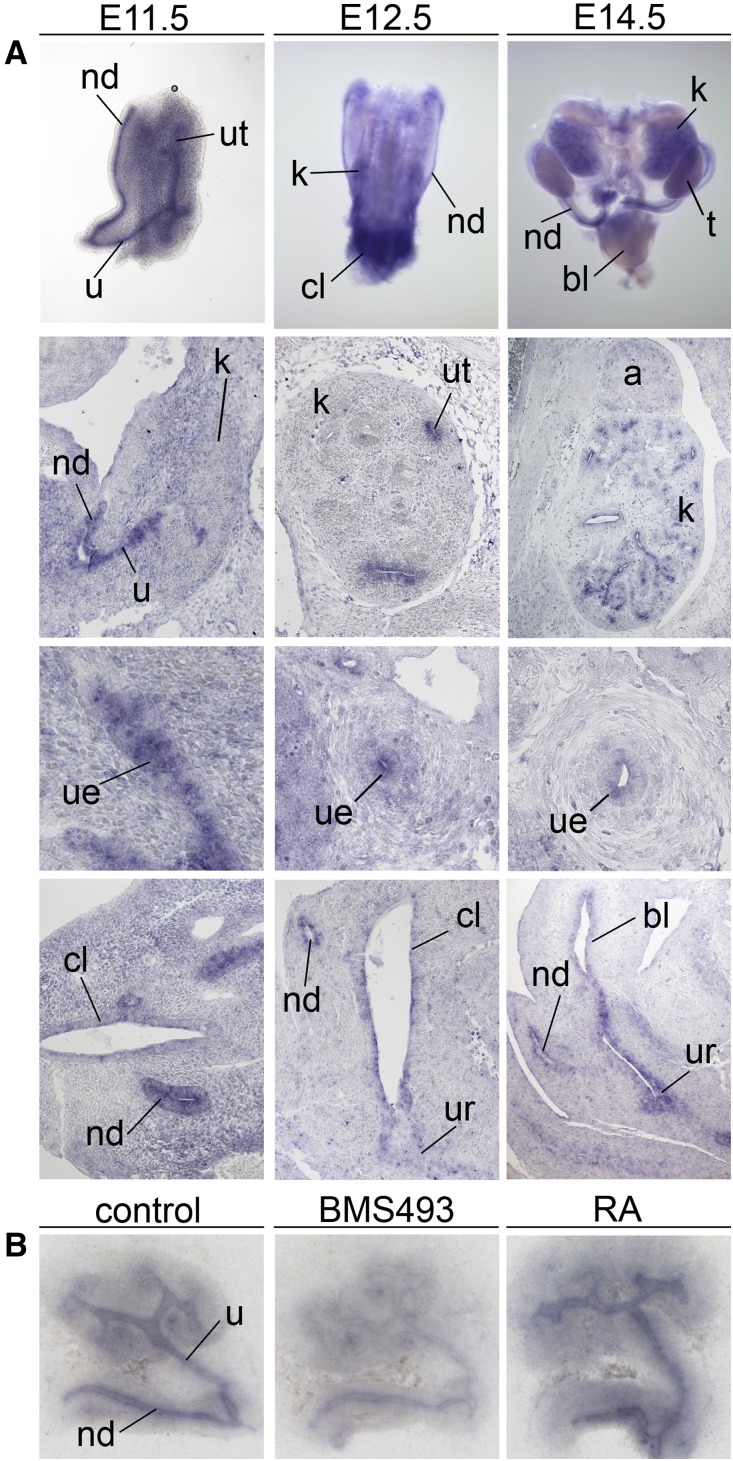
Figure 4.
Nrip1 is a target of RA in the nephric duct and ureter epithelium. (A) In situ hybridization analysis of expression of Nrip1 in whole urogenital systems (first row), on coronal kidney sections (second row), on transverse ureter sections (third row), and on cloaca/bladder sections (fourth row) of wild-type embryos at E11.5, E12.5, and E14.5. Nrip1 is expressed in the epithelium of the cloaca, the nephric duct, and the ureter from E11.5 to E14.5. a, adrenal gland; bl, bladder; cl, cloaca; k, kidney; nd, nephric duct; t, testis; u, ureter; ue, ureteric epithelium; ur, urethra; ut, ureteric tip. (B) In situ hybridization analysis of Nrip1 expression in kidney rudiments dissected from E11.5 wild-type embryos and cultured for 24 hours in the presence of 1 µM of BMS493 or 1 µM RA. Nrip1 mRNA expression in the nephric duct and the ureteric tree is downregulated after treatment with the pan-RAR antagonist BMS493, and upregulated after treatment with RA.
Functional In Vivo Studies in Xenopus laevis Show a Major Role for NRIP1 during Renal Development Which Is Abolished with the Mutant NRIP1
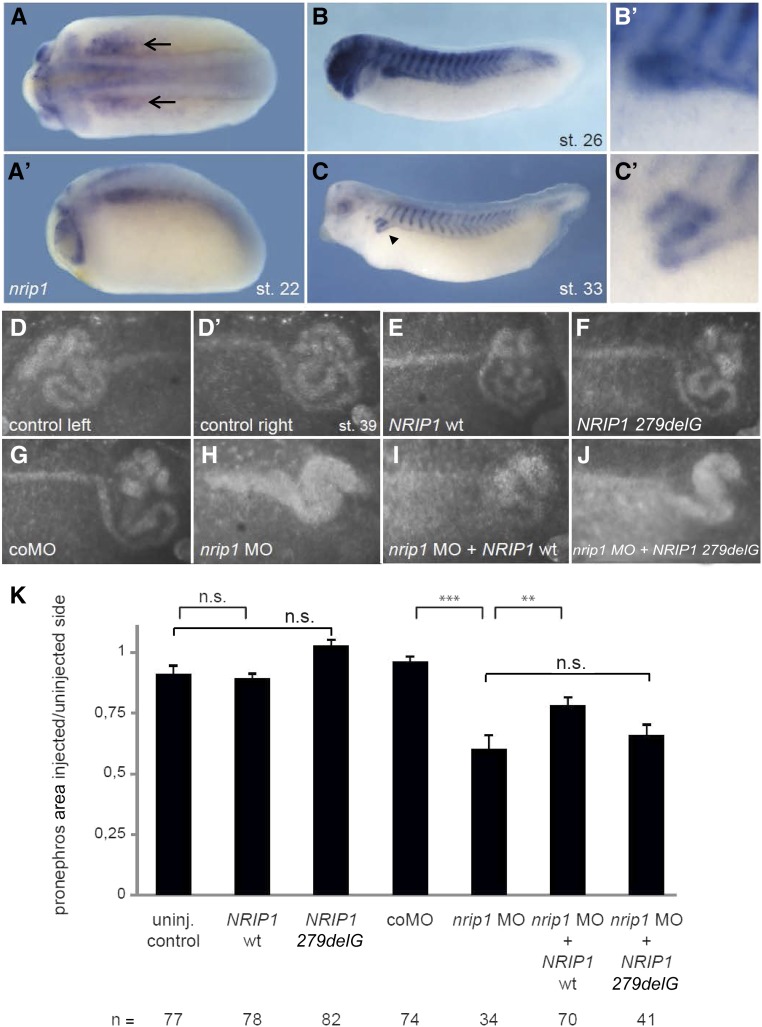
Expression and knockdown experiments in X. laevis further confirmed a role for NRIP1 in renal development. Because unilateral injections allow a tissue-restricted knockdown and analysis of organ-specific phenotypes, we turned to the Xenopus model to analyze the developmental events in renal formation in further detail.31 Nrip1 is expressed during Xenopus development at neurula and tadpole stages. Strong expression was detected in the condensing pronephric tubules and somites (Figure 5, A–C′). Neither overexpression of the wild type NRIP1 nor the altered protein significantly interfered with pronephric development, again arguing against a a dominant-negative effect of the mutant construct (Figure 5, E, F, and K). Knockdown with a translation-blocking nrip1 MO inhibited renal development and resulted in distorted pronephric structures (Figure 5, H and K; P<0.001). This could partially be rescued with the wild-type NRIP1 mRNA (Figure 5, I and K; P=0.01) but not with the mutant (NRIP1 279delG) mRNA (Figure 5, J and K; P=0.43).
Figure 5.
Nrip1 deficiency affects pronephros development in Xenopus. (A–C) WM-ISH of nrip1 expression in X. laevis shows its occurrence in the pronephros during development. Expression starts in the pronephric anlage at stage 22 (A, A’) (black arrows) and is present in the tubules of stage 26 (B, B’) and stage 33 tadpoles (C, C’) (black arrow head). (D–K) Functional analysis of nrip1 in X. laevis. Embryos were unilaterally injected with nrip1 MO and/or NRIP1-encoding mRNA at the four-cell stage. Stage 39 tadpoles were stained with fluorescein-conjugated lectin to visualize the pronephros (D–J). The size of the pronephros was measured and the proportion between the injected (right) and uninjected (left) side calculated (K). The structure of the pronephros was not changed by microinjections of the NRIP1 wt (E/K) mRNA or its truncated variant (NRIP1 279delG; [F]) compared with the controls (D’/K). Knockdown with a translation-blocking nrip1 MO inhibited pronephros development (H/K; P<0.001) and could be partially rescued with NRIP1 wt (I/K; P=0.01) but not with NRIP1 G279 del (J/K; P=0.43). Error bars represent SEM. **P<0.01, ***P<0.001 (MWU-test). coMO, control morpholino oligonucleotide; del, deletion; MO, antisense morpholino oligonucleotide; st., stage according to Nieuwkoop and Faber50; wt, wild-type.
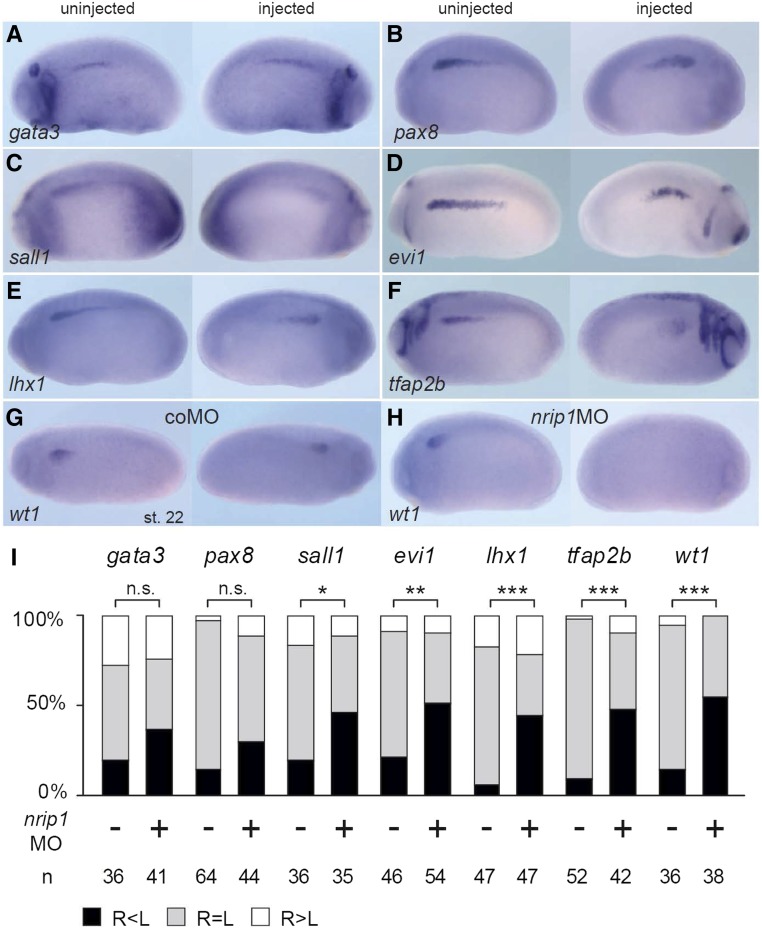
Further analysis of early pronephros markers after nrip1 MO injection resulted in significantly reduced expression of transcription factors implicated in pronephros specification and patterning, such as wt1, lhx1, tfap2b, sall1, and evi1 (Figure 6). No significant difference was observed for gata3 or pax8. We conclude that nrip1 is necessary for early specification events in pronephros formation in Xenopus. Taken together, these data support a role for nrip1 during early tubular morphogenesis, and are consistent with the human CAKUT phenotype.
Figure 6.
Nrip1 deficiency affects early pronephros marker gene expression in Xenopus embryos. (A–H) Pronephros marker gene analysis of Nrip1-depleted embryos at stage 22. Embryos were injected with nrip1 MO (+) or control MO (−) at the four-cell stage targeting their right sided pronephros. An in situ hybridization was performed for early pronephros markers (gata3, pax8, sall1, emx1, evi1, lhx1, tfap2b, and wt1) followed by the examination of their expression intensity on the injected (right) side compared with the uninjected side and quantified as shown in (I) (R, right side, injected; L, left side, uninjected). The expression of gata3 was not affected (P=0.16 z-test). The expression of pax8 (P=0.09) was slightly, but not significantly, reduced. Significant differences were observed for sall1 ([C]; P=0.035 z-test), evi1 ([D]; P=0.004), lhx1, tfap2b, and wt1 ([E–H]; P<0.001). n, number of embryos analyzed. *p<0.05; **p<0.01; ***p<0.001. coMO, control morpholino oligonucleotide; MO, antisense morpholino oligonucleotide; n.s., not significant.
CAKUT Phenotype in Nrip1+/− Mice
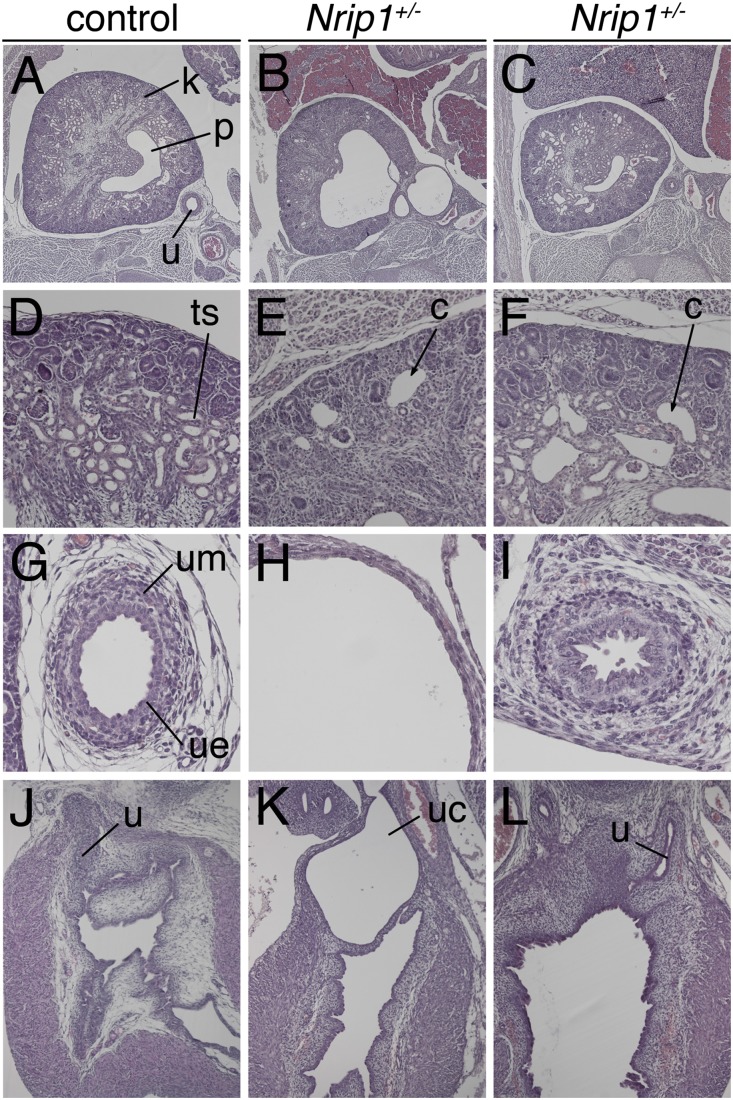
Previous work reported that mice homozygous for a null allele of Nrip1 exhibit defective ovulation in females as well as reduced body weight and body fat content.32 The genitourinary system was not yet analyzed for defects. To uncover a possible involvement of Nrip1 in the development of the murine urinary tract, we analyzed mice heterozygous for a null allele of Nrip1 at E18.5 for phenotypic changes. In all three specimens analyzed we detected dysplastic kidneys with cystic dilations. One of the specimens exhibited severe hydoureter with hydronephrosis and ureterocele (Figure 7). Hence, heterozygous loss of Nrip1 results in a spectrum of CAKUT phenotypes in mice similar to the human situation.
Figure 7.
Mice heterozygous for an Nrip1 null allele exhibit a spectrum of CAKUT phenotypes at E18.5. (A–L) Hematoxylin and eosin stainings of transverse sections of kidney and the ureter (A–C), kidney cortex (D–F), proximal ureter (G–I), and of sagittal sections of the embryonic bladder (J–L) from Nrip1+/− E18.5 embryos. One of three Nrip1+/− embryos analyzed exhibits a strong hydroureter, hydronephrosis, and ureterocele (middle panel). In two more specimens, kidneys are dysplastic and have microcystic dilations (right panel). c, cysts; k, kidney; p, pelvis; ts, tubular structures of the kidney; u, ureter; uc, ureterocele; ue, ureteric epithelium; um, ureteric mesenchyme.
Discussion
We identified a truncating mutation of NRIP1 as a novel autosomal dominant cause of human CAKUT. By studying NRIP1 cellular localization, transcriptional activation, and protein-protein interaction, we demonstrate loss of function for a truncating NRIP1 mutation (c.279delG; p.Trp93fs*) that segregated in a large CAKUT pedigree. We show that this mutation acts via haploinsufficiency rather than in a dominant-negative manner. By expression and knockdown experiments in X. laevis, as well as by analyzing the genitourinary system in mice with Nrip1 heterozygous deletions, we generated additional data that suggest that dysregulation of NRIP1-dependent RA signaling during kidney and ureter morphogenesis causes CAKUT.
NRIP1, also known as receptor-interacting protein 140 (RIP140), is a transcriptional coregulator that plays an integral role in fine-tuning the activity of a large number of transcription factors during development.27 NRIP1 has been mainly implicated in transcriptional repression by interacting with different nuclear receptors, including the RARs.28,29,33,34 Nonetheless, the role of NRIP1 during kidney and ureter development, as well as its relation to RA signaling, has remained unclear. In this study, our finding of a germline NRIP1 mutation as a novel cause of human CAKUT provides a link between the well documented association of vitamin A/RA and renal malformations.20,21,24
RA is a signaling molecule that is crucial in the development of many organs.35 In human21,36 and rodent20,37 embryos, both excess and deficiency (which was shown mainly in animal models) of RA can cause CAKUT. Classic mouse-model studies have highlighted the importance of RA signaling for proper ureter maturation and ureter-bladder connectivity.22–24 Perturbation of this process in mice can result in hypo/dysplastic kidneys as well as in backflow of urine to the ureters, clinically known as VUR. Interestingly, renal dysplasia and VUR were both the predominant phenotypes in the kindred we have studied (Figure 1, Supplemental Figure 1, Table 1). Furthermore, we detected a ureterocele in one Nrip1+/− embryo, pointing to a role of NRIP1 in regulating RA signaling during ureter maturation. Our results indicate that NRIP1 inhibits RA-induced transcription. Because Nrip1 is a target of RA signaling in the nephric duct and ureter, NRIP1 most likely serves as a feedback inhibitor for this pathway in ureter development. We postulate that loss of this feedback inhibition results in an excess of RA and RA signaling, leading to congenital renal and urinary tract malformations. A negative-feedback regulatory mechanism for NRIP1 is also supported by previous reports.38
NRIP1 mutations have never been implicated before in human disease. Furthermore, early NRIP1 truncating mutations are absent from all available WES databases, supporting intolerance for this gene for loss of function (see ExAC: pLI score of 0.99 [i.e., probability of being loss-of-function intolerant]). This is consistent with our finding that the early truncating NRIP1 mutation, which we identified, results in a pathomechanism of haploinsufficiency rather than a dominant-negative effect. The fact that we did not find a second CAKUT family with NRIP1 mutations supports the general notion that CAKUT is probably caused by a very large number of different and extremely rare disease-causing mutations in large numbers of different genes.1,17
Our study provides several insights into the complex genetic basis of CAKUT.4 In addition to the genetic heterogeneity, low penetrance mutations, and variable expressivity already described in monogenic CAKUT, our results emphasize possible gene-environment interactions as an additional level of complexity in the pathogenesis of human CAKUT and provide insight into potential therapeutic targets.
The loss-of-function mutation we identified in the NRIP1 gene may cause developmental renal abnormalities in humans and could indicate that NRIP1 is an important player in the development of the kidneys and urinary tract. Any generalizations regarding its direct causal role, however, must await the description and characterization of mutations in additional patients.
Concise Methods
Study Participants
After informed consent, we obtained clinical data, pedigree data, and blood samples from individuals with CAKUT from worldwide sources using a standardized questionnaire. Approval for human subjects’ research was obtained from the Institutional Review Boards of the University of Michigan, Boston Children’s Hospital, Sheba Medical Center, and from other relevant local Ethics Review Boards. Informed consent was obtained from the individuals and/or parents, as appropriate. The diagnosis of CAKUT was made by (pediatric) nephrologists and/or urologists on the basis of relevant imaging.
WES
To identify a causative mutated gene for CAKUT, we investigated family members from a three-generation Yemenite Jewish family (Figure 1) with an autosomal dominant form of CAKUT characterized predominantly by lower urinary tract involvement, renal hypodysplasia, and/or ectopia (Table 1). DNA samples from six affected individuals (Figure 1) were subjected to WES using Agilent SureSelect human exome capture arrays (Life Technologies) with next-generation sequencing on an Illumina sequencing platform. Sequence reads were mapped against the human reference genome (NCBI build 37/hg19) using CLC Genomics Workbench (version 6.5.1) software (CLC bio). Mutation calling under an autosomal dominant model was performed by geneticists and cell biologists, who had knowledge regarding clinical phenotypes, pedigree structure, genetic mapping, and WES evaluation (Supplemental Table 1), and in line with proposed guidelines.39,40 Sequence variants that remained after the WES evaluation process were examined for segregation.
WES Analysis
After WES, genetic variants were first filtered to retain only heterozygous, nonsynonymous variants that were shared between the six affected relatives of family H subjected to WES (Supplemental Table 1). Second, filtering was performed to retain only alleles with a MAF<0.1%, a widely accepted cutoff for autosomal dominant disorders.41,42 MAF was estimated using combined datasets incorporating all available data from the 1000 Genomes Project, the Exome Variant Server (EVS) project, dbSNP138, and the Exome Aggregation Consortium (ExAC). Third, observed sequence variants were analyzed using the UCSC Human Genome Bioinformatics Browser for the presence of paralogous genes, pseudogenes, or misalignments. Fourth, we scrutinized all variants with MAF<0.1% within the sequence alignments of the CLC Genomic Workbench software program for poor sequence quality and for the presence of mismatches that indicate potential false alignments. Fifth, we employed web-based programs to assess variants for evolutionary conservation, to predict the effect of disease candidate variants on the encoded protein, and to predict whether these variants represented known disease-causing mutations. Finally, Sanger sequencing was performed to confirm the remaining variants in original DNA samples and to test for familial segregation of phenotype with genotype. Variants were also tested for absence from in-house control populations of 200 individuals with nephronophthisis and 429 individuals with steroid-resistant nephrotic syndrome (Supplemental Table 1).
CNV Analysis
One individual from the index family (family H individual IV:8; Figure 1A, Table 1) was analyzed for copy number variations. CNV analysis was performed on a 180K genome-wide array, using array comparative genomic hybridization with approximately 180,000 oligonucleotides covering the whole genome at an average resolution of 30 kb, with denser coverage at disease loci. The array was designed by Baylor Medical Genetics Laboratories and manufactured by Agilent. The CNV calls were determined with generalized copy number variation algorithms. CNVs were mapped to the human reference genome hg19, and annotated with UCSC RefGene. Polymorphic variants were excluded on the basis of the database of genomic variants (http://projects.tcag.ca/variation/).
High-Throughput NRIP1 Mutation Analysis
Massively parallel sequencing of all NRIP1 exons was performed in 155 sporadic cases and 253 familial cases of CAKUT (total of 724 affected individuals) from different pediatric nephrology centers worldwide using microfluidic PCR (Fluidigm) and next-generation sequencing (MiSeq; Illumina), as described previously.43,44 Variants were filtered against public variant databases (http://evs.gs.washington.edu/EVS) and only novel heterozygous variants were considered, confirmed by Sanger sequencing, and tested for segregation with the CAKUT phenotype.
cDNA Cloning
Full-length human NRIP1 cDNA (cDNA clone MGC:9257 IMAGE:3918685) and RARα cDNA (cDNA clone MGC:1651 IMAGE:3163891) were subcloned by PCR from full-length cDNA clones. Expression vectors were generated using LR Clonase (Thermo Fisher) according to the manufacturer’s instructions. The following expression vectors were used in this study: pRK5-N-Myc, pcDNA6.2-N-GFP, and pDest69-FLAG. Mutagenesis was performed using the QuikChange II XL site-directed mutagenesis kit (Agilent Technologies) to generate a clone with the NRIP1 mutation identified in family H.
Cell Culture, Transient Transfections, and RA Treatment
The experiments described were performed in HEK293 cells purchased from the American Type Culture Collection biologic resource center. For transient transfections, HEK293 cells were seeded at 60%–70% confluency in DMEM, supplemented with 10% fetal calf serum and 1% penicillin/streptomycin, and grown overnight. Transfections were carried out using Lipofectamine2000 (Thermo Fisher) and OptiMEM (Thermo Fisher) following the manufacturer’s instructions. For experiments involving treatment with RA, HEK293 cells were treated with 500 nM RA 24 hours after transfection. Experiments were then carried out 24 hours after continuous treatment with RA.
NRIP1 Reporter Gene Assays
Reporter assays (Dual luciferase reporter assay system; Promega) were carried out using HEK293 cells seeded in 24-well dishes and transfected with constant amounts of reporter plasmids and 100 ng of pRL-TK Renilla luciferase for normalization. The total amount of expression plasmid was kept constant by adding empty pcDNA6.2-N-GFP. Per transfection, 500 ng of pGL3-RARE (RAREs) luciferase (https://www.addgene.org, plasmid, 13458; Addgene, Cambridge, MA) and 500 ng of pcDNA6.2-N-GFP.NRIP1 expression plasmid were used. For competition experiments, the amount of pcDNA6.2-N-GFP.NRIP1_WT was held constant and increasing amounts of pcDNA6.2-N-GFP.NRIP1_p.Trp93fs* were added. Firefly luciferase and Renilla luciferase activities were measured 24 hours after transfection. All transfections were performed in triplicate, and individual experiments were repeated at least three times. In addition, all experiments were repeated with a different RARE reporter plasmid (RARE-tk-luciferase, kind gift from Li-Na Wei). After normalization, the mean luciferase activities and standard deviations were plotted as “fold activation” when compared with the empty expression plasmid. P values were determined using the paired t test.
Immunofluorescence and Confocal Microscopy in Cell Lines
For immunostaining, HEK293 cells were seeded on fibronectin-coated coverslips in 6-well plates. After 16–24 hours, cells were transiently transfected using Lipofectamine2000 (Thermo Fisher) according to the manufacturer’s instructions. Experiments were performed 24–48 hours after transfection. Cells were fixed for 15 minutes using 4% paraformaldehyde and permeabilized for 15 minutes using 0.5% Triton X-100. After blocking with 10% donkey serum + BSA, cells were incubated with primary antibody overnight at 4°C. The following day, cells were incubated in secondary antibody for 60 minutes at room temperature, and subsequently stained for 5 minutes with 4',6-diamidino-2-phenylindole (DAPI) in PBS. Confocal imaging was performed using the Leica SP5 × system with an upright DM6000 microscope, and images were processed with the Leica AF software suite. Immunofluorescence experiments were repeated at least two times in independent experiments. The following antibodies were used for immunostaining: mouse anti-Myc (sc-40; Santa Cruz Biotechnology) and rabbit anti-NRIP1 (ab42125; Abcam), both diluted 1:100. Donkey anti-mouse secondary antibodies conjugated to Alexa Fluor 488 or 594 and donkey anti-rabbit secondary antibody conjugated to Alexa Fluor 594 were purchased from Thermo Fisher Scientific.
Coimmunoprecipitation Assays
Coimmunoprecipitation experiments were performed using protein lysates from transfected HEK293 cells. Cell lysates were precleared with Recombinant Protein G Sepharose beads (Thermo Fisher) at 4°C overnight in IP lysis buffer (Thermo Fisher) containing Halt Protease Inhibitor Cocktail (Thermo Fisher) and EDTA (Thermo Fisher). Coimmunoprecipitation of Myc-tagged proteins was performed using Myc agarose beads (Sigma). The beads were washed four times with lysis buffer and proteins were eluted from the beads by incubating in loading buffer for 30 minutes at 30°C, shaking at 300 rpm. Samples were analyzed by western blot with anti-Myc (sc-40; Santa Cruz Biotechnology) and anti-FLAG (F3165; Sigma) antibodies at 1:1000 dilutions. Horseradish peroxidase–labeled secondary antibodies were purchased from Santa Cruz Biotechnology. Ten percent of the input was loaded as a control. Experiments were repeated at least three times in independent experiments and quantification of band intensity was performed using ImageJ (https://imagej.nih.gov/ij/).
Organ Cultures
E11.5 kidney rudiments were dissected from the embryo, explanted on 0.4-µm polyester membrane Transwell supports (Corning), and cultured at the air-liquid interface for 24 hours with DMEM/F12 (Gibco) supplemented with 10% FCS (Biochrom), 1× penicillin/streptomycin (Gibco), 1× pyruvate (Gibco), and 1× glutamax (Gibco). The pan-RA receptor antagonist BMS493 (Tocris) or RA (Tocris) was dissolved in DMSO and used at a final concentration of 1 µM in the culture medium.
In Situ Hybridization Analysis
Whole-mount in situ hybridization (WM-ISH) was performed with a digoxigenin-labeled antisense riboprobe corresponding to position 3102–4030 of the Nrip1 mRNA (NM_173440.2) following a standard procedure.45 Stained specimens were cleared in 80% glycerol before documentation. In situ hybridization on 10-µm paraffin sections was done essentially as described.46 For each stage, at least three independent specimens were analyzed.
Xenopus Embryo Manipulations
Xenopus embryos were cultured and their stages determined as described before.47 Microinjections were performed in the ventrolateral region of 4- and 8-cell stage Xenopus embryos targeting the pronephros anlagen. Five-hundred picograms of RNA and/or 10 ng MO in a volume of 10 nl were injected. A standard control MO was used as negative control. GFP-mRNA or RFP-mRNA were coinjected as lineage tracers and embryos showing fluorescence in the pronephros were sorted for further analysis.
Staining and Imaging of Xenopus Embryos
Xenopus embryos were fixed with MEMFA (0.1 mol/L Mops, 2 mmol/L EGTA, 1 mmol/L MgSO4, 3.7% formaldehyde, pH 7.4) for 2 hours at room temperature. For WM-ISH, nrip1 in pBS II KS (−) was linearized with NotI. The digoxigenin-labeled antisense probe was transcribed with SP6 (Roche) and bound probes detected with an alkaline phosphatase–conjungated secondary antibody (Roche). WM-ISH was performed as described before.48 For immunofluorescence, embryos were fixed at stage 39 and their pronephros stained with fluorescein or Texas Red–labeled Lycopersicon esculentum lectin (1:100 dilution; Vector Laboratories). Embryos were imaged with SteREO Discovery.V8 from Zeiss and Zen 2011 Blue Edition. The pronephros size was measured with ImageJ and the ratio of the injected and uninjected side calculated.47 SigmaStat was used to show statistical significance with the Wilcoxon–Mann–Whitney test. Significances are quoted in the figure legend and above the bars with ***P<0.001, **P<0.01, and *P<0.05.
Xenopus Plasmids, MOs, and mRNA Synthesis
Full-length human NRIP1 CDS was obtained from BIOCAT and subcloned into VF1049 for overexpression in Xenopus after addition of a stop-codon. The deletion construct NRIP1 279delG was generated by site-directed mutagenesis (New England BioLabs). mRNAs for microinjections were synthesized by in vitro transcription of linearized plasmids using the mMessage Machine kit (Ambion, Kassel, Germany). For WM-ISH full-length CDS of X. laevis nrip1 (NM_001090238.1) was cloned with MluI/NotI restriction enzyme sites in pBS II KS (−). The nrip1 translation-blocking morpholino oligonucleotide (5′-AGCTCTTCTCCATAAGTCATGTTCA-3′) was designed by and ordered from GeneTools.
Analysis of Nrip1-Deficient Mice
Mice with a null allele of Nrip1 (RIPKO mice) were generated in Professor MG Parker's laboratory (Imperial College London, London, UK).32 They were maintained at the ICRM under standard conditions, on a 12:12-hour light/dark schedule and fed a chow diet ad libitum, according to European Union guidelines for use of laboratory animals. In compliance with the French guidelines for experimental animal studies (agreement B34–172–27), heterozygous mice were mated to wild-type C57BL/6J mice. At E18.5, females were euthanized to obtain heterozygous Nrip1 embryos which were fixed in 4% PFA and processed into methanol before paraffin-embedding and sectioning to 5 µm. Hematoxylin and eosin staining was performed according to standard procedures. Three embryos of each genotype were used for each analysis.
Web Resources
UCSC Genome Browser, http://genome.ucsc.edu/cgi-bin/hgGateway
1000 Genomes Browser, http://browser.1000genomes.org
Ensembl Genome Browser, http://www.ensembl.org
Exome Variant Server, http://evs.gs.washington.edu/EVS
Polyphen2, http://genetics.bwh.harvard.edu/pph2/
Sorting Intolerant From Tolerant (SIFT), http://sift.jcvi.org
Online Mendelian Inheritance in Man (OMIM), http://www.omim.org/
ExAC Browser Beta, http://exac.broadinstitute.org
Gudmap (GenitoUrinary Molecular Anatomy Project), http://www.gudmap.org
Mutation Taster, http://www.mutationtaster.org/
Disclosures
None.
Supplementary Material
Acknowledgments
We are grateful to the family who contributed to this study. We thank Li-Na Wei for providing us with the RARE luciferase reporter.
This research was supported by grants from the National Institutes of Health to R.P.L and to F.H. (DK088767), and from the March of Dimes to F.H. A.V. is a recipient of the Fulbright postdoctoral scholar award for 2013 and is also supported by grants from the Manton Center Fellowship Program, Boston Children’s Hospital, Boston, Massachusetts, and the Mallinckrodt Research Fellowship Award. N.M. was supported by a Fred Lovejoy Resident Research and Education Award. A.T.v.d.V. is the recipient of a postdoctoral research fellowship from the German Research Foundation (DFG), VE916/1-1. R.P.L. is an Investigator of the Howard Hughes Medical Institute and is supported by a grant from the Yale Center for Mendelian Genomics, grant U54HG006504. Work in the laboratory of A.K. was supported by a grant from the German Research Council (DFG KI728/9-1). S.S.L is supported by the Emmy Noether Programme (LI1817/2-1) and Project B07 of the collaborative research initiative (SFB 1140/KIDGEM) by the German Research Foundation (DFG). E.W. is supported by the Leopoldina Fellowship Program, German National Academy of Sciences Leopoldina (LPDS 2015-07). F.H. is the William E. Harmon Professor of Pediatrics at Harvard Medical School.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016060694/-/DCSupplemental.
References
- 1.Vivante A, Kohl S, Hwang DY, Dworschak GC, Hildebrandt F: Single-gene causes of congenital anomalies of the kidney and urinary tract (CAKUT) in humans. Pediatr Nephrol 29: 695–704, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vivante A, Hildebrandt F: Exploring the genetic basis of early-onset chronic kidney disease. Nat Rev Nephrol 12: 133–146, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dressler GR: Advances in early kidney specification, development and patterning. Development 136: 3863–3874, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nicolaou N, Renkema KY, Bongers EM, Giles RH, Knoers NV: Genetic, environmental, and epigenetic factors involved in CAKUT. Nat Rev Nephrol 11: 720–731, 2015 [DOI] [PubMed] [Google Scholar]
- 5.Caruana G, Bertram JF: Congenital anomalies of the kidney and urinary tract genetics in mice and men. Nephrology (Carlton) 20: 309–311, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Blake J, Rosenblum ND: Renal branching morphogenesis: Morphogenetic and signaling mechanisms. Semin Cell Dev Biol 36: 2–12, 2014 [DOI] [PubMed] [Google Scholar]
- 7.Van Esch H, Groenen P, Nesbit MA, Schuffenhauer S, Lichtner P, Vanderlinden G, Harding B, Beetz R, Bilous RW, Holdaway I, Shaw NJ, Fryns JP, Van de Ven W, Thakker RV, Devriendt K: GATA3 haplo-insufficiency causes human HDR syndrome. Nature 406: 419–422, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Clissold RL, Hamilton AJ, Hattersley AT, Ellard S, Bingham C: HNF1B-associated renal and extra-renal disease-an expanding clinical spectrum. Nat Rev Nephrol 11: 102–112, 2015 [DOI] [PubMed] [Google Scholar]
- 9.Nie X, Sun J, Gordon RE, Cai CL, Xu PX: SIX1 acts synergistically with TBX18 in mediating ureteral smooth muscle formation. Development 137: 755–765, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma R, Sanchez-Ferras O, Bouchard M: Pax genes in renal development, disease and regeneration. Semin Cell Dev Biol 44: 97–106, 2015 [DOI] [PubMed] [Google Scholar]
- 11.Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM Jr, Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F: SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A 101: 8090–8095, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kohlhase J, Wischermann A, Reichenbach H, Froster U, Engel W: Mutations in the SALL1 putative transcription factor gene cause Townes-Brocks syndrome. Nat Genet 18: 81–83, 1998 [DOI] [PubMed] [Google Scholar]
- 13.Paces-Fessy M, Fabre M, Lesaulnier C, Cereghini S: Hnf1b and Pax2 cooperate to control different pathways in kidney and ureter morphogenesis. Hum Mol Genet 21: 3143–3155, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Vivante A, Kleppa MJ, Schulz J, Kohl S, Sharma A, Chen J, Shril S, Hwang DY, Weiss AC, Kaminski MM, Shukrun R, Kemper MJ, Lehnhardt A, Beetz R, Sanna-Cherchi S, Verbitsky M, Gharavi AG, Stuart HM, Feather SA, Goodship JA, Goodship TH, Woolf AS, Westra SJ, Doody DP, Bauer SB, Lee RS, Adam RM, Lu W, Reutter HM, Kehinde EO, Mancini EJ, Lifton RP, Tasic V, Lienkamp SS, Jüppner H, Kispert A, Hildebrandt F: Mutations in TBX18 cause dominant urinary tract malformations via transcriptional dysregulation of ureter development. Am J Hum Genet 97: 291–301, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vivante A, Mark-Danieli M, Davidovits M, Harari-Steinberg O, Omer D, Gnatek Y, Cleper R, Landau D, Kovalski Y, Weissman I, Eisenstein I, Soudack M, Wolf HR, Issler N, Lotan D, Anikster Y, Dekel B: Renal hypodysplasia associates with a WNT4 variant that causes aberrant canonical WNT signaling. J Am Soc Nephrol 24: 550–558, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanna-Cherchi S, Sampogna RV, Papeta N, Burgess KE, Nees SN, Perry BJ, Choi M, Bodria M, Liu Y, Weng PL, Lozanovski VJ, Verbitsky M, Lugani F, Sterken R, Paragas N, Caridi G, Carrea A, Dagnino M, Materna-Kiryluk A, Santamaria G, Murtas C, Ristoska-Bojkovska N, Izzi C, Kacak N, Bianco B, Giberti S, Gigante M, Piaggio G, Gesualdo L, Kosuljandic Vukic D, Vukojevic K, Saraga-Babic M, Saraga M, Gucev Z, Allegri L, Latos-Bielenska A, Casu D, State M, Scolari F, Ravazzolo R, Kiryluk K, Al-Awqati Q, D’Agati VD, Drummond IA, Tasic V, Lifton RP, Ghiggeri GM, Gharavi AG: Mutations in DSTYK and dominant urinary tract malformations. N Engl J Med 369: 621–629, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicolaou N, Pulit SL, Nijman IJ, Monroe GR, Feitz WF, Schreuder MF, van Eerde AM, de Jong TP, Giltay JC, van der Zwaag B, Havenith MR, Zwakenberg S, van der Zanden LF, Poelmans G, Cornelissen EA, Lilien MR, Franke B, Roeleveld N, van Rooij IA, Cuppen E, Bongers EM, Giles RH, Knoers NV, Renkema KY: Prioritization and burden analysis of rare variants in 208 candidate genes suggest they do not play a major role in CAKUT. Kidney Int 89: 476–486, 2016 [DOI] [PubMed] [Google Scholar]
- 18.Hwang DY, Dworschak GC, Kohl S, Saisawat P, Vivante A, Hilger AC, Reutter HM, Soliman NA, Bogdanovic R, Kehinde EO, Tasic V, Hildebrandt F: Mutations in 12 known dominant disease-causing genes clarify many congenital anomalies of the kidney and urinary tract. Kidney Int 85: 1429–1433, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Groen In ’t Woud S, Renkema KY, Schreuder MF, Wijers CH, van der Zanden LF, Knoers NV, Feitz WF, Bongers EM, Roeleveld N, van Rooij IA: Maternal risk factors involved in specific congenital anomalies of the kidney and urinary tract: A case-control study. Birth Defects Res A Clin Mol Teratol 106: 596–603, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Wilson JG, Warkany J: Malformations in the genito-urinary tract induced by maternal vitamin A deficiency in the rat. Am J Anat 83: 357–407, 1948 [DOI] [PubMed] [Google Scholar]
- 21.Rothman KJ, Moore LL, Singer MR, Nguyen US, Mannino S, Milunsky A: Teratogenicity of high vitamin A intake. N Engl J Med 333: 1369–1373, 1995 [DOI] [PubMed] [Google Scholar]
- 22.Batourina E, Gim S, Bello N, Shy M, Clagett-Dame M, Srinivas S, Costantini F, Mendelsohn C: Vitamin A controls epithelial/mesenchymal interactions through Ret expression. Nat Genet 27: 74–78, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Batourina E, Choi C, Paragas N, Bello N, Hensle T, Costantini FD, Schuchardt A, Bacallao RL, Mendelsohn CL: Distal ureter morphogenesis depends on epithelial cell remodeling mediated by vitamin A and Ret. Nat Genet 32: 109–115, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Batourina E, Tsai S, Lambert S, Sprenkle P, Viana R, Dutta S, Hensle T, Wang F, Niederreither K, McMahon AP, Carroll TJ, Mendelsohn CL: Apoptosis induced by vitamin A signaling is crucial for connecting the ureters to the bladder. Nat Genet 37: 1082–1089, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Sanna-Cherchi S, Kiryluk K, Burgess KE, Bodria M, Sampson MG, Hadley D, Nees SN, Verbitsky M, Perry BJ, Sterken R, Lozanovski VJ, Materna-Kiryluk A, Barlassina C, Kini A, Corbani V, Carrea A, Somenzi D, Murtas C, Ristoska-Bojkovska N, Izzi C, Bianco B, Zaniew M, Flogelova H, Weng PL, Kacak N, Giberti S, Gigante M, Arapovic A, Drnasin K, Caridi G, Curioni S, Allegri F, Ammenti A, Ferretti S, Goj V, Bernardo L, Jobanputra V, Chung WK, Lifton RP, Sanders S, State M, Clark LN, Saraga M, Padmanabhan S, Dominiczak AF, Foroud T, Gesualdo L, Gucev Z, Allegri L, Latos-Bielenska A, Cusi D, Scolari F, Tasic V, Hakonarson H, Ghiggeri GM, Gharavi AG: Copy-number disorders are a common cause of congenital kidney malformations. Am J Hum Genet 91: 987–997, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behar DM, Yunusbayev B, Metspalu M, Metspalu E, Rosset S, Parik J, Rootsi S, Chaubey G, Kutuev I, Yudkovsky G, Khusnutdinova EK, Balanovsky O, Semino O, Pereira L, Comas D, Gurwitz D, Bonne-Tamir B, Parfitt T, Hammer MF, Skorecki K, Villems R: The genome-wide structure of the Jewish people. Nature 466: 238–242, 2010 [DOI] [PubMed] [Google Scholar]
- 27.Augereau P, Badia E, Carascossa S, Castet A, Fritsch S, Harmand PO, Jalaguier S, Cavaillès V: The nuclear receptor transcriptional coregulator RIP140. Nucl Recept Signal 4: e024, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farooqui M, Franco PJ, Thompson J, Kagechika H, Chandraratna RA, Banaszak L, Wei LN: Effects of retinoid ligands on RIP140: Molecular interaction with retinoid receptors and biological activity. Biochemistry 42: 971–979, 2003 [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Kerimo A, Khan S, Wei LN: Real-time analysis of molecular interaction of retinoid receptors and receptor-interacting protein 140 (RIP140). Mol Endocrinol 16: 2528–2537, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Cunningham TJ, Duester G: Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nat Rev Mol Cell Biol 16: 110–123, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lienkamp SS: Using Xenopus to study genetic kidney diseases. Semin Cell Dev Biol 51: 117–124, 2016 [DOI] [PubMed] [Google Scholar]
- 32.White R, Leonardsson G, Rosewell I, Ann Jacobs M, Milligan S, Parker M: The nuclear receptor co-repressor nrip1 (RIP140) is essential for female fertility. Nat Med 6: 1368–1374, 2000 [DOI] [PubMed] [Google Scholar]
- 33.Castet A, Boulahtouf A, Versini G, Bonnet S, Augereau P, Vignon F, Khochbin S, Jalaguier S, Cavaillès V: Multiple domains of the receptor-interacting protein 140 contribute to transcription inhibition. Nucleic Acids Res 32: 1957–1966, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Hu X, Wei LN: Molecular interaction of retinoic acid receptors with coregulators PCAF and RIP140. Mol Cell Endocrinol 226: 43–50, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Duester G: Retinoic acid synthesis and signaling during early organogenesis. Cell 134: 921–931, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zomerdijk IM, Ruiter R, Houweling LM, Herings RM, Sturkenboom MC, Straus SM, Stricker BH: Isotretinoin exposure during pregnancy: A population-based study in The Netherlands. BMJ Open 4: e005602, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee LM, Leung CY, Tang WW, Choi HL, Leung YC, McCaffery PJ, Wang CC, Woolf AS, Shum AS: A paradoxical teratogenic mechanism for retinoic acid. Proc Natl Acad Sci USA 109: 13668–13673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kerley JS, Olsen SL, Freemantle SJ, Spinella MJ: Transcriptional activation of the nuclear receptor corepressor RIP140 by retinoic acid: A potential negative-feedback regulatory mechanism. Biochem Biophys Res Commun 285: 969–975, 2001 [DOI] [PubMed] [Google Scholar]
- 39.MacArthur DG, Manolio TA, Dimmock DP, Rehm HL, Shendure J, Abecasis GR, Adams DR, Altman RB, Antonarakis SE, Ashley EA, Barrett JC, Biesecker LG, Conrad DF, Cooper GM, Cox NJ, Daly MJ, Gerstein MB, Goldstein DB, Hirschhorn JN, Leal SM, Pennacchio LA, Stamatoyannopoulos JA, Sunyaev SR, Valle D, Voight BF, Winckler W, Gunter C: Guidelines for investigating causality of sequence variants in human disease. Nature 508: 469–476, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richards CS, Bale S, Bellissimo DB, Das S, Grody WW, Hegde MR, Lyon E, Ward BE; Molecular Subcommittee of the ACMG Laboratory Quality Assurance Committee : ACMG recommendations for standards for interpretation and reporting of sequence variations: Revisions 2007. Genet Med 10: 294–300, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Bamshad MJ, Ng SB, Bigham AW, Tabor HK, Emond MJ, Nickerson DA, Shendure J: Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet 12: 745–755, 2011 [DOI] [PubMed] [Google Scholar]
- 42.Lee H, Deignan JL, Dorrani N, Strom SP, Kantarci S, Quintero-Rivera F, Das K, Toy T, Harry B, Yourshaw M, Fox M, Fogel BL, Martinez-Agosto JA, Wong DA, Chang VY, Shieh PB, Palmer CG, Dipple KM, Grody WW, Vilain E, Nelson SF: Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA 312: 1880–1887, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halbritter J, Diaz K, Chaki M, Porath JD, Tarrier B, Fu C, Innis JL, Allen SJ, Lyons RH, Stefanidis CJ, Omran H, Soliman NA, Otto EA: High-throughput mutation analysis in patients with a nephronophthisis-associated ciliopathy applying multiplexed barcoded array-based PCR amplification and next-generation sequencing. J Med Genet 49: 756–767, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Halbritter J, Porath JD, Diaz KA, Braun DA, Kohl S, Chaki M, Allen SJ, Soliman NA, Hildebrandt F, Otto EA; GPN Study Group : Identification of 99 novel mutations in a worldwide cohort of 1,056 patients with a nephronophthisis-related ciliopathy. Hum Genet 132: 865–884, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilkinson DG, Nieto MA: Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol 225: 361–373, 1993 [DOI] [PubMed] [Google Scholar]
- 46.Moorman AF, Houweling AC, de Boer PA, Christoffels VM. Sensitive nonradioactive detection of mRNA in tissue sections: Novel application of the whole-mount in situ hybridization protocol. J Histochem Cytochem 49:1–8, 2001. [DOI] [PubMed] [Google Scholar]
- 47.Lienkamp S, Ganner A, Boehlke C, Schmidt T, Arnold SJ, Schäfer T, Romaker D, Schuler J, Hoff S, Powelske C, Eifler A, Krönig C, Bullerkotte A, Nitschke R, Kuehn EW, Kim E, Burkhardt H, Brox T, Ronneberger O, Gloy J, Walz G: Inversin relays Frizzled-8 signals to promote proximal pronephros development. Proc Natl Acad Sci U S A 107: 20388–20393, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sive HL, Grainger RM, Harland RM: Early Development of Xenopus laevis: A Laboratory Manual. Plainview, NY, Cold Spring Harbor Laboratory Press, 2000 [Google Scholar]
- 49.Hoff S, Halbritter J, Epting D, Frank V, Nguyen TM, van Reeuwijk J, Boehlke C, Schell C, Yasunaga T, Helmstädter M, Mergen M, Filhol E, Boldt K, Horn N, Ueffing M, Otto EA, Eisenberger T, Elting MW, van Wijk JA, Bockenhauer D, Sebire NJ, Rittig S, Vyberg M, Ring T, Pohl M, Pape L, Neuhaus TJ, Elshakhs NA, Koon SJ, Harris PC, Grahammer F, Huber TB, Kuehn EW, Kramer-Zucker A, Bolz HJ, Roepman R, Saunier S, Walz G, Hildebrandt F, Bergmann C, Lienkamp SS: ANKS6 is a central component of a nephronophthisis module linking NEK8 to INVS and NPHP3. Nat Genet 45: 951–956, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nieuwkoop J and Faber PD: Normal Table of Xenopus Laevis (Daudin). New York, Garland Publishing, 1994
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.