Abstract
Although spontaneous kidney transplant acceptance/tolerance occurs in mice and occasionally in humans, mechanisms remain unclear. Herein we test the hypothesis that EPO, a hormone predominantly produced by the adult kidney, has immunomodulating properties that are required for spontaneous kidney graft acceptance. In vitro, in a manner dependent on the EPO receptor and CD131 on antigen-presenting cells, EPO induced the secretion of active TGFβ by antigen-presenting cells, which in turn converted naïve CD4+ T cells into functional Foxp3+ regulatory T cells (Treg). In murine transplant models, pharmacologic downregulation of kidney-derived EPO prevented spontaneous Treg generation. In a controlled, prospective cohort clinical study, EPO administration at doses used to correct anemia augmented the frequency of peripheral CD4+CD25+CD127lo T cells in humans with CKD. Furthermore, EPO directly inhibited conventional T cell proliferation in vitro via tyrosine phosphatase SHP-1–dependent uncoupling of IL-2Rβ signaling. Conversely, EPO-initiated signals facilitated Treg proliferation by augmenting IL-2Rγ signaling and maintaining constitutively quenched IL-2Rβ signaling. In additional murine transplant models, recombinant EPO administration prolonged heart allograft survival, whereas pharmacologic downregulation of kidney-derived EPO reduced the expression of TGFβ mRNA and abrogated kidney allograft acceptance. Together, our findings delineate the protolerogenic properties of EPO in inhibiting conventional T cells while simultaneously promoting Treg induction, and suggest that manipulating the EPO/EPO receptor signaling axis could be exploited to prevent and/or treat T cell-mediated pathologies, including transplant rejection.
Keywords: erythropoietin, transplantation, acute allograft rejection, Regulatory T cell
Current protolerogenic strategies being investigated to prevent rejection of transplanted grafts and treat autoimmune disease include antibody-mediated T cell depletion,1 blockade of T cell activation through costimulatory blockade,2 and myeloablative-conditioning in conjunction with allogeneic donor bone marrow transplantation to induce hematopoietic chimerism.3 To date, however, these strategies have proven to be either ineffective or associated with significant morbidity.4–6
The ability of Foxp3+ regulatory T cells (Treg) to regulate responses to self and nonself antigens7,8 has driven research aimed at correcting suboptimal Treg numbers or function in patients with transplantation9–11 or autoimmune diseases.12 Although early results are promising, this approach is costly, labor intensive, and the longer-term outcomes remain unknown.6 An alternative approach is to administer reagents that directly induce and expand endogenous Treg in patients. In small studies, administration of low dose IL-2 augmented endogenous Treg and improved graft versus host disease after bone marrow transplantation13 or disease severity in patients with lupus.14 However, IL-2 can expand conventional T cells (Tconv), reducing its therapeutic window and likely accounting for the related morbidity.15 These initial studies support strategies to promote intrinsic Treg-mediated immunoregulation, but raise the need to develop other reagents and/or strategies that directly promote Treg function while simultaneously inhibiting the Tconv.
Erythropoietin (EPO) is a 34 kD glycoprotein produced predominantly by pericapillary fibroblasts within the kidney and is required for red blood cell (RBC) development.16 The effects of EPO are mediated through a homodimeric, Janus kinase 2 (JAK2)-linked EPO receptor (EPO-R) expressed on myeloid–erythroid precursors that initiates STAT5-dependent signaling pathways to induce gene transcription and maturation to erythrocytes.17 EPO also binds to a second heterodimeric receptor that consists of one EPO-R chain plus CD131, the common β chain of receptors for CSF1, IL-3, and IL-5.18 The EPO-R/CD131 heterodimer is expressed by immune cells and by parenchymal cells in multiple organs, including the heart, kidney, and brain.
Emerging evidence suggests that EPO ligation of EPO-R homodimers and of EPO-R/CD131 heterodimers induces a number of nonerythropoietic effects,19 including improved kidney transplant outcomes in animal models20 and humans21 by mechanisms that are distinct from correction of anemia but are not otherwise elucidated. The studies described herein document that endogenous, kidney-derived EPO promotes the induction of Treg that in turn mediate local immune regulation and heart and kidney allograft survival in mice, and that pharmacologic EPO administration expands Treg in human subjects. These findings provide novel mechanistic insights into Treg physiology and present an innovative therapeutic strategy for establishing and/or reinstating immunologic tolerance in humans by pharmacologic targeting of the EPO/EPO-R signaling axis.
Results
EPO Promotes Treg Generation via Antigen-Presenting Cell Production and Activation of TGFβ
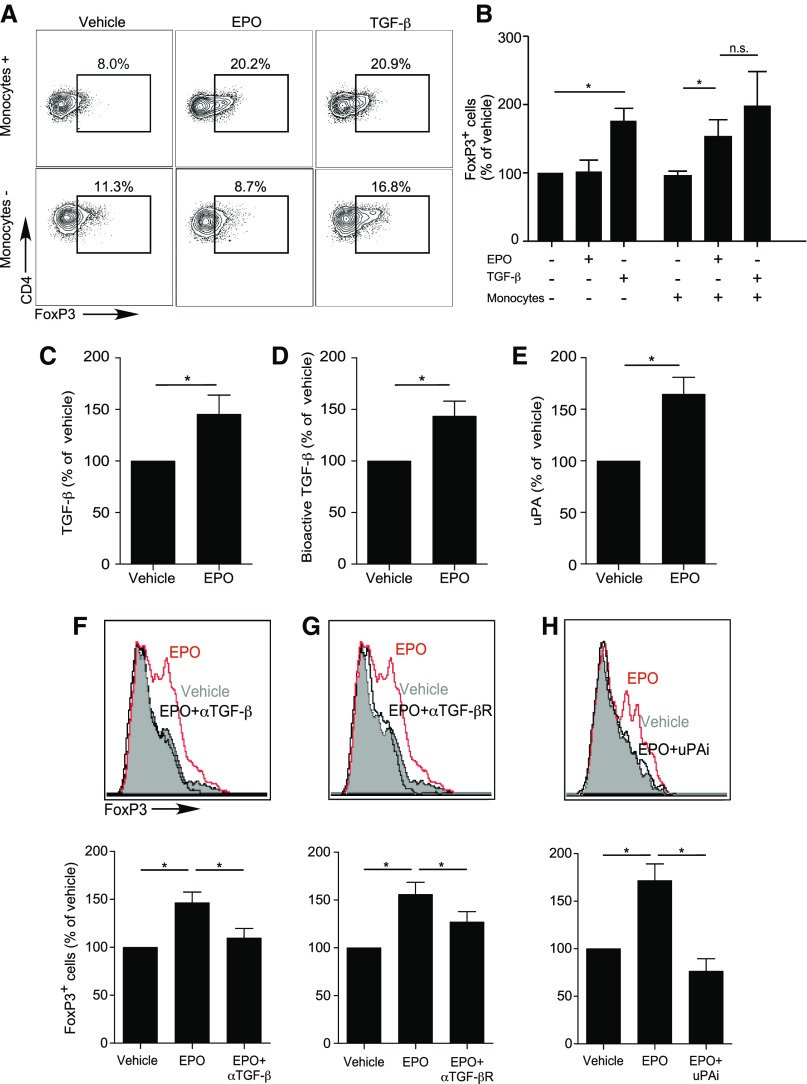
CD4+ Treg can be generated in the periphery from naïve CD4+ T cells (induced or iTreg) through Transforming Growth Factor β–dependent (TGFβ-dependent) induction and stabilization of FOXP3. To formally test whether EPO induces Treg, we mixed naïve human CD4+ T cells with or without CD14+ monocytes as antigen-presenting cells (APCs), both of which express EPO-R,22 with anti-CD3 mAb and IL-2, with or without EPO (but without TGFβ) for 5 days (Figure 1, A and B). Parallel cultures included TGFβ without EPO as controls. In the absence of TGFβ, EPO increased the frequencies of CD4+CD25+FOXP3+ T cells to those obtained in control wells containing recombinant TGFβ (Figure 1, A and B). EPO-dependent induction of CD4+CD25+FOXP3+ Tregs required APCs (Figure 1, A and B). The EPO-induced CD4+CD25+FOXP3+ Tregs suppressed proliferation of anti–CD3- and anti–CD28-stimulated Tconv with equal efficacy as TGFβ-induced iTreg (Supplemental Figure 1, A and B).
Figure 1.
EPO promotes in vitro human Treg induction. (A and B) Enriched naïve human CD4+ T cells were cultured for 5 days in the presence (top) or absence (bottom) of CD14+ monocytes, anti-CD3 (1 μl/ml), IL-2 (100 IU/ml), and EPO (1000 IU/ml) or vehicle control. Naïve CD4+ T cells cultured in the presence of anti–CD3- and anti–CD28-coated beads (25 μl/106 cells), IL-2, and TGFβ (5 ng/ml) were used as positive control (right panel in each row). Representative flow plots for FOXP3 expression gated on CD4+CD25+ T cells (A) and summarized normalized quantification (B) (see Statistical Analyses) of 15–22 experiments using different donors. (C–E) Human monocytes were cultured in serum-free media with EPO (1000 IU/ml) or vehicle control for 2 days and culture supernatants tested for total TGFβ by ELISA (C), bioactive TGFβ using SMAD3-SMAD4 reporter cells (D), and uPA protein (ELISA) (E). (F–H) Enriched human naïve CD4+ T cells were cultured for 5 days in the presence of monocytes, anti-CD3 mAb (1 μl/ml), IL-2 (100 IU/ml), and EPO (1000 IU/ml) or vehicle control in serum-free media. (F–H) Treg induction experiments as performed in (A and B), in which (F) anti-TGFβ antibody (10 μg/ml), (G) anti-TGFβ receptor neutralizing antibody (10 μg/ml), or (H) uPA chemical inhibitor (100 μM) were added to the cultures and compared with relative isotype or vehicle controls. Top panels show representative flow plots for FOXP3 expression gated on CD4+CD25+ T cells, bottom panels show summarized quantification of three independent experiments from nine different donors. *P<0.05 (paired t test).
TGFβ is produced as an inactive precursor bound to latency active peptide (LAP), and is converted to its active form by urokinase-type plasminogen activator (uPA) (or thrombospondin, among other proteases) -dependent LAP cleavage.23 When we stimulated human monocytes with EPO, we detected significant increases in TGFβ mRNA (Supplemental Figure 2A), and total and active TGFβ protein24 (Figure 1, C and D). Addition of a blocking anti–EPO-R antibody abrogated the EPO-induced increase in TGFβ production by monocytes (Supplemental Figure 2A). EPO stimulation of human CD14+ monocytes induced upregulation of uPA mRNA (Supplemental Figure 2B) and protein (Figure 1E). We observed similar effects of EPO on TGFβ production by cultured human kidney tubular cells (Supplemental Figure 2C). Adding blocking antibodies for TGFβ, TGFβ receptor, or uPA inhibitor prevented EPO-induced iTreg generation (Figure 1, F–H), thereby verifying functional links.
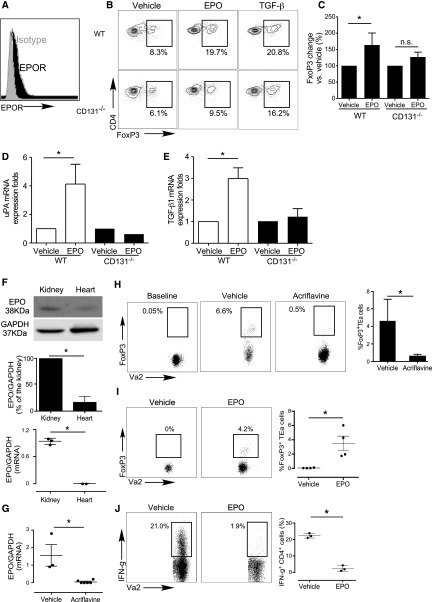
Similarly, when we cultured naïve CD4+CD44loCD62LhiFoxp3neg EPO-R+ murine T cells (Figure 2, A–C) with syngeneic APCs, IL-2, and either TGFβ or EPO, we observed that EPO induced Foxp3 expression within the CD4+ T cells to the same degree as TGFβ. EPO had minimal effect on murine Treg generation in parallel cultures containing CD131−/− murine APCs (Figure 2, B and C). EPO induced WT, but not CD131−/− murine APCs, to upregulate TGFβ and uPA gene expression (Figure 2, D and E). Together with the human data (Figure 1), these findings indicate that EPO ligation of the EPO-R/CD131 heterodimer on APCs induces production and activation of TGFβ, driving iTreg generation.
Figure 2.
EPO promotes in vivo murine antigen-specific Treg generation. (A) Representative flow plot (of four individual experiments) depicting EPO-R expression gated on naïve murine B6 CD4+ T cells (black). Isotype control staining is shown in gray. (B and C) Murine naïve B6 CD4+ T cells were stimulated with WT or CD131−/− B6 APCs, anti-CD3 mAb, and IL-2 with or without EPO, with or without TGFβ for 5 days. (B) Representative flow plots (gated on CD4+ T cells) and (C) quantification of Foxp3 expression (n=3). (D and E) TGFβ1 (D) and uPA (E) gene expression in murine B6 WT or CD131−/− monocytes treated with EPO or vehicle control for 24 hours analyzed by qRT-PCR, representative of two independent experiments. (F) Lysates of BALB/c kidney and heart tissue were tested for EPO by immunoblot (immunoblots for GAPDH verified equal amounts of protein added to each lane). Representative of three independent experiments (top panel) and data quantification (middle panel). Lower panel shows results of qRT-PCR for EPO gene mRNA expression obtained from the same organs used for immunoblotting. (G) BALB/c mice were treated with acriflavine (2 mg/kg per day, administered intraperitoneally) or vehicle for 7 days and kidneys were harvested, RNA isolated, and EPO gene expression analyzed by qRT-PCR (n=3 per group). (H) Foxp3GFPneg CD4+ TEa cells (left panel) were injected into B6rag1−/− mice on day 2 after transplantation of a kidney from control- or acriflavine-treated BALB/c donors (recipient native kidney left in place, no acriflavine was administered post-transplant). Representative flow plots gated on CD4+ T cells in the spleen as indicated and summarized quantified results (n=3 per group) obtained on day 3 after transfers, showing Foxp3 and Vα2+ (TEa is Vα2+). (I) Foxp3GFPneg CD4+ TEa cells (>99% purity) were injected into B6rag1−/− mice on day 2 after transplantation of a BALB/c heart. Recipients were given EPO (1000 IU/kg per day) or vehicle control for 5 days. Representative flow plots gated on CD4+ T cells in the spleen (left) and summarized quantified results (right; n=3 per group) obtained on day 5 after transfers, showing Foxp3 and Vα2+ (TEa is Vα2+). (J) Intracellular IFNγ expression (flow cytometry) in splenocytes from the same animals of (I) reactivated ex vivo with TEa peptide 52–68 (100 μM) for 4 hours. Representative flow plots gated on CD4+ T cells and summarized quantified results of IFNγ-producing cells (n=3 per group). *P<0.05.
Using murine systems, we next tested whether kidney-produced EPO (Figure 2F) physiologically (in the absence of exogenous recombinant EPO) generates antigen-specific Treg. We adoptively transferred naïve (Foxp3neg) TEaFoxp3GFP cells, specific for peptide I-Edα52–68 complexed with I-Ab, into B6rag1−/− recipients of untreated BALB/c kidneys or of BALB/c kidneys from donors that had been treated with acriflavine, a HIF1/2 antagonist that prevents kidney EPO production.25 Quantitative RT-PCR (qRT-PCR) analyses demonstrated EPO mRNA in kidney but not heart tissue (Figure 2F), and immunoblots of organ lysates confirmed significantly more EPO protein in WT kidneys (Figure 2F). Additional qRT-PCR analyses showed that acriflavine treatment markedly downregulated kidney EPO gene expression (Figure 2G). Three days after adoptive transfer of Foxp3GFPneg TEa T cells into the animals, 3%–4% of them spontaneously became Foxp3GFPpos in recipients of control kidneys, whereas <0.5% were Foxp3GFPpos in acriflavine-treated kidney recipients (Figure 2H).
To test effects of therapeutic recombinant EPO on iTreg generation in vivo, we adoptively transferred naïve CD4+Foxp3GFPneg TEa T cell receptor (TCR) transgenic T cells into B6rag1−/− recipients of BALB/c (I-Ed+) EPO-negative heart allografts (Figure 2I) with or without recombinant EPO, and measured the frequency of Foxp3GFPpos TEa cells in the recipients 5 days later (Figure 2I). These analyses showed approximately 4% of splenic TEa cells became GFPpos in the EPO-treated heart transplant recipients, a frequency similar to that reported by others after costimulatory blockade-induced (no EPO) murine heart transplantation.26 Of the adoptively transferred naïve TEa T cells within the recipient spleens on day 5, only 2.4%±1.7% underwent differentiation into proinflammatory IFNγ-producing Tconv. In contrast, analysis of the splenic TEa cells in the spleens of vehicle-treated controls showed no detectable Foxp3GFPpos TEa cells and 22.3%±1.4% IFNγ producers (P<0.05 for each, versus EPO-treated recipients; Figure 2J).
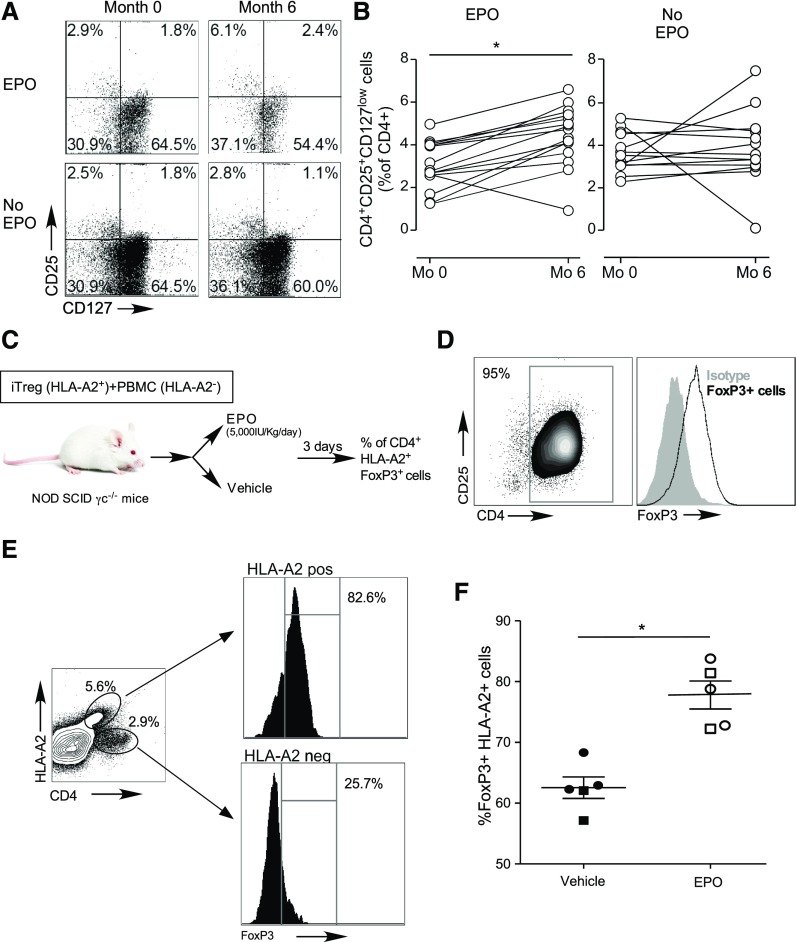
To evaluate effects of therapeutic doses of EPO on Treg in humans, we performed a prospective cohort study where we quantified changes in circulating CD4+CD25+CD127low Treg27,28 in patients with stage 429 CKD who were given EPO to treat anemia. Blood samples were obtained before, and 6 months after EPO therapy initiation. Control samples were obtained from age- and sex-matched subjects with nonanemic stage 4 CKD (not treated with EPO, Supplemental Table 1). Hemoglobin level significantly increased in EPO-treated patients (month 0 versus 6: 9.7±2.8 versus 11.8±1.3 g/dl; P<0.05), although it did not change in controls (13.5±1.6 versus 13.2±1.5 g/dl). Flow cytometric analyses revealed marked and significant increases in the frequencies of peripheral CD4+CD25+CD127low Treg (confirmed to be predominantly FOXP3+, see Supplemental Figure 3) in subjects given EPO, whereas no changes were observed in the controls (Figure 3, A and B). In the patients for whom data on lymphocyte counts were available, we found that EPO increased the absolute numbers of circulating Treg (15.9±8.6/μl at time 0 versus 24.2±12.1/μl at 6 months; n=8; P<0.05), with no observed effect on absolute numbers in the control group (32.0±26.2/μl at time 0 versus 29.6±17.9/μl at 6 months; n=12; P=0.67).
Figure 3.
EPO augments human Treg in vivo. (A and B) Blood samples were obtained from patients with CKD with anemia before and after 6 months of EPO therapy and from matched CKD controls without anemia (and not given EPO) at the same time points (see Supplemental Table 1 for patients’ baseline characteristics). (A) Representative flow plots and (B) data quantification of CD4+CD25+CD127low Treg at study enrollment and after 6 months in the EPO treatment and in the control group. (C) Schematic of experimental design in NOD SCIDγc−/− mice: 1 million CD4+CD25+FOXP3+ human, in vitro generated iTregs from HLA-A2pos donors. FOXP3 expression before injection shown in (D) were injected together with 5 million PBMC from HLA-A2– donors in NOD SCIDγc−/− mice. Mice were treated with EPO or vehicle control for 3 days and then euthanized for analyses. (E) Representative plots of FOXP3 expression in HLA-A2neg and HLA-A2+ cells and (F) percentage of splenic HLA-A2pos cells that are FOXP3+ in the vehicle and EPO treatment groups. Data are from two experiments (indicated by squares and circles) with two to three mice per group in each (error bars, SEM). *P<0.05 (paired t test).
In contrast to the stable phenotype of thymic Treg, iTreg are susceptible to inflammation-induced reprogramming into pathogenic Tconv.30 To test the effects of EPO on in vivo FOXP3 expression of human iTreg,31 we coinjected HLA-A2pos (A2 functions as a traceable marker) iTreg (>90% FOXP3+, Figure 3, C and D) and HLA-A2neg PBMC (effector cells and a source of human IL-2) into NOD scidγc−/− mice. We treated the recipients with EPO (5000 IU/kg per day, administered intraperitoneally) or vehicle control (Figure 3C), and quantified the frequencies of human CD4+FOXP3+ T cells within the HLA-A2pos gate 3 days later. EPO treatment resulted in higher frequencies of HLA-A2pos FOXP3+ cells (Figure 3, E and F), suggesting Treg-stabilizing effects in vivo.
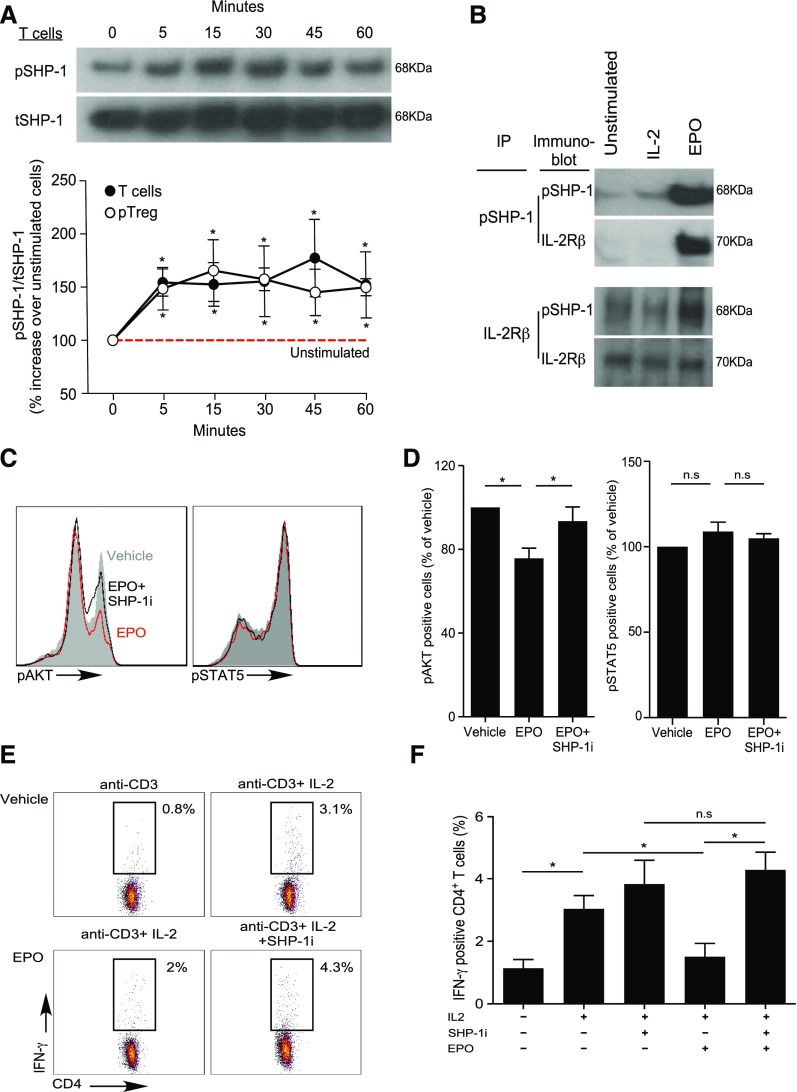
EPO Uncouples IL-2Rβ Signaling in Tconv but Not Treg through SHP-1–Dependent Molecular Crosstalk
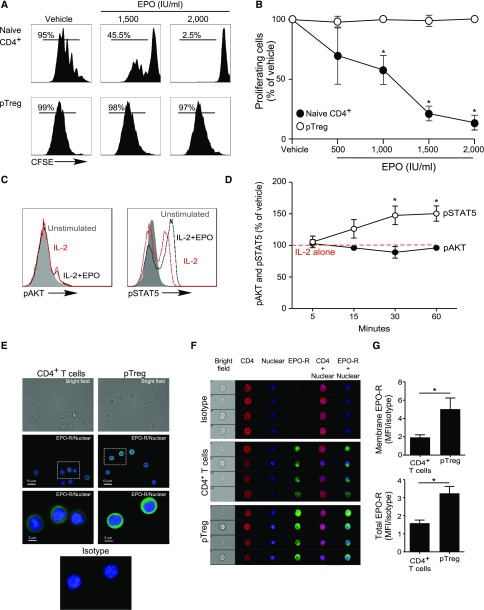
Our previous work revealed that EPO binds to EPO-R expressed on Tconv and directly inhibits their proliferation by uncoupling IL-2R signaling,22 yet analyses of human peripheral blood CD4+CD25+CD127intFOXP3+ Treg or in vitro generated iTreg stimulated with anti-CD3, anti-CD28, and IL-2 revealed that the same concentrations of recombinant EPO had no effect on Treg proliferation (Figures 4 and 5, A and B, Supplemental Figure 4).
Figure 4.
EPO inhibits proliferation of human Tconv but not Treg. (A and B) CFSE-labeled naïve human CD4+ T cells and ex vivo expanded Treg were activated with anti–CD3- and anti–CD28-coated beads (25 μl/106 cells) and IL-2 (100 IU/ml) for 5 days with or without increasing EPO concentrations or vehicle control. (A) Representative flow cytometric analyses gated on live cells and (B) summarized data quantification of CFSE dilution for six independent experiments using different donors (mean and SEM) and at least 100,000 events per experiment; *P<0.05 versus vehicle at the same dose (two-way ANOVA). (C and D) pAKT and pSTAT5 expression in CD4+ Treg activated with anti–CD3- and anti–CD28-coated beads (25 μl/106 cells) and IL-2 (100 IU/ml) plus EPO (1000 IU/ml) or vehicle control for 5–60 minutes. (C) Representative flow plots at 30 minutes and (D) data quantification show levels of pAKT and pSTAT5 assayed by flow cytometry at times indicated. The results are expressed as percentage of pAKT or pSTAT5 in EPO-treated cells versus IL-2 alone (set at 100%, red dashed line). Data are from five to nine independent experiments with different donors (mean and SEM) with >100,000 analyzed events per experiment. *P<0.05 versus vehicle at the same time point (two-way ANOVA). (E–G) Expression of EPO-R in naïve human CD4+ Tconv and Treg. (E) Immunofluorescence microscopy images of naïve CD4+ T cells and Treg attached to coverslips coated with laminin and poly-D-lysin: (top) bright field, (middle and bottom) stained with AF488-anti–EPO-R antibody or matching isotype (green) together with the Hoechst 33342 nuclear dye (blue). Images are representative of four independent experiments. (F) Representative Imaging Flow single-cell images depicting bright field (column 1), CD4 expression (APC-anti–CD4, column 2), nucleus location (Hoechst 33342, column 3), and EPO-R expression (AF488-anti–EPO-R or matching isotype, column 4) on CD4+ T cells and Treg and (G) quantification of the membrane EPO-R (obtained applying a contour mask derived from the bright field image) and total EPO-R expression for four independent experiments from different donors (mean and SEM). The same number of events (5000–30,000) for CD4+ T cells and Tregs were recorded for each donor. *P<0.05 (paired t test).
Figure 5.
EPO/EPO-R binding inhibits IL-2Rβ signaling in Tconv via SHP-1 molecular crosstalk. (A) Enriched human total T cells and CD4+CD25+ Treg were stimulated with EPO (1000 IU/ml) for the indicated times and the lysates were immunoblotted for pSHP-1 and tSHP-1. The top panel shows a representative gel using total T cells and the bottom panel shows ratios of densitometry measurements of pSHP-1 to tSHP-1 band intensity for T cells and Treg as indicated. *P<0.05 versus unstimulated cells (two-way ANOVA). (B) Immunoprecipitation experiments. Cell lysates were produced from human resting total T cells or from total T cells activated with 100 IU/ml IL-2 (labeled IL-2) or IL-2 plus 1000 IU/ml EPO (labeled EPO) for 60 minutes. The cell lysates were immunoprecipitated with either anti–pSHP-1 (B) (top) or anti–IL-2Rβ (B) (bottom), and blotted with anti–pSHP-1 or anti–IL-2Rβ as indicated. Representative of three individual experiments. (C and D) Total T cells were activated with anti–CD3- and anti–CD28-coated beads (25 μl/106 cells), IL-2 (100 IU/ml), and EPO (1000 IU/ml), EPO plus SHP-1 inhibitor (NCS-87877; 175 μg/ml), or vehicle control for 30 minutes. pAKT and pSTAT5 were assayed by flow cytometry. (C) Representative plots and (D) summarized data quantification of six to nine experiments. *P<0.05 (paired t test). (E and F) Human PBMC were activated with anti-CD3 (1 μg/ml) with or without IL-2 (100 IU/ml), with or without EPO (1000 IU/ml), and with or without SHP-1 inhibitor (NCS-87877; 37.5 μg/ml) for 24 hours. (E) Representative flow plots (gated on CD4+ T cells) and (F) summarized quantification of intracellular IFNγ expression from four independent experiments with different donors. *P<0.05.
To address this apparent paradox, we compared intracellular signaling pathway activity initiated by IL-2/IL-2R ligation in human naïve CD4+ Tconv and Treg with or without EPO, focusing on a kinetic analysis of AKT (downstream of the IL-2Rβ)32 and STAT5 (downstream of the IL-2Rγ chain).32 Consistent with previous reports,33,34 IL-2 induced AKT phosphorylation (pAKT) and STAT5 phosphorylation (pSTAT5) in naïve CD4+ T cells (Supplemental Figure 5, A and B). In Treg, IL-2 induced pSTAT5 but not pAKT (Figure 4, C and D), consistent with the presence of constitutive phosphatase activities35,36 preventing pAKT. Whereas EPO inhibited IL-2–induced pAKT in naïve human CD4+ T cells (Supplemental Figure 5, A and B), the same concentrations of EPO did not reduce constitutively low levels of pAKT in Treg (Figure 4, C and D). EPO did not alter IL-2–induced pSTAT5 in naïve CD4+ T cells but increased IL-2–initiated pSTAT5 in Treg. Imaging flow analyses revealed higher total EPO-R expression, and more membrane-associated EPO-R on Treg compared with Tconv (Figure 4, E–G), excluding the possibility that the resistance of Treg to EPO’s inhibitory effects is related to absent or lower EPO-R expression. Together, the data support the interpretation that EPO/EPO-R ligations inhibit IL-2Rβ signaling crucial for proliferation of Tconv cells, but despite EPO-R expression and signaling in Treg, EPO/EPO-R ligations maintain and/or enhance IL-2Rγ/STAT5 signaling required for Treg proliferation.
In erythrocyte precursors, EPO-R signaling induces SHP-1 activation,37 a phosphatase that negatively regulates TCR and IL-2R signaling in T cells.38 This raised the possibility that EPO’s inhibitory effects on Tconv are mediated through EPO-R–induced activation of SHP-1, which subsequently dephosphorylates signaling intermediaries downstream of the IL-2Rβ chain via molecular crosstalk.
To test this, we performed immunoblots for total SHP-1 (tSHP-1) and active phosphorylated SHP-1 (pSHP-1) using EPO-stimulated human T cells and Treg (Figure 5A). These assays showed EPO induced pSHP-1 in total T cells and in Treg, peaking at 30–45 minutes. To test whether the EPO-induced pSHP-1 physically interacts with IL-2Rβ, we performed immunoprecipitation with anti–IL-2Rβ mAb on lysates from EPO/IL-2–stimulated CD3+ Tconv and detected both the IL-2Rβ chain and pSHP-1 by immunoblot (Figure 5B). Converse assays in which we performed IP with anti–SHP-1 mAb yielded both pSHP-1 and IL-2Rβ by immunoblot (Figure 5B). No pSHP-1 was detected in the absence of EPO, indicating the physical interactions between pSHP-1 and IL-2Rβ are EPO-dependent.
To test for a functional link, we activated CD4+ T cells with EPO with or without a specific SHP-1 inhibitor, NSC-87877, and quantified IL-2–induced pAKT (Figure 5, C and D), IFNγ production (Figure 5, E and F), and cell proliferation (Supplemental Figure 6, A and B). NSC-87877 rescued EPO-induced pAKT inhibition, and overcame EPO-induced inhibition of T cell IFNγ production and proliferation. In contrast, NSC-87877 did not alter Treg proliferation in the presence or absence of EPO (Supplemental Figure 6, C and D). These data indicate that EPO uncouples the IL-2Rβ signaling (critical for Tconv cell activation) through a SHP-1–dependent mechanism that leaves the IL-2Rγ (crucial for Treg proliferation) unaffected.
EPO Promotes In Vivo Allograft Acceptance
To directly test the in vivo impact of EPO-mediated immunoregulation, we utilized mouse models of organ transplantation. Consistent with the above described effects of EPO on human cells, recombinant EPO inhibited in vitro proliferation of naïve murine CD4+ T cells (Supplemental Figure 7, A and B).
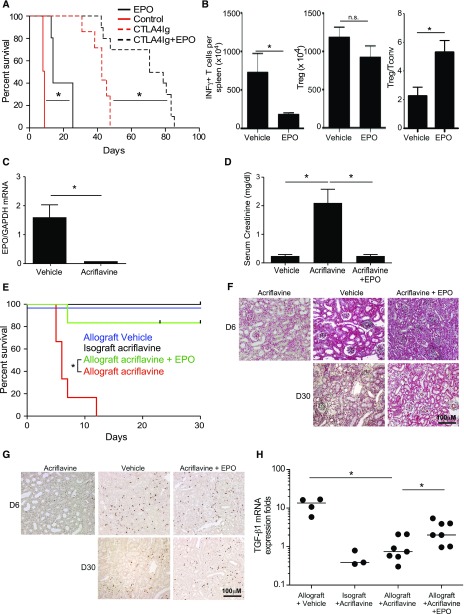
We treated BALB/c recipients of B6 heart transplants with recombinant EPO or vehicle control for the initial 3 days after transplantation, with or without a single perioperative dose of CTLA4-Ig (Figure 6A). Whereas vehicle-treated recipients invariably rejected the grafts by day 8, EPO administration prolonged graft survival for >14 days. CTLA4-Ig prolonged graft survival to a median of 42 days and combination therapy with CTLA4-Ig plus EPO synergized to prolong graft survival to >70 days. We performed additional sets of transplants with or without perioperative EPO (no CTLA4-Ig), euthanizing animals on day 7 post-transplant (all grafts beating) and performed immunologic analyses. These assays showed fewer donor-reactive IFNγ-producing, donor-reactive splenic T cells, similar numbers of splenic Foxp3+CD4+ T cells, and higher ratios of total CD4+CD25+Foxp3+ Treg/IFNγ-producing, donor-reactive T cells in the spleens of EPO-treated recipients (Figure 6B).
Figure 6.
EPO mediates murine allograft survival. (A) Survival of allogeneic B6 heart transplants in BALB/c recipients given EPO (n=5) or vehicle (n=4), and CTLA4Ig (n=7) or CTA4Ig with or without EPO (n=10). (B) Numbers of donor-reactive INFγ-producing T cells (left), total splenic CD4+CD25+Foxp3+ Treg (middle), and ratio of the frequency of Treg over donor-reactive IFNγ producers (right) in spleens of EPO- or vehicle-treated heart transplant recipient mice studied on day 7 after transplantation. IFNγ producers were measured by ELISPOT in response to overnight donor APC stimulation (four vehicle-treated and three EPO-treated animals) and CD4+CD25+Foxp3+ Treg were analyzed by flow cytometry. *P<0.05 (paired t test, mean and SEM). (C) EPO gene expression in A/J kidneys transplanted into B6 analyzed 7 days after acriflavine initiation (qRT-PCR). (D and E) Serum Cr levels on day 7 (D) and graft survival (E) of allogeneic A/J kidney transplants in B6 recipients given acriflavine (n=6), acriflavine plus EPO (n=5), or vehicle (n=13). Syngeneic B6 kidneys transplanted into B6 recipients given acriflavine (n=6) are also included in the survival analysis. (F and G) Representative images of day 5–9 allografts from mice treated with vehicle (n=6), acriflavine (n=6), or acriflavine plus EPO (n=3), and of day 30 allografts from mice treated with vehicle (n=6), or acriflavine plus EPO (n=3). (F) Representative Trichrome staining and (G) representative Foxp3 staining (brown) from grafts of each treatment group/time post-transplant as indicated. (H) TGFβ1 gene expression in renal grafts analyzed by qRT-PCR on day 7–9 in mice treated with acriflavine, EPO, or vehicle control. Each dot represents an individual mouse. *P<0.05 (by one-way ANOVA with Tukey test).
Kidney allografts are accepted spontaneously by selected MHC-disparate recipients via mechanisms thought to be Treg- and TGFβ-dependent.39 To link kidney-derived EPO to kidney transplant acceptance, we transplanted MHC-disparate A/J kidneys into B6 recipients, a strain combination that results in spontaneous acceptance.40 We transplanted B6 isografts as controls. All recipients underwent native nephrectomies. We treated the recipients with acriflavine (2 mg/kg per day initiated 48 hours post-transplant) with or without recombinant EPO (10,000 IU/kg on day 2–5) and monitored kidney function by serum creatinine (Cr) measurements. qRT-PCR analysis of mRNA from transplanted kidneys analyzed 7 days after acriflavine initiation confirmed undetectable EPO transcripts (Figure 6C). Whereas serum Cr levels remained at approximately 0.3 mg/dl in untreated recipients of allogeneic kidneys (similar to naïve mice) for >30 days, serum Cr values in acriflavine-treated recipients were 2.6±0.8 mg/dl by day 7 post-transplant (Figure 6D). EPO therapy prevented the acriflavine-induced increase in serum Cr in five out of six animals, with Cr remaining <0.4 mg/dl for >30 days (Figure 6D). In control experiments we observed that serum Cr levels in recipients of acriflavine-treated B6 kidney isografts were 0.5±0.6 mg/dl on day 7 post-transplant, ruling out nonspecific injurious effects of acriflavine on kidney function.
Survival analyses showed that acriflavine precipitated rejection in allograft recipients by day 10 after transplant, whereas combined acriflavine and EPO treatment rescued kidney graft survival to those of vehicle-treated allografts and acriflavine isograft controls (Figure 6E). Histologic analysis of kidney graft tissue revealed diffuse infiltrating mononuclear cells in the interstitium and periarterial compartments of mice treated with vehicle, acriflavine, or acriflavine and EPO (Figure 6F), but these infiltrates included more Foxp3+ cells in the control mice and mice treated with acriflavine and EPO (Figure 6G). At day 30 post-transplant, histology of grafts from mice treated with vehicle or acriflavine and EPO showed limited mononuclear cell infiltrates interspersed with Foxp3+ cells (Figure 6G). Validating that our in vitro findings linking EPO to TGFβ upregulation (Figures 1 and 2) apply in vivo, we observed lower quantities of TGFβ mRNA in kidneys from acriflavine-treated mice versus vehicle-treated controls or mice treated with acriflavine and EPO (Figure 6H).
Discussion
Our findings support a conceptual paradigm shift regarding the function of EPO, a hormone traditionally considered to be required for RBC differentiation, but now newly identified as having potent protolerogenic properties. We demonstrate that EPO directly inhibits proliferation of Tconv, while simultaneously sparing Treg proliferative capacity (Figure 4) and facilitating Treg generation (Figures 1–3).
The molecular mechanisms underlying the differential effects of EPO on Tconv versus Treg are that EPO/EPO-R ligations on Tconv activate SHP-1, which dephosphorylates signals downstream of the IL-2Rβ chain, including pAKT (Figure 5, see schematic Supplemental Figure 8). Although previous work by others showed that EPO/EPO-R initiated signals activate JAK2/STAT5 and that associated SHP-1 activation dephosphorylates JAK2, providing negative feedback of EPO-R signaling,17 our data uniquely show that the EPO-R–induced pSHP-1 interacts with IL-2R (Figure 5) via molecular crosstalk. These events do not require CD131 because EPO equally inhibited anti–CD3- and anti–CD28-stimulated WT and CD131−/− T cell proliferation (two independent experiments, data not shown). This EPO-induced, SHP-1–dependent inhibition of IL-2Rβ signaling spares Treg because EPO does not impair IL-2Rγ/STAT5 signaling (Figure 5, Supplemental Figure 8) required for IL-2–induced Treg proliferation,41 and because AKT signaling (among other pathways) is constitutively prevented by endogenous phosphatases preferentially expressed in Treg.35,42
Building upon data in rodents showing that EPO induces TGFβ synthesis by monocytes43 and tubular cells,44 we demonstrate that EPO converts naïve CD4+ T cells into functional CD4+CD25+FOXP3+ iTreg by stimulating local TGFβ production. EPO simultaneously upregulates production of uPA (among other proteases), which cleaves LAP, thereby activating TGFβ to drive FOXP3 expression (Figure 1). These effects require CD131 on APCs (Figure 2), supporting the conclusion that they are mediated through EPO ligating the EPO-R/CD131 heterodimer. Whether EPO-R–transmitted signals on naïve T cells interact with and/or synergize with TGFβ-initiated signals to directly support Treg generation remains to be discerned. These new mechanistic insights explain a previous report by others in which spontaneous murine kidney transplant acceptance was associated with kidney-produced uPA and TGFβ.45
Although the EPO doses used for our in vitro experiments are above those used clinically, our data remarkably show that EPO administered at doses used for correction of anemia augment frequencies of CD4+FOXP3+ T cells in patients with CKD (Figure 3). Short-term EPO therapy also augments murine Treg generation in vivo and prolongs heart transplant survival (Figure 6), raising the possibility that similar approaches should be tested as induction therapy to prevent rejection of human organ transplants. Conversely, we showed that acriflavine-induced EPO downregulation prevents spontaneous kidney transplant-induced iTreg generation, lowers intragraft TGFβ gene expression, and prevents acceptance of allogeneic kidney transplants (Figure 6), whereas adding back recombinant EPO rescues these acriflavine-induced deleterious effects (Figure 6), supporting the conclusion that kidney-derived EPO is a crucial regulator of T cell alloimmunity after kidney transplantation.
It is worth noting that WT animals with intact native kidneys capable of producing EPO nonetheless rapidly reject allogeneic WT heart allografts (Figure 6 and Baldwin et al.46), suggesting that kidney-derived, circulating levels of EPO are insufficient to regulate heart transplant-induced alloimmune responses (Figure 6), despite the fact that circulating EPO levels are adequate to drive RBC development in the bone marrow.16 We posit that the high local EPO levels within the kidney (Figure 2) and/or within draining immune organs optimally generate iTreg (and inhibit donor-reactive Tconv) because these relatively high levels of EPO are present directly at sites of allo-antigen recognition.
Our data provide a potential explanation to account for published evidence that EPO therapy improves kidney transplant outcomes.21 EPO-dependent immunoregulation could additionally account for (1) the lower rates of acute rejection in kidney compared with heart or lung transplant recipients47–49 (kidneys produce EPO but hearts and lungs do not); (2) the better outcomes in recipients of combined kidney/heart transplants from the same donor versus recipients of hearts alone50; and (3) the observation that spontaneous human kidney transplant acceptance/tolerance occurs, albeit infrequently,51,52 whereas spontaneous human heart transplant acceptance/tolerance has not been described.
Beyond transplantation, the protolerogenic effects of EPO delineated herein provide potential additional explanations for published observations that EPO reduces clinical expression of experimental arthritis,53 EAE,54,55 experimental colitis,56,57 and lupus nephritis,58 and that EPO improves disease activity in rheumatoid patients with arthritis59 or lupus.60 EPO’s protolerogenic effects could also contribute to tumor growth, as described in patients treated with EPO.61,62
A potential increased risk of thrombotic events possibly because of augmented blood viscosity and endothelial and platelet activation63 is a theoretical risk in transplant recipients who may receive EPO therapy as an immunosuppressant. EPO analogs without hematopoietic function have been developed to overcome this problem, including ARA 290, which has a high specificity for the EPO-R/CD131 heterodimer.64,65 Our data document that activation of this receptor is responsible for production of active TGFβ in monocytes (Figure 2), whereas previous data by others showed that ARA 290 inhibits secretion of IL-6, IL-12, and TNF-α by macrophages stimulated with LPS and IFNγ, and prolongs survival of islet transplantation in mice.66 Building upon the available clinical data showing that ARA 290 can safely improve outcomes in patients with sarcoidosis67 or diabetes,68 ARA 290 has the potential to safely improve outcomes in patients with organ transplants or autoimmune diseases by promoting Treg induction and inhibiting innate immune response.
In conclusion, our data collectively elucidate previously unrecognized protolerogenic effects of EPO, particularly within the kidney. In addition, the findings identify a new clinically viable, pharmacologic strategy capable of inhibiting Tconv and also augmenting endogenous Treg as a means to prevent and/or treat transplant rejection, graft versus host disease, and autoimmune disease.
Concise Methods
Isolation of Human Peripheral Blood Cell and T Cell Subsets
PBMC were isolated from buffy coats obtained from deidentified healthy donors (New York Blood Bank) through Ficoll–Hypaque (SIGMA LifeScience) density gradient centrifugation. CD4+CD45RA+CD45RO− naïve CD4+ T cells and CD4+CD25+CD127low Treg were enriched through magnetic isolation (Miltenyi Biotec) using AutoMACS (Miltenyi Biotech) (>95% purity). Enriched peripheral Treg were directly used to test EPO-R expression. Total CD3+ T cells and CD14+ monocytes were isolated with EasySep (STEMCELL Technologies) selection Kits with a purity >95%.
Frozen aliquots of previously isolated and in vitro expanded Treg were used for functional, biochemical, and signaling studies. These Treg were isolated and expanded under good manufacturing practice conditions as published.69 The expanded Treg maintained the CD4+CD25+FOXP3+ phenotype and their suppressive function (Supplemental Figure 3).
Microscopy and Imaging Flow Cytometry
For microscopy and Imaging flow cytometry experiments cells were stained for 1 hour at room temperature with an anti–EPO-R mouse IgG2a mAb (clone 38407; R&D Systems, Minneapolis, MN) conjugated to Alexa Fluor 488 (Zenon Alexa Fluor 488 Mouse IgG2a Labeling kit Z-25102; Thermo Fisher Scientific) together with an anti-CD4 mouse IgG1 mAb conjugated with APC (clone RPA-T4; BD Biosciences) and the nuclear staining dye Hoechst 33342 (catalog no. 904536; Invitrogen, Carlsbad, CA). Controls were performed by staining the corresponding isotype antibodies: IgG2a conjugated to Alexa Fluor 488 (catalog no. MAB003; R&D Systems) and IgG1 conjugated to APC (catalog no. 555751; BD Biosciences).
Cells adhered on glass coverslips previously coated with laminin and poly-D-lysin as described previously.70 Coverslips were washed with PBS and then were fixed in PBS containing 3.7% (vol/vol) paraformaldehyde for 10 minutes at room temperature. After fixation, cell were washed with PBS and permeabilized with 70% (vol/vol) ethanol for 20 minutes at 4°C. Cells were then stained as described above and coverslips were mounted using an aqueous mounting medium (CTS011; R&D Systems). Cells were imaged with an upright epifluorescence microscope, Axioplan Imager 2 (Zeiss), with a 100× oil immersion objective and a numerical aperture of 1.4. The microscope was controlled by the Zeiss Zen software and was equipped with a Zeiss AxioCam MRc camera. The laser exposure time was 250 ms and 10 ms for the AF488 and Hoechst fluorescence channels, respectively.
Imaging flow cytometry was used to measure EPO-R total and surface expression at a single cell level. Naïve CD4+ T cells and Treg were isolated, permeabilized, and fixed (00–5523–00; eBiosience) before staining to avoid a possible internalization of the receptor because of antibody binding.71 After staining, images of each cell were acquired at a magnification of 60× on an ImageStream flow cytometer (Amnis) and analyzed using IDEAS analysis software. Single-color controls were used for the creation of a compensation matrix that was applied to all files to correct for spectral crosstalk. Surface receptor expression was estimated by constructing a contour mask on the bright-field image and applying it to the EPO-R-AF488 channel, an approach that has been successfully applied to quantify surface expression.72,73
Flow Cytometry
For surface staining, human or murine cells were stained using standard approaches as published.22,30 Antibodies to human antigens were obtained from BD Pharmingen: APC-Cy7-anti–CD4 (clone RPA-T4), PE-anti–CD8 (clone RPA-T8), APC-Cy7-anti–CD8 (clone RPA-T8), Fixable Viability Dye eFlour450, APC-anti–CD25 (clone BC96; eBioscience), and FITC-anti–EPO-R (clone 38407; R&D Systems). Antibodies to murine antigens were obtained from eBioscience: Fixable Viability Dye eFlour480, Pacific Blue-Anti–CD4 (clone RM4–5), PE-anti–CD25 (clone PC61.5), and APC-anti–Vα2 TCR (clone B20.1). Intracellular staining for human or murine Foxp3 and IFNγ were performed as published.22,30
For intracellular signaling, phospho-flow analyses of human T cells or Treg were distributed into round-bottom 96-well plates (2×105 per well) in CTL Free Serum Media (C.T.L., Shaker Heights, OH) and allowed to rest at 37°C for 4 hours. Cells were kept resting or activated with anti-CD3 and anti-CD28 mAb beads (25 μl/106) and IL-2 (100 IU/ml) with or without EPO (1000 IU/ml) for 5, 15, 30, 45, and 60 minutes at 37°C. The reaction was stopped by fixation with 4% paraformaldehyde (Affymetrix) for 10 minutes at 37°C. The cells were washed with PBS and permeabilized by resuspension in 90% cold methanol (Fisher Scientific) for 15 minutes in the rocker. Samples were washed three times with PBS 1% FBS (Thermo Scientific, Wilmington, DE) and stained overnight at 4°C for Pacific Blue anti-CD4 3 μg/ml (clone RPA-T4; BD Pharmingen) and PE anti-pAKT (pS473) 10 μg/ml (clone M89–61; BD Phosflow), or stained for 2 hours at room temperature for Alexa Fluor 647 conjugated anti-pSTAT5 (pY694) 10 μg/ml (clone 47/Stat5; BD Phosflow). In selected experiments the SHP-1 inhibitor NCS-87877 (EMD Millipore, Billerica, MA) was added at 37.5 μg/ml concentration. Data were acquired (10,000–100,000 events) on a three-laser Canto II flow cytometer (Becton, Dickinson) and analyzed using FlowJo software (Tree Star) or CytoBank software (https://www.cytobank.org).
Human T Cell Proliferation and Suppression Assays
Isolated cells (2×105 per well) were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE; Invitrogen) and stimulated with anti-CD3 and anti-CD28 mAb-coated beads (25 μl/106 cells, Dynabeads; Gibco life technologies) and recombinant IL-2 (100 IU/ml; BD Biosciences) and proliferation was assessed by CFSE dilution as published.22 To assess suppressive capacity, CFSE-labeled PBMC (1×105 per well) were stimulated with anti-CD3 mAb (1 μg/ml; BD Biosciences) in the presence of varying ratios of Treg (1:1, 2:1, 4:1, 8:1, 16:1, and 32:1) in complete RPMI media. After 5 days, CD8 T cell proliferation was quantified by CFSE dilution using the Canto II flow cytometer.
Treg Generation In Vitro
Syngeneic CD14+ cells were incubated with naïve CD4+ cells in CTL media with 2% FBS (Thermo Scientific) with anti-CD3 mAb (1 μg/ml; BD Biosciences) and IL-2 (100 IU/ml; BD Biosciences) with and without EPO (1000 IU/ml, Procrit; Janssen–Cilag, Titusville, NJ) and/or TGFβ (5 ng/ml; PeproTech) for 5 days. A blocking anti-TGFβ antibody (Abcam), a blocking anti-TGFβ receptor (R&D Systems) antibody, or an uPA inhibitor (Santa Cruz Biotechnology) was added to selected experiments as indicated. Murine Treg induction was performed as published,30 with and without recombinant EPO.
Immunoprecipitation Assays
T cells were rested at 37°C for 4 hours in CTL Free Serum Media (C.T.L.). Aliquots were activated with IL-2 (100 IU/ml) or EPO (1000 IU/ml) for 60 minutes. Cell lysates were prepared in 25 mM Tris-HCl pH 7.4, 150 mM NaCl, 1% NP-40, 1 mM EDTA, and 5% glycerol, and incubated with anti–pSHP-1 (Tyr564; Cell Signaling Technology) or anti–IL-2Rβ (Santa Cruz Biotechnology) with gentle rotation overnight at 4°C. Protein A/G PLUS-Agarose preblocked with BSA (Santa Cruz Biotechnology) was added for an additional 4 hours. Beads were centrifuged (10,000×g for 5 minutes) and washed three times. Proteins associated with the beads were eluted by boiling in loading buffer and analyzed by SDS-PAGE and immunoblotting.
Immunoblots
We stimulated 3×106 T cells with EPO (1000 IU/ml) for 60, 45, 30, 15, and 5 minutes at 37°C. We stopped the activation by adding phosphatase inhibitor containing lysis buffer for 30 minutes on a rocker. Lysates were centrifuged at 12,000×g for 20 minutes at 4°C, and supernatant was stored at −80°C. Protein concentration was measured by NanoDrop 2000 spectrophotometer (Thermo Scientific). The same amount of protein was loaded in Bolt 4%–12% Bis-Tris Plus gels precasted polyacrylamide gels from Novex (Life Technology) and electrophoresis was performed using Novex manufacturer’s recommendations. Anti–SHP-1 and anti–pSHP-1 (Tyr564) antibodies used were from Cell Signaling Technology.
Real-Time qRT-PCR
RNA isolation was performed using TRIzol (Thermo Fisher Scientific) and cDNA was reverse-transcribed using the High-Capacity cDNA Reverse Transcription kit (Applied Biosystems) as per the manufacturer’s instructions. RT-PCR (TaqMan probes; Applied Biosystems) was performed using the CFX96 Real-Time PCR Detection System (Bio-Rad Laboratories). All human and mouse PCR primers were purchased from Life Technologies. Real-time PCR assays using the TaqMan universal PCR Master Mix and primer sets for human TGFβ1, uPA, and mouse EPO were purchased from Thermo Fisher Scientific. PCR was performed on a C1000 Touch Thermal Cycler from BioRad. All experiments were performed at least in triplicate, and gene expression was normalized to housekeeping gene 18s (for immune cells) or GAPDH (for kidney or heart cells) and expressed as fold change using the ΔΔCt method.
ELISAs
ELISAs for TGFβ or uPA (Quantikine ELISA Kit; R&D Systems) were performed as per manufacturer’s instructions.
Bioactive TGFβ Assays
Active TGFβ was assessed using the HEK-Blue TGFβ sensor reporter cell line (Invitrogen) according to the manufacturer’s instructions.
Mice
NOD scidγc−/−, BALB/c (H-2d), A/J, TEa Foxp3GFP (intercrossed from TEa and Foxp3GFP mice), C57BL/6 (H-2b), B6rag1−/− (Jackson Laboratories, Bar Harbor, ME), and CD131−/− mice (kind gift from Miriam Merad, Icahn School of Medicine at Mount Sinai, New York, NY)74 were housed at the Icahn School of Medicine at Mount Sinai Center for Comparative Medicine and Surgery or at the Cleveland Clinic (Cleveland, Ohio), in accordance with guidelines of the Association for Assessment and Accreditation of Laboratory Animal Care International. The study protocols described herein were reviewed and approved by the Institutional Animal Care and Use Committee at Icahn School of Medicine at Mount Sinai and at Cleveland Clinic.
In Vivo Treg Induction Experiments
Naïve CD4 HLA-A2 cells from two human donors were incubated with anti-CD3 and anti-CD28 mAb-coated beads (25 μl/106 cells, Dynabeads) recombinant IL-2 (100 IU/ml; BD Biosciences), and TGFβ (5 ng/ml; PeproTech) in Complete RPMI media at for 5 days at 37°C and 5% CO2. CD25+ iTreg from HLA-A2 donors (1×106 per mouse) were given as retro-orbital injections together with PBMC from HLA-A2 donors into NOD scidγc−/− mice. Animals were treated with EPOα (5000 IU/kg per day for 3 days) or vehicle control and euthanized at day 3 after injection for flow cytometry analysis of FOXP3+CD4+HLA-A2pos PBMC.
In separate experiments, naïve CD4 GFPFoxp3 TEa cells (1×106 per mouse) were adoptively transferred by retro-orbital injection into RAG B6 mice transplanted with a BALB/c heart three days prior. Starting from the day of TEa cell injection, mice were treated with EPOα (5000 IU/kg per day for 5 days) or vehicle control and euthanized at day 5 after injection for flow cytometry analysis of GFPFoxp3+CD4+ TEa cell.
Kidney Transplant
Murine kidney transplantation with urinary reconstruction was performed as previously detailed.75 On day 4 post-transplant, the native (left) kidney was removed, rendering the recipient dependent on the function of the graft. Graft function and survival were followed by daily examination of animal health and monitoring of serum Cr levels. Whole-blood Cr levels were determined using an I-Stat portable clinical analyzer (Heska). Allograft rejection was deemed ongoing when the recipient showed signs of illness and the Cr level was elevated to ≥1.5 mg/dl, and at this time, mice were euthanized and grafts harvested for histopathology analysis.
Vascularized Heart Transplantation
BALB/c hearts were transplanted as fully vascularized heterotopic grafts into C57BL/6 mice as previously described.46 Mice were treated with EPOα (5000 IU/kg per day for 3 days, administered intraperitoneally, starting perioperatively) or vehicle, with or without a perioperative intraperitoneal dose of CTLA4Ig (250 μg) Graft function was monitored every other day by abdominal palpation. Rejection was defined as complete cessation of a palpable beat and confirmed by direct visualization at laparotomy.
Acriflavine and EPO Administration
Acriflavine (Sigma) was given at the dose of 2 mg/kg via intraperitoneal injection for the specified time periods. EPO (EPOα; Procrit) was given at the dose of 10,000 IU/kg via intraperitoneal injection for the specified time periods.
Patient Enrollment and PBMC Acquisition
Blood samples (20 ml) were obtained from patients with CKD stage 4 (eGFR of 15–30 ml/min per m2)29 who were to initiate EPO therapy for anemia and from control age- and sex-matched subjects with stage 3–4 CKD without anemia (not expected to receive EPO over the 6-month follow-up period) (see Supplemental Table 1 for patients’ baseline characteristics). We included consenting patients aged 18–80 years, who had not received EPO or blood transfusions in the previous 6 months, with sufficient iron (Tsat>20%, ferritin >20 ng/ml) and folic acid stores. Exclusion criteria included presence of autoimmune diseases or current immunosuppression therapy. Blood samples were obtained in the morning of the day EPO was started and after 6 months of treatment. Control subjects also had blood taken at study enrollment and at 6 months thereafter.
All studies were done in adherence to the Declaration of Helsinki. The protocol was approved by Navarra Government Institutional Review Board (Comite Etico de Investigacion Clinica) Pamplona, Spain and the samples were managed by Biobank research unit from Navarrabiomed-Fundacion Miguel Servet.
Statistical Analyses
Because of the heterogeneity of responses in each assay type among the tested human donors, we normalized results of each set of experiments defining the response in vehicle/control for each donor as 100% and the responses in each of the experimental manipulations from that donor as a percent change relative to the 100% vehicle/control value. Group comparisons of paired data were analyzed by paired t tests if not otherwise specified. We used mixed effects two-way ANOVA for multiple repeated measures, and one-way ANOVA (with Tukey test for post hoc pairwise comparisons) for independent samples. The assumptions of normality and homogeneity of variance was assessed using Shapiro–Wilk normality test and by examining plots of residuals (residuals versus fitted value plots, and distributional diagnostic plots). The analysis of TGFβ1 gene expression in mice was carried out after log transformation of the data to improve normality. Two-tailed P values <0.05 were considered significant. All statistical analyses were performed using GraphPad Prism (version 5 for Windows; GraphPad Software, Inc.) and Stata 14 (StataCorp, College Station, TX).
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank Peter Boros, Yansui Li, and Jinhua Liu for their microsurgical support.
This study was funded by support from the US National Institutes of Health grants P01 AI087506 (to R.L.F. and W.M.B.), R01 HL11879 (to B.R.B), P01 AI 123086 (to J.M., R.B.C., and P.S.H.), AI 071185 (to P.S.H.), and 5T32AI078892-07 (to P.C.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2016101100/-/DCSupplemental.
References
- 1.Kuhn C, Weiner HL: Immunology. How does the immune system tolerate food? Science 351: 810–811, 2016 [DOI] [PubMed] [Google Scholar]
- 2.Yamada Y, Ochiai T, Boskovic S, Nadazdin O, Oura T, Schoenfeld D, Cappetta K, Smith RN, Colvin RB, Madsen JC, Sachs DH, Benichou G, Cosimi AB, Kawai T: Use of CTLA4Ig for induction of mixed chimerism and renal allograft tolerance in nonhuman primates. Am J Transplant 14: 2704–2712, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai T, Sachs DH, Sykes M, Cosimi AB; Immune Tolerance Network : HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med 368: 1850–1852, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sykes M: Immune tolerance in recipients of combined haploidentical bone marrow and kidney transplantation. Bone Marrow Transplant 50[Suppl 2]: S82–S86, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias N, Cosimi AB, Kawai T: Clinical trials for induction of renal allograft tolerance. Curr Opin Organ Transplant 20: 406–411, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Safinia N, Scotta C, Vaikunthanathan T, Lechler RI, Lombardi G: Regulatory T cells: Serious contenders in the promise for immunological tolerance in transplantation. Front Immunol 6: 438, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, Jiang S, Kuchroo VK, Mathis D, Roncarolo MG, Rudensky A, Sakaguchi S, Shevach EM, Vignali DA, Ziegler SF: Regulatory T cells: Recommendations to simplify the nomenclature. Nat Immunol 14: 307–308, 2013 [DOI] [PubMed] [Google Scholar]
- 8.van der Veeken J, Arvey A, Rudensky A: Transcriptional control of regulatory T-cell differentiation. Cold Spring Harb Symp Quant Biol 78: 215–222, 2013 [DOI] [PubMed] [Google Scholar]
- 9.Brunstein CG, Miller JS, McKenna DH, Hippen KL, DeFor TE, Sumstad D, Curtsinger J, Verneris MR, MacMillan ML, Levine BL, Riley JL, June CH, Le C, Weisdorf DJ, McGlave PB, Blazar BR, Wagner JE: Umbilical cord blood-derived T regulatory cells to prevent GVHD: Kinetics, toxicity profile, and clinical effect. Blood 127: 1044–1051, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hippen KL, Merkel SC, Schirm DK, Nelson C, Tennis NC, Riley JL, June CH, Miller JS, Wagner JE, Blazar BR: Generation and large-scale expansion of human inducible regulatory T cells that suppress graft-versus-host disease. Am J Transplant 11: 1148–1157, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bluestone JA, Buckner JH, Fitch M, Gitelman SE, Gupta S, Hellerstein MK, Herold KC, Lares A, Lee MR, Li K, Liu W, Long SA, Masiello LM, Nguyen V, Putnam AL, Rieck M, Sayre PH, Tang Q: Type 1 diabetes immunotherapy using polyclonal regulatory T cells. Sci Transl Med 7: 315ra189, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bluestone JA, Trotta E, Xu D: The therapeutic potential of regulatory T cells for the treatment of autoimmune disease. Expert Opin Ther Targets 19: 1091–1103, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Koreth J, Matsuoka K, Kim HT, McDonough SM, Bindra B, Alyea EP 3rd, Armand P, Cutler C, Ho VT, Treister NS, Bienfang DC, Prasad S, Tzachanis D, Joyce RM, Avigan DE, Antin JH, Ritz J, Soiffer RJ: Interleukin-2 and regulatory T cells in graft-versus-host disease. N Engl J Med 365: 2055–2066, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He J, Zhang X, Wei Y, Sun X, Chen Y, Deng J, Jin Y, Gan Y, Hu X, Jia R, Xu C, Hou Z, Leong YA, Zhu L, Feng J, An Y, Jia Y, Li C, Liu X, Ye H, Ren L, Li R, Yao H, Li Y, Chen S, Zhang X, Su Y, Guo J, Shen N, Morand EF, Yu D, Li Z: Low-dose interleukin-2 treatment selectively modulates CD4(+) T cell subsets in patients with systemic lupus erythematosus. Nat Med 22: 991–993, 2016 [DOI] [PubMed] [Google Scholar]
- 15.Kosmaczewska A: Low-dose interleukin-2 therapy: A driver of an imbalance between immune tolerance and autoimmunity. Int J Mol Sci 15: 18574–18592, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bunn HF: Erythropoietin. Cold Spring Harb Perspect Med 3: a011619, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuhrt D, Wojchowski DM: Emerging EPO and EPO receptor regulators and signal transducers. Blood 125: 3536–3541, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Broxmeyer HE: Erythropoietin: Multiple targets, actions, and modifying influences for biological and clinical consideration. J Exp Med 210: 205–208, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vogel J, Gassmann M: Erythropoietic and non-erythropoietic functions of erythropoietin in mouse models. J Physiol 589: 1259–1264, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cassis P, Gallon L, Benigni A, Mister M, Pezzotta A, Solini S, Gagliardini E, Cugini D, Abbate M, Aiello S, Rocchetta F, Scudeletti P, Perico N, Noris M, Remuzzi G: Erythropoietin, but not the correction of anemia alone, protects from chronic kidney allograft injury. Kidney Int 81: 903–918, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Choukroun G, Kamar N, Dussol B, Etienne I, Cassuto-Viguier E, Toupance O, Glowacki F, Moulin B, Lebranchu Y, Touchard G, Jaureguy M, Pallet N, Le Meur Y, Rostaing L, Martinez F; CAPRIT study Investigators : Correction of postkidney transplant anemia reduces progression of allograft nephropathy. J Am Soc Nephrol 23: 360–368, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cravedi P, Manrique J, Hanlon KE, Reid-Adam J, Brody J, Prathuangsuk P, Mehrotra A, Heeger PS: Immunosuppressive effects of erythropoietin on human alloreactive T cells. J Am Soc Nephrol 25: 2003–2015, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fortunel NO, Hatzfeld A, Hatzfeld JA: Transforming growth factor-beta: Pleiotropic role in the regulation of hematopoiesis. Blood 96: 2022–2036, 2000 [PubMed] [Google Scholar]
- 24.Dzieran J, Fabian J, Feng T, Coulouarn C, Ilkavets I, Kyselova A, Breuhahn K, Dooley S, Meindl-Beinker NM: Comparative analysis of TGF-β/Smad signaling dependent cytostasis in human hepatocellular carcinoma cell lines. PLoS One 8: e72252, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shay JE, Imtiyaz HZ, Sivanand S, Durham AC, Skuli N, Hsu S, Mucaj V, Eisinger-Mathason TS, Krock BL, Giannoukos DN, Simon MC: Inhibition of hypoxia-inducible factors limits tumor progression in a mouse model of colorectal cancer. Carcinogenesis 35: 1067–1077, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, Jessberger R, Trinchieri G, Lira SA, Randolph GJ, Bromberg JS: Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol 7: 652–662, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA: CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med 203: 1701–1711, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B: Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203: 1693–1700, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kidney Disease Outcome Quality Initiative: K/DOQI clinical practice guidelines for chronic kidney disease : Evaluation, classification, and stratification. Am J Kidney Dis 39: S17–S31, 2002 [PubMed] [Google Scholar]
- 30.van der Touw W, Cravedi P, Kwan WH, Paz-Artal E, Merad M, Heeger PS: Cutting edge: Receptors for C3a and C5a modulate stability of alloantigen-reactive induced regulatory T cells. J Immunol 190: 5921–5925, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi H, Kanno T, Nakayamada S, Hirahara K, Sciumè G, Muljo SA, Kuchen S, Casellas R, Wei L, Kanno Y, O’Shea JJ: TGF-β and retinoic acid induce the microRNA miR-10a, which targets Bcl-6 and constrains the plasticity of helper T cells. Nat Immunol 13: 587–595, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao W, Lin JX, Leonard WJ: Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38: 13–25, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benczik M, Gaffen SL: The interleukin (IL)-2 family cytokines: Survival and proliferation signaling pathways in T lymphocytes. Immunol Invest 33: 109–142, 2004 [DOI] [PubMed] [Google Scholar]
- 34.Gaffen SL: Signaling domains of the interleukin 2 receptor. Cytokine 14: 63–77, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Huynh A, DuPage M, Priyadharshini B, Sage PT, Quiros J, Borges CM, Townamchai N, Gerriets VA, Rathmell JC, Sharpe AH, Bluestone JA, Turka LA: Control of PI(3) kinase in Treg cells maintains homeostasis and lineage stability. Nat Immunol 16: 188–196, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng G, Yu A, Malek TR: T-cell tolerance and the multi-functional role of IL-2R signaling in T-regulatory cells. Immunol Rev 241: 63–76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klingmüller U, Lorenz U, Cantley LC, Neel BG, Lodish HF: Specific recruitment of SH-PTP1 to the erythropoietin receptor causes inactivation of JAK2 and termination of proliferative signals. Cell 80: 729–738, 1995 [DOI] [PubMed] [Google Scholar]
- 38.Migone TS, Cacalano NA, Taylor N, Yi T, Waldmann TA, Johnston JA: Recruitment of SH2-containing protein tyrosine phosphatase SHP-1 to the interleukin 2 receptor; loss of SHP-1 expression in human T-lymphotropic virus type I-transformed T cells. Proc Natl Acad Sci USA 95: 3845–3850, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ashoor I, Najafian N, Korin Y, Reed EF, Mohanakumar T, Ikle D, Heeger PS, Lin M: Standardization and cross validation of alloreactive IFNγ ELISPOT assays within the clinical trials in organ transplantation consortium. Am J Transplant 13: 1871–1879, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tse GH, Hughes J, Marson LP: Systematic review of mouse kidney transplantation. Transpl Int 26: 1149–1160, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Vogtenhuber C, Bucher C, Highfill SL, Koch LK, Goren E, Panoskaltsis-Mortari A, Taylor PA, Farrar MA, Blazar BR: Constitutively active Stat5b in CD4+ T cells inhibits graft-versus-host disease lethality associated with increased regulatory T-cell potency and decreased T effector cell responses. Blood 116: 466–474, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu A, Snowhite I, Vendrame F, Rosenzwajg M, Klatzmann D, Pugliese A, Malek TR: Selective IL-2 responsiveness of regulatory T cells through multiple intrinsic mechanisms supports the use of low-dose IL-2 therapy in type 1 diabetes. Diabetes 64: 2172–2183, 2015 [DOI] [PubMed] [Google Scholar]
- 43.Mausberg AK, Meyer Zu Hörste G, Dehmel T, Stettner M, Lehmann HC, Sheikh KA, Kieseier BC: Erythropoietin ameliorates rat experimental autoimmune neuritis by inducing transforming growth factor-β in macrophages. PLoS One 6: e26280, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gobe GC, Bennett NC, West M, Colditz P, Brown L, Vesey DA, Johnson DW: Increased progression to kidney fibrosis after erythropoietin is used as a treatment for acute kidney injury. Am J Physiol Renal Physiol 306: F681–F692, 2014 [DOI] [PubMed] [Google Scholar]
- 45.Bickerstaff AA, Wang JJ, Pelletier RP, Orosz CG: Murine renal allografts: Spontaneous acceptance is associated with regulated T cell-mediated immunity. J Immunol 167: 4821–4827, 2001 [DOI] [PubMed] [Google Scholar]
- 46.Baldwin WM 3rd, Su CA, Shroka TM, Fairchild RL: Experimental models of cardiac transplantation: Design determines relevance. Curr Opin Organ Transplant 19: 525–530, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2012 annual data report: Kidney. Am J Transplant 14[Suppl 1]: 11–44, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Colvin-Adams M, Smith JM, Heubner BM, Skeans MA, Edwards LB, Waller C, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2011 annual data report: Heart. Am J Transplant 13[Suppl 1]: 119–148, 2013 [DOI] [PubMed] [Google Scholar]
- 49.Tonsho M, Michel S, Ahmed Z, Alessandrini A, Madsen JC: Heart transplantation: Challenges facing the field. Cold Spring Harb Perspect Med 4: 4, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gill J, Shah T, Hristea I, Chavalitdhamrong D, Anastasi B, Takemoto SK, Bunnapradist S: Outcomes of simultaneous heart-kidney transplant in the US: A retrospective analysis using OPTN/UNOS data. Am J Transplant 9: 844–852, 2009 [DOI] [PubMed] [Google Scholar]
- 51.Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI, Hernandez-Fuentes MP, Turka LA, Seyfert-Margolis VL; Immune Tolerance Network ST507 Study Group : Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120: 1836–1847, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S, Rovis F, Jimenez E, Ballow A, Giral M, Rebollo-Mesa I, Le Moine A, Braudeau C, Hilton R, Gerstmayer B, Bourcier K, Sharif A, Krajewska M, Lord GM, Roberts I, Goldman M, Wood KJ, Newell K, Seyfert-Margolis V, Warrens AN, Janssen U, Volk HD, Soulillou JP, Hernandez-Fuentes MP, Lechler RI: Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120: 1848–1861, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cuzzocrea S, Mazzon E, di Paola R, Genovese T, Patel NS, Britti D, de Majo M, Caputi AP, Thiemermann C: Erythropoietin reduces the degree of arthritis caused by type II collagen in the mouse. Arthritis Rheum 52: 940–950, 2005 [DOI] [PubMed] [Google Scholar]
- 54.Agnello D, Bigini P, Villa P, Mennini T, Cerami A, Brines ML, Ghezzi P: Erythropoietin exerts an anti-inflammatory effect on the CNS in a model of experimental autoimmune encephalomyelitis. Brain Res 952: 128–134, 2002 [DOI] [PubMed] [Google Scholar]
- 55.Yuan R, Maeda Y, Li W, Lu W, Cook S, Dowling P: Erythropoietin: A potent inducer of peripheral immuno/inflammatory modulation in autoimmune EAE. PLoS One 3: e1924, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nairz M, Schroll A, Moschen AR, Sonnweber T, Theurl M, Theurl I, Taub N, Jamnig C, Neurauter D, Huber LA, Tilg H, Moser PL, Weiss G: Erythropoietin contrastingly affects bacterial infection and experimental colitis by inhibiting nuclear factor-κB-inducible immune pathways. Immunity 34: 61–74, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cuzzocrea S, Mazzon E, Di Paola R, Patel NS, Genovese T, Muià C, De Sarro A, Thiemermann C: Erythropoietin reduces the development of experimental inflammatory bowel disease. J Pharmacol Exp Ther 311: 1272–1280, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Luo B, Gan W, Liu Z, Shen Z, Wang J, Shi R, Liu Y, Liu Y, Jiang M, Zhang Z, Wu Y: Erythropoeitin signaling in macrophages promotes dying cell clearance and immune tolerance. Immunity 44: 287–302, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Kaltwasser JP, Kessler U, Gottschalk R, Stucki G, Möller B: Effect of recombinant human erythropoietin and intravenous iron on anemia and disease activity in rheumatoid arthritis. J Rheumatol 28: 2430–2436, 2001 [PubMed] [Google Scholar]
- 60.Kiss E, Kávai M, Csipõ I, Szegedi G: Recombinant human erythropoietin modulates erythrocyte complement receptor 1 functional activity in patients with lupus nephritis. Clin Nephrol 49: 364–369, 1998 [PubMed] [Google Scholar]
- 61.Leyland-Jones B; BEST Investigators and Study Group : Breast cancer trial with erythropoietin terminated unexpectedly. Lancet Oncol 4: 459–460, 2003 [DOI] [PubMed] [Google Scholar]
- 62.Arbuckle RB, Griffith NL, Iacovelli LM, Johnson PE, Jorgenson JA, Kloth DD, Lucarelli CD, Muller RJ: Continued challenges with the use of erythropoiesis-stimulating agents in patients with cancer: Perspectives and issues on policy-guided health care. Pharmacotherapy 28: 1S–15S, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Stohlawetz PJ, Dzirlo L, Hergovich N, Lackner E, Mensik C, Eichler HG, Kabrna E, Geissler K, Jilma B: Effects of erythropoietin on platelet reactivity and thrombopoiesis in humans. Blood 95: 2983–2989, 2000 [PubMed] [Google Scholar]
- 64.Brines M, Patel NS, Villa P, Brines C, Mennini T, De Paola M, Erbayraktar Z, Erbayraktar S, Sepodes B, Thiemermann C, Ghezzi P, Yamin M, Hand CC, Xie QW, Coleman T, Cerami A: Nonerythropoietic, tissue-protective peptides derived from the tertiary structure of erythropoietin. Proc Natl Acad Sci USA 105: 10925–10930, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Collino M, Thiemermann C, Cerami A, Brines M: Flipping the molecular switch for innate protection and repair of tissues: Long-lasting effects of a non-erythropoietic small peptide engineered from erythropoietin. Pharmacol Ther 151: 32–40, 2015 [DOI] [PubMed] [Google Scholar]
- 66.Watanabe M, Lundgren T, Saito Y, Cerami A, Brines M, Östenson CG, Kumagai-Braesch M: A nonhematopoietic erythropoietin analogue, ARA 290, inhibits macrophage activation and prevents damage to transplanted islets. Transplantation 100: 554–562, 2016 [DOI] [PubMed] [Google Scholar]
- 67.Dahan A, Dunne A, Swartjes M, Proto PL, Heij L, Vogels O, van Velzen M, Sarton E, Niesters M, Tannemaat MR, Cerami A, Brines M: ARA 290 improves symptoms in patients with sarcoidosis-associated small nerve fiber loss and increases corneal nerve fiber density. Mol Med 19: 334–345, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brines M, Dunne AN, van Velzen M, Proto PL, Ostenson CG, Kirk RI, Petropoulos IN, Javed S, Malik RA, Cerami A, Dahan A: ARA 290, a nonerythropoietic peptide engineered from erythropoietin, improves metabolic control and neuropathic symptoms in patients with type 2 diabetes. Mol Med 20: 658–666, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hippen KL, Merkel SC, Schirm DK, Sieben CM, Sumstad D, Kadidlo DM, McKenna DH, Bromberg JS, Levine BL, Riley JL, June CH, Scheinberg P, Douek DC, Miller JS, Wagner JE, Blazar BR: Massive ex vivo expansion of human natural regulatory T cells (T(regs)) with minimal loss of in vivo functional activity. Sci Transl Med 3: 83ra41, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Patil S, Fribourg M, Ge Y, Batish M, Tyagi S, Hayot F, Sealfon SC: Single-cell analysis shows that paracrine signaling by first responder cells shapes the interferon-β response to viral infection. Sci Signal 8: ra16, 2015 [DOI] [PubMed] [Google Scholar]
- 71.Porritt RA, Hertzog PJ: Dynamic control of type I IFN signalling by an integrated network of negative regulators. Trends Immunol 36: 150–160, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Ralston KS, Solga MD, Mackey-Lawrence NM, Somlata, Bhattacharya A, Petri WA Jr: Trogocytosis by Entamoeba histolytica contributes to cell killing and tissue invasion. Nature 508: 526–530, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang SY, Song JH, Guleng B, Cotoner CA, Arihiro S, Zhao Y, Chiang HS, O’Keeffe M, Liao G, Karp CL, Kweon MN, Sharpe AH, Bhan A, Terhorst C, Reinecker HC: Circulatory antigen processing by mucosal dendritic cells controls CD8(+) T cell activation. Immunity 38: 153–165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kwan WH, Hashimoto D, Paz-Artal E, Ostrow K, Greter M, Raedler H, Medof ME, Merad M, Heeger PS: Antigen-presenting cell-derived complement modulates graft-versus-host disease. J Clin Invest 122: 2234–2238, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Abe T, Ishii D, Gorbacheva V, Kohei N, Tsuda H, Tanaka T, Dvorina N, Nonomura N, Takahara S, Valujskikh A, Baldwin WM 3rd, Fairchild RL: Anti-huCD20 antibody therapy for antibody-mediated rejection of renal allografts in a mouse model. Am J Transplant 15: 1192–1204, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.