Abstract
The cause of spontaneous preterm labor (sPTL) is not known, but it could be due to epigenetic alterations that increase the sensitivity of decidual tissue to inflammatory stimuli. We collected decidual tissue from women at term not in labor (TNL), women at term in labor (TL), and women with sPTL. Illumina Infinium HumanMethylation450 BeadChip analysis revealed significantly reduced DNA methylation for TLR-2 and TLR-9 in sPTL as compared to TL. Immunohistochemical staining documented significantly increased expression of TLR-2 and TLR-9 in decidual tissue of women with sPTL as compared to TL or TNL. TLR expression was not present in decidual cells, but localized to tissue leukocytes as revealed by staining for CD14, a macrophage antigen, and neutrophil elastase. Microarray analysis of inflammatory genes to assess innate immune response demonstrated marked increases in expression of inflammatory cytokines and chemokines in women with TL as compared to TNL. However, when sPTL was compared to TL, there was a further increase in inflammatory cytokines, and a remarkable increase in neutrophil chemokines. These results suggest that epigenetic mechanisms could play a role in increasing leukocyte infiltration, and increasing the sensitivity of decidual tissue to inflammatory stimuli that could precipitate labor prematurely.
Keywords: preterm labor, DNA methylation, toll-like receptors, decidua, macrophages, neutrophils
1. Introduction
Preterm birth is the leading cause of neonatal morbidity and mortality worldwide accounting for as many as 75% of perinatal deaths (2007, Beck et al., 2010, Goldenberg et al., 2008). It is responsible for acute complications of the newborn, as well as long-term sequelae. In the United States 12.5% of births are preterm. Women experiencing preterm labor (PTL) present a number of distinct and independent clinical phenotypes, including infection, uterine over-distension (e.g., twins), hemorrhage, short cervix, stress, clinically diagnosed maternal or fetal abnormalities, but 40–45% are spontaneous PTL (sPTL) that is not explained by other factors (Myatt et al., 2012, Voltolini et al., 2013). A common factor of all births, whether term or preterm, is inflammation.
Our group recently demonstrated the presence of subclinical intrauterine infection and preserved ex vivo inflammatory status in decidual cells of women with sPTL who had no signs of clinical infection (Castro-Leyva et al., 2012). Both term and preterm intrauterine tissues can have bacteria present without overt infection and evidence is accruing that the intrauterine environment is not sterile as once thought (Jones et al., 2009, Romero et al., 2006, Steel et al., 2005, Stout et al., 2013). The presence of bacteria in both term and preterm labor suggests that the presence of bacteria alone is not sufficient to cause sPTL and raises the possibility that sPTL without clinical infection may be due to an increased sensitivity to inflammation by vaginal flora present in intrauterine tissues.
Recent evidence suggests that interaction between vaginal microbial flora and host immune response plays a significant role in sPTL (Ganu et al., 2013). The innate immune system responds to the microbiome by detecting pathogen-associated molecular patterns (PAMPs) through various germ-line encoded pattern recognition receptors. Toll-like receptors (TLRs) are the most widely studied innate sensors and play a fundamental role in pathogen recognition and activation of innate immunity (Janeway and Medzhitov, 2002, Kumar et al., 2011, Underhill and Ozinsky, 2002). Recent studies implicate TLR activated signaling pathways in fetal membranes in preterm birth associated with infection (Abrahams et al., 2013, Gillaux et al., 2011, Hoang et al., 2014). TLR-2 was markedly increased in chorioamniotic membranes in women with sPTL (Kim et al., 2004) and TLR-9 was specifically implicated in increased host response to fetal DNA in sPTL (Scharfe-Nugent et al., 2012, Phillippe, 2014).
Very little information is available concerning epigenetics and sPTL (Burris et al., 2012, Liu et al., 2013, Liu et al., 2012, Menon et al., 2012, Parets et al., 2013), although a number of factors implicate epigenetic factors (Menon et al., 2012). Stress and stress-related behaviors, such as poor diet, could play a role in influencing birth outcomes through epigenetic alterations. Maternal psychosocial stress is a main risk factors for preterm birth (Cardwell, 2013, Sanchez et al., 2013), and recent studies link psychological stress to oxidative stress (Aschbacher et al., 2013, Clerici et al., 2012, Epel, 2009, Hsieh et al., 2012, Szanton et al., 2012, Wang et al., 2007), which can affect DNA methylation patterns (Franco et al., 2008, Hitchler and Domann, 2007, Weitzman et al., 1994). Poor diet can also affect methylation patterns (Zaina et al., 2005, Zaina and Lund, 2011), so stress and stress-related behaviors could trigger epigenetic changes.
Epigenetic alterations in TLRs could increase a women’s sensitivity to inflammatory stimuli leading to sPTL. To determine if epigenetic alterations might play a role in sPTL, we determined the DNA methylation patterns in decidual tissue of women with sPTL (no clinical signs of infection) and compared the results to women with normal term labor (TL), and then correlated the expression levels of hypomethylated TLRs to leukocyte infiltration and markers of innate immune response.
2. Materials and Methods
2.1 Study Subjects
sPTL placentas were collected from pregnant women who gave birth prior to 37 weeks of gestation and had no clinical signs of infection (fever ≥100.4° F, uterine tenderness, malodorous vaginal discharge, maternal or fetal tachycardia) or preterm premature rupture of the membranes (n = 14). Although sPTL patients did not have signs of clinical infection, histologic examination of the decidua revealed evidence of acute chorioamnionitis in 6 patients. Eight sPTL patients were treated with antibiotics and 3 with betamethasone. Labor was induced in one sPTL patient, and two had Cesarean sections, one for Herpes and one for placental abruption. TL placentas were collected from women with normal pregnancies who gave vaginal birth between 37–40 weeks of gestation (n = 16). Labor was induced in 3 TL patients. Term not in labor (TNL) placentas were collected from women who underwent non-emergency Caesarian sections between 37–40 weeks of gestation for a previous Cesarean section (n = 8). Exclusion criteria were: smokers, HIV/AIDS, drug/alcohol users, pregnancies with stillborn babies, multiple fetuses, preeclampsia, lupus, congenital abnormalities, and hemorrhage. Demographic patient data are given in Table 1.
Table 1.
Clinical Characteristics of Patient Groups
| Variable | TNL n = 8 | TL n = 16 | sPTL n = 14 |
|---|---|---|---|
|
| |||
| Maternal age (y) | 29.9±6.9 | 23.9±6.9 | 26.3±5.3 |
|
| |||
| Pre-pregnancy BMI (kg/m2) | 28.6±8.9 | 25.6±5.2 | 23.7±8.5 |
|
| |||
| BMI at sample collection (kg/m2) | 33.3±7.5 | 30.5±4.9 | 27.5±7.0 |
|
| |||
| Race | |||
| White | 2 | 2 | 3 |
| Black | 4 | 11 | 10 |
| Asian | 0 | 0 | 1 |
| Hispanic | 0 | 1 | 0 |
| Other | 2 | 2 | 0 |
|
| |||
| Primiparous | 0 | 9 | 3 |
|
| |||
| Multiparous | 8 | 7 | 11 |
|
| |||
| Gestational age (wk) | 39.0±0.0 | 39.1±1.1 | 33.1±2.5**** |
|
| |||
| Infant birth weight (g) | 3230±343 | 3168±386 | 2268±510**** |
|
| |||
| Delivery Method | |||
| Vaginal | 0 | 13 | 12 |
| C-section | 8 | 3 | 2 |
Values are mean ± SD,
p < 0.0001 compared to TL or TNL
2.2 Methylation Assay
For DNA methylation analysis, decidual tissue was carefully scraped from the chorion surface of the fetal membranes. Decidual tissue from 10 women with normal pregnancy and 8 women with sPTL were processed for DNA extraction. DNA was extracted (~10 mg by weight) using QuickGene DNA tissue kit and QuickGene-Mini80 system (AutoGen, Holliston, MA). DNA was treated with RNase A (Qiagen, Valencia, CA). DNA (1μg) was bisulfite treated and run in Illumina Infinium HumanMethylation450 BeadChip (Illumina, San Diego, CA). HudsonAlpha Institute for Biotechnology (Huntsville, AL) ran the BeadChip using the protocol provided by Illumina.
2.3. Cell culture
THP-1 cells (ATCC), a monocyte/macrophage cell line, were cultured in RPMI-1640 medium supplemented with 2 mM L-glutamine, 10% fetal bovine serum (FBS), 50 μM 2-mercaptoethanol and 1% antibiotics and antimycotics (100 U/ml penicillin, 100 μg/ml streptomycin, 25 μg/ml amphotericin B) at 37°C in a humidified 5% CO2 atmosphere. Approximately 500,000 cells per ml were seeded in 5 ml of media in T-25 flasks. Cells were grown for 72 h in clean media or with 10 μM 5-Aza-2-deoxycytidine (5-Aza, Sigma-Aldrich, Saint-Louis, MO), an agent that inhibits DNA methylation when incorporated into DNA during cell division (Kendrew, 1994). Because 5-Aza degrades in solution, four separate doses were added (0 h, 24 h, 48 h and 72 h).
2.4 Immunohistochemistry
For immunohistochemistry, a rectangular section of the fetal membranes (approximately 6 cm × 4 cm) including amnion, chorion, and decidua was removed with surgical scissors. A roll with the decidua oriented towards the interior was prepared for paraffin embedding. Decidual tissue was collected for immunohistochemical staining from 7 TNL, 6 TL and 5 sPTL women. Tissues were formalin-fixed, paraffin embedded and cut into 8 μm sections with a microtome as previously described (Leik and Walsh, 2004, Shah et al., 2010, Shah and Walsh, 2007). To quench endogenous tissue peroxidase activity, slides were incubated in 3% hydrogen peroxide in methanol for 30 minutes. For antigen retrieval, slides were heat treated in 10 mM citrate buffer for 5 minutes with a pressure cooker. Tissues were immunostained with 2 μg/ml rabbit polyclonal antibody to TLR-2 (Abcam, Cambridge, MA Cat. # ab24192), 2 μg/ml rabbit polyclonal antibody to TLR-9 (Abcam, Cat. # ab37154), a 1:200 titer of rabbit polyclonal antibody to CD14 (Proteintech, Cat. #17000-1-AP), a 1:200 titer of rabbit polyclonal antibody to neutrophil elastase (Abcam, Cat. #ab21595), or rabbit IgG isotype negative control pre-diluted in phosphate buffered saline (Invitrogen, Cat #086199) using SuperPicTure Kit with diaminobenzidine (Invitrogen, Cat #87663), which results in a brown stain (Life Technologies, Grand Island, NY). Slides were counterstained with 1:5 dilution of Hematoxylin QS (Vector Laboratories, Burlingame, CA). The staining protocol was the same for all samples with regard to processing, incubation times and temperature.
2.5 Quantitative RT-PCR
For cell cultures, RNA extraction was performed using QuickGene RNA cultured cell kit with QuickGene Mini-80 system (AutoGen, Holliston, MA). DNase treatment was performed using Turbo DNAse kit (Ambion, Austin, TX). RNA (0.25–1 μg) was reverse transcribed to cDNA using the iScript cDNA Synthesis Kit (Bio-Rad Laboratories, Hercules, CA). Quantitative RT-PCR reactions were performed with RT2 SYBR® Green qPCR Mastermix (SABiosciences, Frederick, MD) on an Eppendorf Realplex Thermal Cycler. For each reaction, 8 ng of cDNA was used. Primers for TLR-2, TLR-9 and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), used as a housekeeping gene, were purchased from Integrated DNA Technologies, Coralville, IA (TLR-2 F: 5′-CCT GGC CCT CTC TAC AAA CTT-3′, R: 5′-ACT GTG TAT TCG TGT GCT GGA TA-3′; TLR-9 F: 5′-CTG CCA CAT GAC CAT CGA G-3′, R: 5′-GGA CAG GGA TAT GAG GGA TTT-3′; GAPDH F: 5′-GAT TCC ACC CAT GGC AAA TT-3′, R: 5′-AGA TGG TGA TGG GAT TTC CAT-3′). Primers for interleukin-8 (IL-8) were purchased commercially (SABiosciences, Valencia. CA). Data were normalized to GAPDH by the ΔΔCt method. Melting curve analysis confirmed specificity of the primers.
2.6 Inflammatory Gene Profiling
To assess innate immune response, microarray analysis of inflammatory genes and chemokines was carried out on decidual tissue samples from each group. RNA was extracted from homogenized decidual tissue using the RiboPure kit (Ambion, Austin, TX). Reverse transcription was performed with 0.2 μg RNA using the RT2 First Strand kit (SABiosciences, Frederick, MD). Gene profiling was generated using the Human Inflammatory Cytokines and Receptors PCR Array (SABiosciences, PAHS-011). Data were analyzed with the RT2 Profiler PCR Array Data Analysis Template v3.0 (SABiosciences).
2.7 Data Analysis
Slides were analyzed with an Olympus BH-2 microscope (Olympus, Center Valley, PA) attached to a digital camera (Olympus QColor5) using image analysis software (cellSens, Olympus). Relative differences in staining were identified by highlighting specific stained areas with yellow overlay using the “Measuring Images” tool. Data were quantified and reported as area stained (μm2).
2.8 Statistical Analysis
Data analysis of the Infinium HumanMethylation450 BeadChip was performed using the beadarray package in R programming environment (Dunning et al., 2007). To control for multiple hypothesis testing, the p-values were subsequently used in estimating the false discovery rates (FDR) using the q-value method (Storey and Tibshirani, 2003). Methylation values (β values) range from 0 to 1 where 0 means unmethylated and 1 means fully methylated. Δβ-values indicate the difference in average methylation values between TL and sPTL women.
One-way ANOVA with Bonferroni multiple comparisons test was used to make comparisons for non-methylation data. Demographic data are presented as mean ± SD. Quantitative data are presented as mean ± SE. A probability level of P < 0.05 was considered significant. A statistical software application was used (Prism 6.0 for Macintosh, GraphPad Software, Inc., San Diego, CA).
3. Results
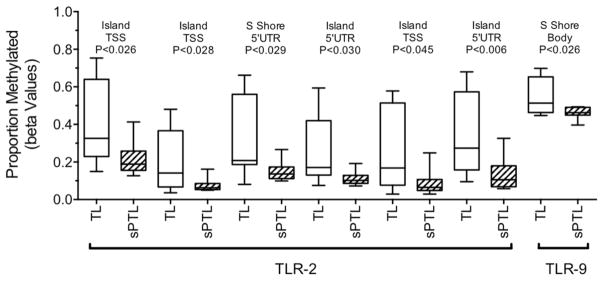
The Illumina Infinium HumanMethylation450 BeadChip analysis revealed significant decreases in DNA methylation for decidual tissue collected from women with sPTL as compared to women with TL for TLR-2 and TLR-9. For TLR-2, there were six CpG sites and for TLR-9 one CpG site with significantly reduced methylation (Fig. 1). Decreased DNA methylation was specific for TLR-2 and TLR-9. No significant differences in methylation were detected for other TLR genes, nor for other pattern recognition receptors genes, such as C-type lectin receptors, NOD-like receptors or RIG-I-like receptors.
Figure 1.
Boxplots of proportion methylated (beta values) of the TLR-2 and TLR-9 genes in decidual tissue from women with term labor (TL, n = 10) and women with spontaneous preterm labor (sPTL, n = 8) as determined by illumina Infinium HumanMethylation450 BeadChip analysis. DNA methylation was significantly lower in sPTL than TL. The results for TLR-2 are especially noteworthy because there were six gene related sites with significantly reduced methylation and TLR-2 was in the top 3% of hypomethylated genes.
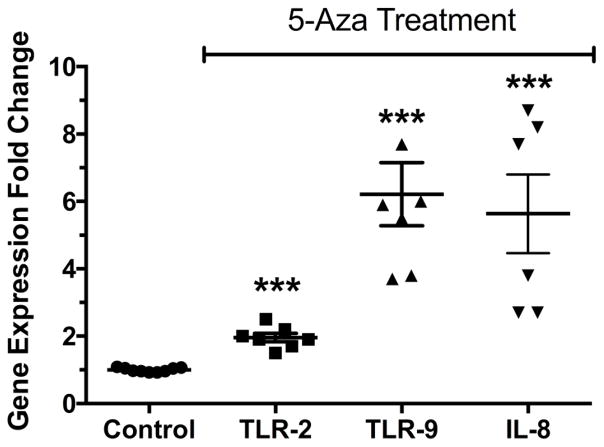
To examine if DNA methylation regulates gene expression of TLR-2 and TLR-9, we treated THP-1 cells, a macrophage cell line, with 5-Aza to induce hypomethylation. It was necessary to use a cell line because 5-Aza induces hypomethylation by being incorporated into genomic DNA during cell division. Macrophages isolated from patients do not divide. Treatment of THP-1 cells with 5-Aza resulted in significant increases in TLR-2 and TLR-9 gene expression and this was associated with a significant increase in the expression of the neutrophil chemokine, IL-8 (Fig. 2, P<0.001).
Figure 2.
TLR-2, TLR-9 and IL-8 gene expression in THP-1 cells, a monocyte/macrophage cell line treated with 5-Aza-2-deoxycytidine, a hypomethylating agent. Treatment with 5-Aza resulted in significant increases in expression of both TLRs, as well as IL-8, suggesting TLR-2 and TLR-9 are regulated by DNA methylation. *** P < 0.001 as compared to control.
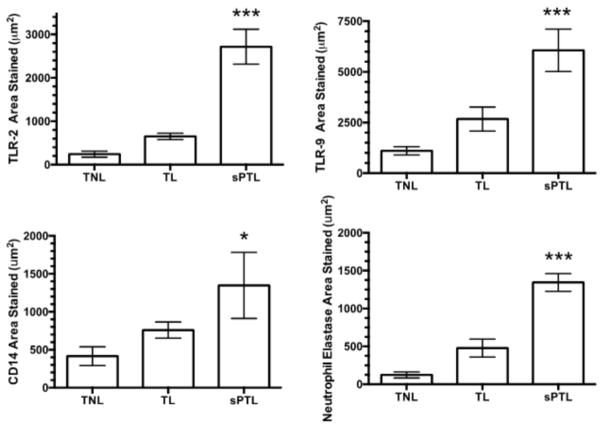
Reduced methylation for the TLR-2 and TLR-9 genes was associated with their increased expression in decidual tissue of women with sPTL as compared to women with TL or TNL. Figure 3 shows the area of staining in decidual tissue. Very little staining was present in TNL. There was more staining in TL, but the increase was not statistically significant. However, in sPTL there were marked and statistically significant increases for both TLR-2 and TLR-9 as compared to TL or TNL (P<0.001). Paralleling the increase in area stained for the TLRs, the area of staining for CD14 and neutrophil elastase were also significantly greater for sPTL than TL or TNL.
Figure 3.
Area of staining in decidual tissue for women who were at term not in labor (TNL, n = 7), women who were at term in labor (TL, n = 6), and women who delivered spontaneously preterm with no clinical signs of infection (sPTL, n = 5). The areas of staining for TLR-2, TLR-9, CD14 and neutrophil elastase were small in women with TNL or TL. However, there was a significant increase in the area stained for all markers in women with sPTL. The increase in area stained for the TLRs paralleled the increase in the area stained for macrophages as evidenced by CD14 staining and for neutrophils as evidenced by neutrophil elastase staining. Data represent mean ± SE, * P<0.05, *** P < 0.001
Figure 4 shows representative pictures of the areas of staining. TLR-2 and TLR-9 were primarily localized to leukocytes, with little or no staining in decidual cells. Increased expression of TLR-2 and TLR-9 in sPTL paralleled increases in the presence of decidual macrophages as evidenced by CD14 staining and of neutrophils as evidenced by neutrophil elastase staining. Macrophage infiltration in sPTL was for the most part uniform throughout decidual tissue, whereas the infiltration of neutrophils in sPTL was more localized.
Figure 4.
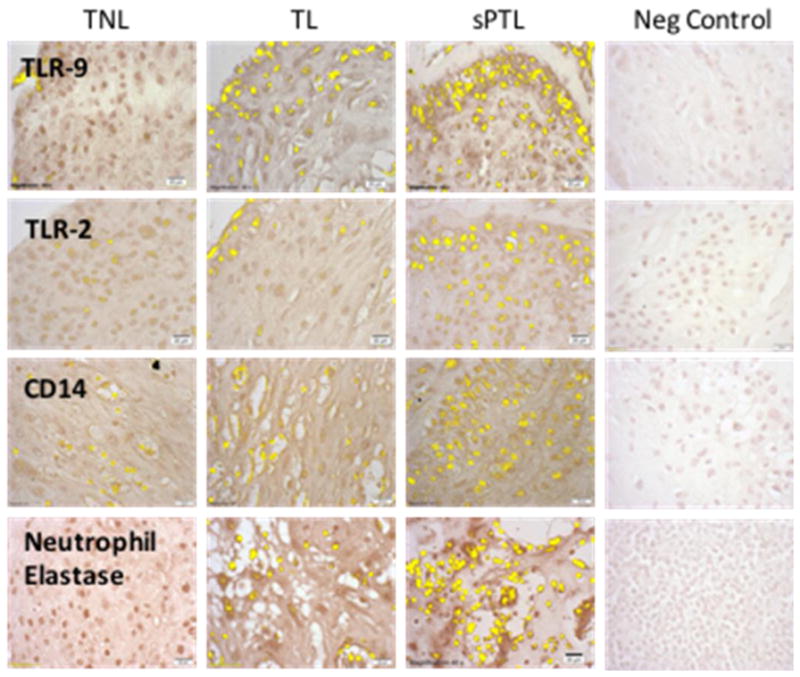
Representative sections of decidual tissue from women with TNL, TL or sPTL immunostained for TLR-2, TLR-9, CD14 and neutrophil elastase. Specific antigen staining was highlighted in yellow using the Measuring Images tool in the cellSens software, which was used to determine the area of staining. There was little staining for TLR-2 or TLR-9 in women with TNL or TL and this correlated with little staining for the macrophage and neutrophils markers, CD14 and neutrophil elastase, respectively. However, in women with sPTL there was extensive infiltration of macrophages and neutrophils and significant increases in the expression of TLR-2 and TLR-9. TLR staining was primarily localized to leukocytes, rather than decidual cells. All pictures and analyses were done with a 40X lens.
We used microarray to determine the gene expression of inflammatory cytokines and chemokines in decidual tissue as indicators of innate immune response. Table 2 shows the fold-change in expression for selected genes. Chemokine targets listed in the Table are from Luster AD (Luster, 1998). We found marked increases in the expression of inflammatory cytokines and chemokines in women with TL as compared to TNL. However, when we compared women with sPTL to women with normal TL, there was a further increase in inflammatory cytokines and a remarkable increase in neutrophil chemokines. Results for array genes with significant increases or decreases are presented in Supplemental Tables 1 and 2 in the online supplement.
Table 2.
Fold Change in Selected Inflammatory and Chemokine Genes
| TNL vs. TL | TL vs. sPTL (no clinical infection) | ||||
|---|---|---|---|---|---|
|
| |||||
| Gene | Target | Fold Increase | Gene | Target | Fold Increase |
|
| |||||
| CCL20 | Dendritic cells | 134 | CXCL5 | Neutrophils | 111 |
|
| |||||
| IL-8 | Neutrophils | 20 | CXCL6 | Neutrophils | 42 |
|
| |||||
| CCL2 | Monocytes | 17 | CXCL3 | Neutrophils | 33 |
|
|
|
||||
| CCL7 | Activated T cells | 9 | CCL20 | Dendritic cells | 25 |
|
|
|
||||
| CCL8 | NK cells | 7 | IL-8 | Neutrophils | 9 |
|
|
|||||
| Dendritic cells | CXCL1 | Neutrophils | 7 | ||
|
| |||||
| IL-17C | Inflammation | 7 | IL-17C | Inflammation | 5 |
|
| |||||
| TNF | Inflammation | 7 | TNF | Inflammation | 4 |
|
| |||||
| IL1B | Inflammation | 6 | IL1B | Inflammation | 3 |
TNL, term not in labor; TL, term labor; sPTL, spontaneous preterm labor.
The full list of up-regulated and down-regulated genes is given in Supplemental Tables in the online supplement.
4. Discussion
The cause of sPTL is not known, but epigenetic mechanisms might provide an answer by enhancing innate inflammatory responses of TLRs. The Illumina BeadChip analysis revealed significant decreases in DNA methylation for TLR-2 and TLR-9. TLR-2 is especially interesting because 6 CpG sites associated with the TLR-2 gene were significantly hypomethylated and TLR-2 was in the top 3% of the most hypomethylated genes. TLR-2 is of interest because it is activated by the most common species of bacteria identified in women with sPTL, which are Gram-positive and mycoplasma vaginal organisms of relatively low virulence (Goldenberg et al., 2000). Therefore, increased expression of TLR-2 could increase sensitivity of intrauterine tissue to inflammation by common species of vaginal microorganisms. TLR-9 is important because microbial unmethylated CpG dinucleotides activate it, and these DNA activators would be generated as intrauterine immune cells, such as macrophages, phagocytose invading vaginal microorganisms. Activated macrophages then release chemokines to signal the infiltration of additional leukocytes. TLR-9 is also unique as it can engage both viral and bacterial nucleic acids.
Alternatively, endogenous molecules produced in response to inflammation or physiological processes recognized as damage-associated molecular patterns could activate TLR-2 and TLR-9 (Chavez-Sanchez et al., 2014, Ding et al., 2013, Gill et al., 2010, Janeesh et al., 2014, Jialal et al., 2014, Solinas and Karin, 2010, Huang et al., 2011). This would provide a sterile mechanism for engaging the innate immune system.
Reduced DNA methylation of TLR-2 and TLR-9 in sPTL was associated with marked and significant increases in their expression in decidual tissue. This is consistent with known epigenetic actions of DNA methylation to silence gene expression and de-methylation to increase gene expression (Seton-Rogers, 2013, Zaina et al., 2005). To evaluate if reduced DNA methylation could lead to an increase in the expression of TLR-2 and TLR-9, we used THP-1 cells, a monocyte/macrophage cell line commonly used to study macrophage function in pregnancy. Experimentally reducing DNA methylation resulted in a significant increase in gene expression for both TLR-2 and TLR-9, as well as IL-8, a potent neutrophil chemokine that was highly expressed in decidual tissue of women with sPTL. Although terminally differentiated macrophages in the decidua may respond differently, and other factors may be involved, the significantly increased expression of TLR-2 and TLR-9 in decidua of women with sPTL is consistent with reduced methylation playing a role.
The remarkable increase in expression of TLRs in women with sPTL without clinical signs of infection or rupture of membranes is noteworthy because it suggests a premature start of the inflammatory process of labor. Our data suggest this premature start could be mediated by macrophages because decidual staining for CD14 was increased with sPTL, and our most hypomethylated gene was TLR-2, which is expressed most abundantly in monocytes/macrophages (Muzio et al., 2000). In addition, as resident sentinels, macrophages are the first alert system in decidual tissue to warn the body of danger, however if their sensitivity to microorganisms was heightened, they might initiate a premature inflammatory response precipitating sPTL.
We used a gene array to determine the expression of inflammatory cytokines and chemokines as markers of innate immune response. We found inflammatory cytokines and leukocyte chemokines were markedly increased in decidual tissue of women with TL as compared to women with TNL. When we compared TL with sPTL, we found a further increase in inflammatory cytokines and leukocyte chemokines, but what was most noteworthy was the dominance of neutrophil chemokines in sPTL and this was associated with a significant increase in the infiltration of neutrophils. It is documented that term labor is associated with inflammation and infiltration of leukocytes into myometrium and intrauterine tissues (Challis et al., 2009, Shynlova et al., 2013, Thomson et al., 1999) with a strong gene signature for neutrophils (Sharp et al., 2016). Other investigators have shown marked infiltration of leukocytes with PTL associated with infection and preterm premature rupture of the membranes (Giaglis et al., 2016, Hamilton et al., 2012). We show here that there is also marked infiltration of leukocytes with sPTL not associated with clinical symptoms of infection or rupture of the membranes. The extensive infiltration of leukocytes in sPTL could increase sensitivity of the decidua because there would be more immune cells to respond to inflammatory stimuli.
A limitation of our study is that although clinical signs of infection were not present, subclinical intrauterine infection cannot be ruled out. Six of the sPTL samples had a histological diagnosis of acute chorioamnionitis. The presence of bacteria alone, however, is not sufficient to cause sPTL because certain bacteria have been identified in both term and preterm intrauterine tissues. This was a rationale for the present study because it raises the possibility that sPTL without clinical infection may be due to epigenetic mechanisms that increase the sensitivity of decidual macrophages to the microbiota present in intrauterine tissues. Our findings of significantly reduced DNA methylation of TLR-2 and TLR-9 in sPTL being associated with a significant increase in their expression in decidual tissue are consistent with epigenetic mechanisms playing a role.
Another limitation of our study is that gestational age at sampling could have affected the TLR methylation profile since sPTL patients delivered earlier than TNL or TL patients. Gestational age is a potential confounder in studies dealing with preterm intrauterine tissues because there are no accessible gestational-age matched controls. We do not think that gestational age explains our data because decidual cells did not express TLRs, and immunostaining for TLRs correlated with leukocyte infiltration. Our methylation findings are most likely related to the infiltration of leukocytes, which express TLRs, rather than to gestational age related changes. Little is known as to how DNA methylation is affected by gestational age. One report found an overall increase in methylation from the first to the third trimester in human placental tissue (Novakovic et al., 2011), and two reports related genome-wide neonatal DNA methylation to gestational age (Bohlin et al., 2016, Knight et al., 2016). Several of the pathways identified in cord blood were related to cell aging and cellular senescence (Bohlin et al., 2016).
In conclusion, these data are consistent with epigenetic mechanisms playing a role in increasing the sensitivity of decidual tissue via increased leukocyte infiltration and expression of TLRs that might lead to a premature inflammatory response to trigger sPTL in the absence of clinical infection.
Supplementary Material
TLR Manuscript (Walsh) – Highlights.
DNA methylation was significantly reduced for TLR-2 and TLR-9 genes in decidual tissue of women with spontaneous preterm labor not associated with clinical symptoms of infection as compared to women with normal term labor.
Reduced DNA methylation was associated with increased expression of TLR-2 and TLR-9, which was associated with a marked infiltration of macrophages and neutrophils into decidual tissue.
Gene expression of inflammatory cytokines and chemokines was remarkably increased in women with spontaneous preterm labor.
These data are consistent with epigenetic mechanisms playing a role in increasing the sensitivity of decidual tissue via increased leukocyte infiltration and expression of TLRs that might trigger labor prematurely in the absence of clinical infection.
Acknowledgments
We acknowledge the contribution of Emily L. Jumet, M.S. for the conduct of microarrays.
Funding
This research was supported in part by grants from NHLBI, R01 HL069851 (SWW), NCMHD P60 MD002256 (JFS, SWW), R01 HD073555 (JFS), NIDCR R01 DE022836 (SES) and R03 DE025037 (SES).
Footnotes
Conflict of Interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Institute of Medicine, Preterm birth: Causes, consequences, and prevention. National Academy of Sciences; Washington DC: [Google Scholar]
- 2.Abrahams VM, Potter JA, Bhat G, Peltier MR, Saade G, Menon R. Bacterial modulation of human fetal membrane Toll-like receptor expression. Am J Reprod Immunol. 2013;69:33–40. doi: 10.1111/aji.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aschbacher K, O’donovan A, Wolkowitz OM, Dhabhar FS, Su Y, Epel E. Good stress, bad stress and oxidative stress: Insights from anticipatory cortisol reactivity. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck S, Wojdyla D, Say L, Betran AP, Merialdi M, Requejo JH, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull World Health Organ. 2010;88:31–8. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bohlin J, Haberg SE, Magnus P, Reese SE, Gjessing HK, Magnus MC, et al. Prediction of gestational age based on genome-wide differentially methylated regions. Genome Biol. 2016;17:207. doi: 10.1186/s13059-016-1063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burris HH, Rifas-Shiman SL, Baccarelli A, Tarantini L, Boeke CE, Kleinman K, et al. Associations of LINE-1 DNA Methylation with Preterm Birth in a Prospective Cohort Study. J Dev Orig Health Dis. 2012;3:173–181. doi: 10.1017/s2040174412000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cardwell MS. Stress: pregnancy considerations. Obstet Gynecol Surv. 2013;68:119–29. doi: 10.1097/OGX.0b013e31827f2481. [DOI] [PubMed] [Google Scholar]
- 8.Castro-Leyva V, Espejel-Nunez A, Barroso G, Zaga-Clavellina V, Flores-Pliego A, Morales-Mendez I, et al. Preserved ex vivo inflammatory status in decidual cells from women with preterm labor and subclinical intrauterine infection. PLoS One. 2012;7:e43605. doi: 10.1371/journal.pone.0043605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009;16:206–15. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 10.Chavez-Sanchez L, Garza-Reyes MG, Espinosa-Luna JE, Chavez-Rueda K, Legorreta-Haquet MV, Blanco-Favela F. The role of TLR2, TLR4 and CD36 in macrophage activation and foam cell formation in response to oxLDL in humans. Hum Immunol. 2014;75:322–9. doi: 10.1016/j.humimm.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Clerici G, Slavescu C, Fiengo S, Kanninen TT, Romanelli M, Biondi R, et al. Oxidative stress in pathological pregnancies. J Obstet Gynaecol. 2012;32:124–7. doi: 10.3109/01443615.2011.637139. [DOI] [PubMed] [Google Scholar]
- 12.Ding Z, Liu S, Wang X, Khaidakov M, Dai Y, Mehta JL. Oxidant stress in mitochondrial DNA damage, autophagy and inflammation in atherosclerosis. Sci Rep. 2013;3:1077. doi: 10.1038/srep01077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunning MJ, Smith ML, Ritchie ME, Tavare S. beadarray: R classes and methods for Illumina bead-based data. Bioinformatics. 2007;23:2183–4. doi: 10.1093/bioinformatics/btm311. [DOI] [PubMed] [Google Scholar]
- 14.Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens) 2009;8:7–22. doi: 10.14310/horm.2002.1217. [DOI] [PubMed] [Google Scholar]
- 15.Franco R, Schoneveld O, Georgakilas AG, Panayiotidis MI. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008;266:6–11. doi: 10.1016/j.canlet.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 16.Ganu RS, Ma J, Aagaard KM. The role of microbial communities in parturition: is there evidence of association with preterm birth and perinatal morbidity and mortality? Am J Perinatol. 2013;30:613–24. doi: 10.1055/s-0032-1329693. [DOI] [PubMed] [Google Scholar]
- 17.Giaglis S, Stoikou M, Grimolizzi F, Subramanian BY, Van Breda SV, Hoesli I, et al. Neutrophil migration into the placenta: Good, bad or deadly? Cell adhesion & migration. 2016;10:208–25. doi: 10.1080/19336918.2016.1148866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill R, Tsung A, Billiar T. Linking oxidative stress to inflammation: Toll-like receptors. Free Radic Biol Med. 2010;48:1121–32. doi: 10.1016/j.freeradbiomed.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillaux C, Mehats C, Vaiman D, Cabrol D, Breuiller-Fouche M. Functional screening of TLRs in human amniotic epithelial cells. J Immunol. 2011;187:2766–74. doi: 10.4049/jimmunol.1100217. [DOI] [PubMed] [Google Scholar]
- 20.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldenberg RL, Hauth JC, Andrews WW. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–7. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 22.Hamilton S, Oomomian Y, Stephen G, Shynlova O, Tower CL, Garrod A, et al. Macrophages infiltrate the human and rat decidua during term and preterm labor: evidence that decidual inflammation precedes labor. Biol Reprod. 2012;86:39. doi: 10.1095/biolreprod.111.095505. [DOI] [PubMed] [Google Scholar]
- 23.Hitchler MJ, Domann FE. An epigenetic perspective on the free radical theory of development. Free Radic Biol Med. 2007;43:1023–36. doi: 10.1016/j.freeradbiomed.2007.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoang M, Potter JA, Gysler SM, Han CS, Guller S, Norwitz ER, et al. Human Fetal Membranes Generate Distinct Cytokine Profiles in Response to Bacterial Toll-like Receptor and Nod-like Receptor Agonists. Biol Reprod. 2014 doi: 10.1095/biolreprod.113.115428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsieh TT, Chen SF, Lo LM, Li MJ, Yeh YL, Hung TH. The association between maternal oxidative stress at mid-gestation and subsequent pregnancy complications. Reprod Sci. 2012;19:505–12. doi: 10.1177/1933719111426601. [DOI] [PubMed] [Google Scholar]
- 26.Huang H, Evankovich J, Yan W, Nace G, Zhang L, Ross M, et al. Endogenous histones function as alarmins in sterile inflammatory liver injury through Toll-like receptor 9 in mice. Hepatology. 2011;54:999–1008. doi: 10.1002/hep.24501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janeesh PA, Sasikala V, Dhanya CR, Abraham A. Robinin modulates TLR/NF-kappaB signaling pathway in oxidized LDL induced human peripheral blood mononuclear cells. Int Immunopharmacol. 2014;18:191–7. doi: 10.1016/j.intimp.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 29.Jialal I, Kaur H, Devaraj S. Toll-like Receptor Status in Obesity and Metabolic Syndrome: A Translational Perspective. J Clin Endocrinol Metab. 2014;99:39–48. doi: 10.1210/jc.2013-3092. [DOI] [PubMed] [Google Scholar]
- 30.Jones HE, Harris KA, Azizia M, Bank L, Carpenter B, Hartley JC, et al. Differing prevalence and diversity of bacterial species in fetal membranes from very preterm and term labor. PLoS One. 2009;4:e8205. doi: 10.1371/journal.pone.0008205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kendrew JC, editor. The Encyclopedia of Molecular Biology. Cambridge: Blackwell Science; 1994. [Google Scholar]
- 32.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H, et al. Toll-like receptor-2 and -4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol. 2004;191:1346–55. doi: 10.1016/j.ajog.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Knight AK, Craig JM, Theda C, Baekvad-Hansen M, Bybjerg-Grauholm J, Hansen CS, et al. An epigenetic clock for gestational age at birth based on blood methylation data. Genome Biol. 2016;17:206. doi: 10.1186/s13059-016-1068-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30:16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 35.Leik CE, Walsh SW. Neutrophils infiltrate resistance-sized vessels of subcutaneous fat in women with preeclampsia. Hypertension. 2004;44:72–7. doi: 10.1161/01.HYP.0000130483.83154.37. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Hoyo C, Murphy S, Huang Z, Overcash F, Thompson J, et al. DNA methylation at imprint regulatory regions in preterm birth and infection. Am J Obstet Gynecol. 2013;208:395 e1–7. doi: 10.1016/j.ajog.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu Y, Murphy SK, Murtha AP, Fuemmeler BF, Schildkraut J, Huang Z, et al. Depression in pregnancy, infant birth weight and DNA methylation of imprint regulatory elements. Epigenetics. 2012;7:735–46. doi: 10.4161/epi.20734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luster AD. Chemokines--chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–45. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 39.Menon R, Conneely KN, Smith AK. DNA methylation: an epigenetic risk factor in preterm birth. Reprod Sci. 2012;19:6–13. doi: 10.1177/1933719111424446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muzio M, Bosisio D, Polentarutti N, D’amico G, Stoppacciaro A, Mancinelli R, et al. Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells. J Immunol. 2000;164:5998–6004. doi: 10.4049/jimmunol.164.11.5998. [DOI] [PubMed] [Google Scholar]
- 41.Myatt L, Eschenbach DA, Lye SJ, Mesiano S, Murtha AP, Williams SM, et al. A standardized template for clinical studies in preterm birth. Reprod Sci. 2012;19:474–82. doi: 10.1177/1933719111426602. [DOI] [PubMed] [Google Scholar]
- 42.Novakovic B, Yuen RK, Gordon L, Penaherrera MS, Sharkey A, Moffett A, et al. Evidence for widespread changes in promoter methylation profile in human placenta in response to increasing gestational age and environmental/stochastic factors. BMC Genomics. 2011;12:529. doi: 10.1186/1471-2164-12-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parets SE, Conneely KN, Kilaru V, Syed TA, Saade G, Smith AK, et al. DNA methylation differences in umbilical cord blood from a preterm birth population. Reprod Sci. 2013;20(Suppl):123A. [Google Scholar]
- 44.Phillippe M. Cell-free Fetal DNA - A Trigger for Parturition. N Engl J Med. 2014;370:2534–2536. doi: 10.1056/NEJMcibr1404324. [DOI] [PubMed] [Google Scholar]
- 45.Romero R, Espinoza J, Kusanovic JP, Gotsch F, Hassan S, Erez O, et al. The preterm parturition syndrome. Br J Obstet Gynaecol. 2006;113(Suppl 3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sanchez SE, Puente GC, Atencio G, Qiu C, Yanez D, Gelaye B, et al. Risk of spontaneous preterm birth in relation to maternal depressive, anxiety, and stress symptoms. J Reprod Med. 2013;58:25–33. [PMC free article] [PubMed] [Google Scholar]
- 47.Scharfe-Nugent A, Corr SC, Carpenter SB, Keogh L, Doyle B, Martin C, et al. TLR9 provokes inflammation in response to fetal DNA: mechanism for fetal loss in preterm birth and preeclampsia. J Immunol. 2012;188:5706–12. doi: 10.4049/jimmunol.1103454. [DOI] [PubMed] [Google Scholar]
- 48.Seton-Rogers S. Epigenetics: Methylation reboot. Nat Rev Cancer. 2013;13:292. doi: 10.1038/nrc3513. [DOI] [PubMed] [Google Scholar]
- 49.Shah TJ, Leik CE, Walsh SW. Neutrophil infiltration and systemic vascular inflammation in obese women. Reprod Sci. 2010;17:116–24. doi: 10.1177/1933719109348252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah TJ, Walsh SW. Activation of NF-kappaB and expression of COX-2 in association with neutrophil infiltration in systemic vascular tissue of women with preeclampsia. Am J Obstet Gynecol. 2007;196:48 e1–8. doi: 10.1016/j.ajog.2006.08.038. [DOI] [PubMed] [Google Scholar]
- 51.Sharp GC, Hutchinson JL, Hibbert N, Freeman TC, Saunders PT, Norman JE. Transcription Analysis of the Myometrium of Labouring and Non-Labouring Women. PLoS One. 2016;11:e0155413. doi: 10.1371/journal.pone.0155413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shynlova O, Lee YH, Srikhajon K, Lye SJ. Physiologic uterine inflammation and labor onset: integration of endocrine and mechanical signals. Reprod Sci. 2013;20:154–67. doi: 10.1177/1933719112446084. [DOI] [PubMed] [Google Scholar]
- 53.Solinas G, Karin M. JNK1 and IKK{beta}: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- 54.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P, et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res. 2005;57:404–11. doi: 10.1203/01.PDR.0000153869.96337.90. [DOI] [PubMed] [Google Scholar]
- 55.Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100:9440–5. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am J Obstet Gynecol. 2013;208:226 e1–7. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Szanton SL, Rifkind JM, Mohanty JG, Miller ER, 3rd, Thorpe RJ, Nagababu E, et al. Racial discrimination is associated with a measure of red blood cell oxidative stress: a potential pathway for racial health disparities. Int J Behav Med. 2012;19:489–95. doi: 10.1007/s12529-011-9188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thomson AJ, Telfer JF, Young A, Campbell S, Stewart CJ, Cameron IT, et al. Leukocytes infiltrate the myometrium during human parturition: further evidence that labour is an inflammatory process. Hum Reprod. 1999;14:229–36. [PubMed] [Google Scholar]
- 59.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Curr Opin Immunol. 2002;14:103–10. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 60.Voltolini C, Torricelli M, Conti N, Vellucci FL, Severi FM, Petraglia F. Understanding Spontaneous Preterm Birth: From Underlying Mechanisms to Predictive and Preventive Interventions. Reprod Sci. 2013;20:1274–1292. doi: 10.1177/1933719113477496. [DOI] [PubMed] [Google Scholar]
- 61.Wang L, Muxin G, Nishida H, Shirakawa C, Sato S, Konishi T. Psychological stress-induced oxidative stress as a model of sub-healthy condition and the effect of TCM. Evid Based Complement Alternat Med. 2007;4:195–202. doi: 10.1093/ecam/nel080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weitzman SA, Turk PW, Milkowski DH, Kozlowski K. Free radical adducts induce alterations in DNA cytosine methylation. Proc Natl Acad Sci U S A. 1994;91:1261–4. doi: 10.1073/pnas.91.4.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zaina S, Lindholm MW, Lund G. Nutrition and aberrant DNA methylation patterns in atherosclerosis: more than just hyperhomocysteinemia? J Nutr. 2005;135:5–8. doi: 10.1093/jn/135.1.5. [DOI] [PubMed] [Google Scholar]
- 64.Zaina S, Lund G. Epigenetics: a tool to understand diet-related cardiovascular risk? J Nutrigenet Nutrigenomics. 2011;4:261–74. doi: 10.1159/000334584. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.