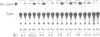Abstract
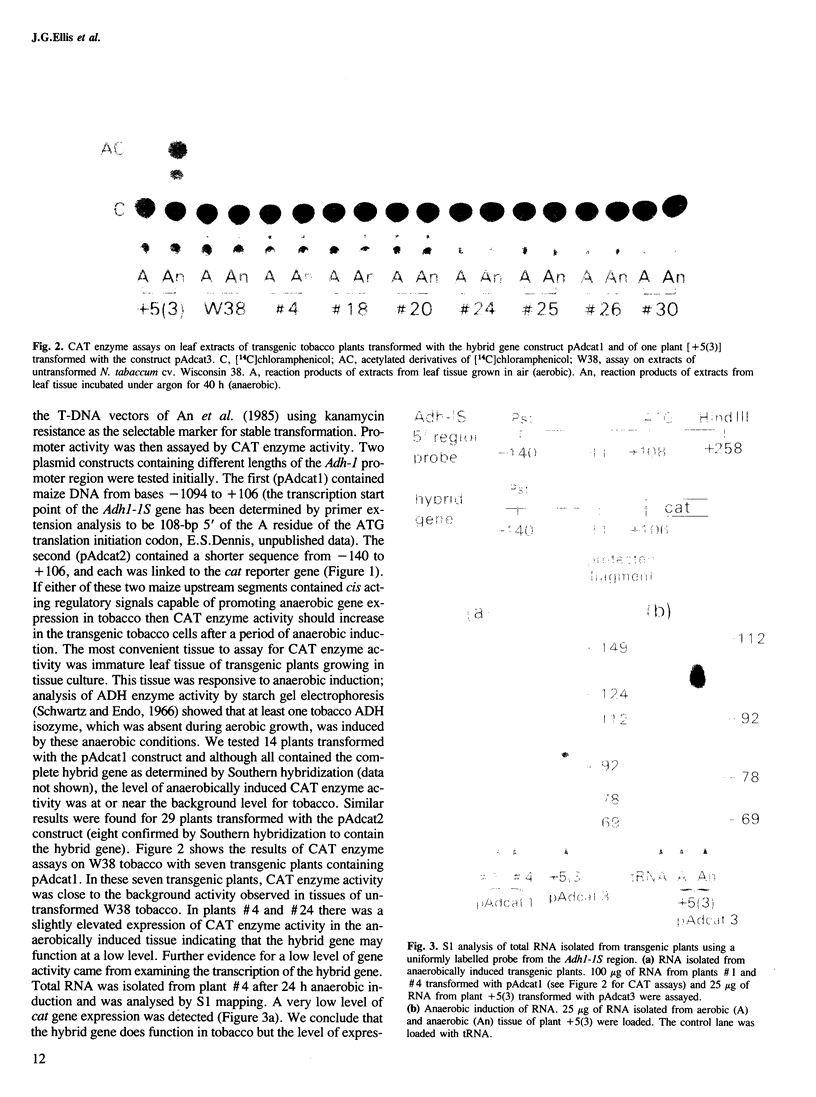
The promoter region of a maize alcohol dehydrogenase gene (Adh-1) was linked to a reporter gene encoding chloramphenicol acetyl transferase (CAT) and transformed stably into tobacco cells using T-DNA vectors. No CAT enzyme activity could be detected in transgenic tobacco plants unless upstream promoter elements from the octopine synthase gene or the cauliflower mosaic virus 35S promoter were supplied in addition to the maize promoter region. CAT enzyme activity and transcription of the chimaeric gene were then readily detected after anaerobic induction. The first 247 bp upstream of the translation initiation codon of the maize Adh-1 gene were sufficient to impose anaerobic regulation on the hybrid gene and S1 nuclease mapping confirmed mRNA initiation is from the normal maize Adh-1 transcription start point.
Keywords: alcohol dehydrogenase, anaerobiosis, maize, plant enhancers, T-DNA transformation
Full text
PDF

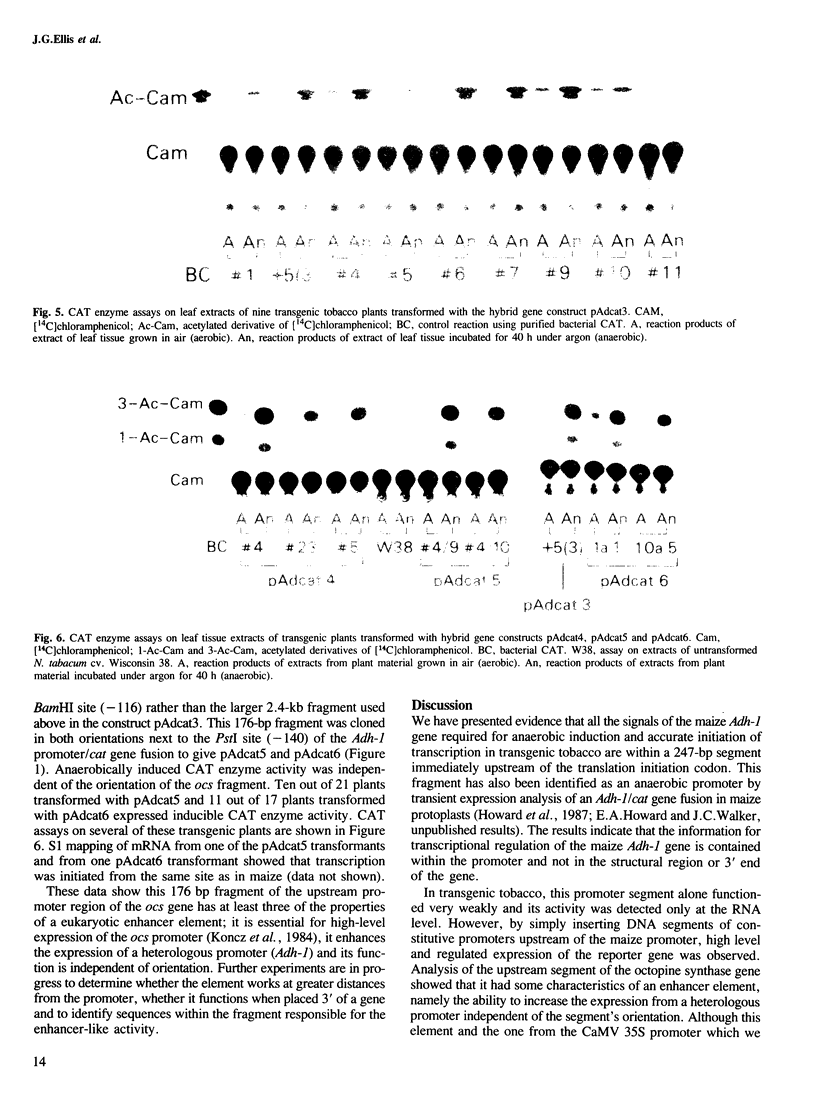


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- An G., Watson B. D., Stachel S., Gordon M. P., Nester E. W. New cloning vehicles for transformation of higher plants. EMBO J. 1985 Feb;4(2):277–284. doi: 10.1002/j.1460-2075.1985.tb03626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan M., Barnes W. M., Chilton M. D. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucleic Acids Res. 1983 Jan 25;11(2):369–385. doi: 10.1093/nar/11.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique Eco RI sites for selection of Eco RI generated recombinant DNA molecules. Gene. 1978 Oct;4(2):121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- De Vos G., De Beuckeleer M., Van Montagu M., Schell J. Restriction endonuclease mapping of the octopine tumor-inducing plasmid pTiAch5 of Agrobacterium tumefaciens. Plasmid. 1981 Sep;6(2):249–253. doi: 10.1016/0147-619x(81)90070-6. [DOI] [PubMed] [Google Scholar]
- Dennis E. S., Gerlach W. L., Pryor A. J., Bennetzen J. L., Inglis A., Llewellyn D., Sachs M. M., Ferl R. J., Peacock W. J. Molecular analysis of the alcohol dehydrogenase (Adh1) gene of maize. Nucleic Acids Res. 1984 May 11;12(9):3983–4000. doi: 10.1093/nar/12.9.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Depicker A., Stachel S., Dhaese P., Zambryski P., Goodman H. M. Nopaline synthase: transcript mapping and DNA sequence. J Mol Appl Genet. 1982;1(6):561–573. [PubMed] [Google Scholar]
- Ditta G., Stanfield S., Corbin D., Helinski D. R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franck A., Guilley H., Jonard G., Richards K., Hirth L. Nucleotide sequence of cauliflower mosaic virus DNA. Cell. 1980 Aug;21(1):285–294. doi: 10.1016/0092-8674(80)90136-1. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Pryor A. J., Dennis E. S., Ferl R. J., Sachs M. M., Peacock W. J. cDNA cloning and induction of the alcohol dehydrogenase gene (Adh1) of maize. Proc Natl Acad Sci U S A. 1982 May;79(9):2981–2985. doi: 10.1073/pnas.79.9.2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAGEMAN R. H., FLESHER D. The effect of an anaerobic environment on the activity of alcohol dehydrogenase and other enzymes of corn seedings. Arch Biochem Biophys. 1960 Apr;87:203–209. doi: 10.1016/0003-9861(60)90161-2. [DOI] [PubMed] [Google Scholar]
- Herrera-Estrella L., Block M. D., Messens E., Hernalsteens J. P., Montagu M. V., Schell J. Chimeric genes as dominant selectable markers in plant cells. EMBO J. 1983;2(6):987–995. doi: 10.1002/j.1460-2075.1983.tb01532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G. S., Farrell P. J., Barrell B. G. Two related but differentially expressed potential membrane proteins encoded by the EcoRI Dhet region of Epstein-Barr virus B95-8. J Virol. 1985 Feb;53(2):528–535. doi: 10.1128/jvi.53.2.528-535.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones J. D., Dunsmuir P., Bedbrook J. High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J. 1985 Oct;4(10):2411–2418. doi: 10.1002/j.1460-2075.1985.tb03949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize fructose-1,6-diphosphate aldolase. J Biol Chem. 1984 Nov 25;259(22):14180–14183. [PubMed] [Google Scholar]
- Kelley P. M., Freeling M. Anaerobic expression of maize glucose phosphate isomerase I. J Biol Chem. 1984 Jan 10;259(1):673–677. [PubMed] [Google Scholar]
- Koncz C., Kreuzaler F., Kalman Z., Schell J. A simple method to transfer, integrate and study expression of foreign genes, such as chicken ovalbumin and alpha-actin in plant tumors. EMBO J. 1984 May;3(5):1029–1037. doi: 10.1002/j.1460-2075.1984.tb01923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamppa G., Nagy F., Chua N. H. Light-regulated and organ-specific expression of a wheat Cab gene in transgenic tobacco. Nature. 1985 Aug 22;316(6030):750–752. doi: 10.1038/316750a0. [DOI] [PubMed] [Google Scholar]
- Matzke M. A., Susani M., Binns A. N., Lewis E. D., Rubenstein I., Matzke A. J. Transcription of a zein gene introduced into sunflower using a Ti plasmid vector. EMBO J. 1984 Jul;3(7):1525–1531. doi: 10.1002/j.1460-2075.1984.tb02006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Otten L. A., Schilperoort R. A. A rapid micro scale method for the detection of lysopine and nopaline dehydrogenase activities. Biochim Biophys Acta. 1978 Dec 8;527(2):497–500. doi: 10.1016/0005-2744(78)90363-7. [DOI] [PubMed] [Google Scholar]
- Otten L., De Greve H., Hernalsteens J. P., Van Montagu M., Schieder O., Straub J., Schell J. Mendelian transmission of genes introduced into plants by the Ti plasmids of Agrobacterium tumefaciens. Mol Gen Genet. 1981;183(2):209–213. doi: 10.1007/BF00270619. [DOI] [PubMed] [Google Scholar]
- Rochester D. E., Winer J. A., Shah D. M. The structure and expression of maize genes encoding the major heat shock protein, hsp70. EMBO J. 1986 Mar;5(3):451–458. doi: 10.1002/j.1460-2075.1986.tb04233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M. M., Freeling M., Okimoto R. The anaerobic proteins of maize. Cell. 1980 Jul;20(3):761–767. doi: 10.1016/0092-8674(80)90322-0. [DOI] [PubMed] [Google Scholar]
- Schwartz M., Sofer W. Alcohol dehydrogenase-negative mutants in Drosophila: defects at the structural locus? Genetics. 1976 May;83(1):125–136. doi: 10.1093/genetics/83.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]