Abstract
Purpose
Our objective was to delineate components of motor performance in specific language impairment (SLI); specifically, whether deficits in timing precision in one effector (unimanual tapping) and in two effectors (bimanual clapping) are observed in young children with SLI.
Method
Twenty-seven 4- to 5-year-old children with SLI and 21 age-matched peers with typical language development participated. All children engaged in a unimanual tapping and a bimanual clapping timing task. Standard measures of language and motor performance were also obtained.
Results
No group differences in timing variability were observed in the unimanual tapping task. However, compared with typically developing peers, children with SLI were more variable in their timing precision in the bimanual clapping task. Nine of the children with SLI performed greater than 1 SD below the mean on a standardized motor assessment. The children with low motor performance showed the same profile as observed across all children with SLI, with unaffected unimanual and impaired bimanual timing precision.
Conclusions
Although unimanual timing is unaffected, children with SLI show a deficit in timing that requires bimanual coordination. We propose that the timing deficits observed in children with SLI are associated with the increased demands inherent in bimanual performance.
Specific language impairment (SLI) has long been considered a developmental disorder that is constrained to the processing and production of language (see Leonard, 2014). These children are traditionally defined by “a significant deficit in language ability that cannot be attributed to hearing loss, low nonverbal intelligence, or neurological damage” (Leonard, 2014, p. 3). However, it has become evident that children with SLI demonstrate compromised performance in many cognitive domains (Johnston, 1994), such as memory (e.g., Archibald & Gathercole, 2006; Archibald & Joanisse, 2009; Dodwell & Bavin, 2008; Lum, Conti-Ramsden, Page, & Ullman, 2012; Weismer, Evans, & Hesketh, 1999), speed of processing (e.g., Johnston & Weismer, 1983; Leonard et al., 2007), and, most relevant to the current study, motor performance (Brumbach & Goffman, 2014; Hill, 2001; McPhillips, Finlay, Bejerot, & Hanley, 2014; Zelaznik & Goffman, 2010).
The relationship between language and motor deficits in children with SLI, though documented in the literature, remains poorly understood. A large percentage of children with SLI meet criteria for developmental coordination disorder, a disorder in which “the acquisition and execution of coordinated motor skills is substantially below that expected given the individual's chronological age and opportunity for skill learning and use” (American Psychiatric Association, 2013, p. 74). A subset of children with developmental coordination disorder also show a profile of language difficulties that is similar to children with SLI (e.g., Archibald & Alloway, 2008). It has become evident that language and motor disorders may overlap in important ways.
Standardized motor assessments have documented impairments in fine and gross motor skill (Brumbach & Goffman, 2014; Hill, 2001; McPhillips et al., 2014; Zelaznik & Goffman, 2010), typically in 50% to 90% of children with SLI (Hill, 2001). Standardized assessments test performance on a myriad of tasks across multiple dimensions of motor skill, but specific profiles of performance are not well documented from these broad measures. Results from experimental motor tasks, however, allow researchers to specify particular areas of weakness in children with SLI. Motor deficits have been documented in experimental tasks such as peg moving (Bishop, 2002; Bishop & Edmundson, 1987; Powell & Bishop, 1992), fine motor tasks such as cutting out shapes, copying shapes, and tracing mazes without crossing the lines (Schwartz & Regan, 1996), and miming gestures (Hill, 1998). The overarching goal of our research program is to specify the profile of motor decrements, and potentially strengths, observed in children with SLI, and to delineate how this motor profile relates to children's language impairment. The aim of the current study was to specify components of the motor deficit in SLI through the assessment of two key motor behaviors: timing and coordination. We also include a standardized measure of gross and fine motor performance to assess motor skill more broadly.
Timing and the Procedural Deficit Hypothesis
Several hypotheses have emerged regarding the motor deficit in SLI. Some view these motor difficulties as a comorbidity that does not tie mechanistically to the language disorder (e.g., Hill, 2001; Locke, 1997). In this view, motor deficits may reflect a general neuromaturational delay seen in children with SLI, but these motor deficits may have no significant or causal relationship to language deficits. Rather, under this view SLI is regarded as a modular disorder that is confined to the language domain.
An alternative to the comorbidity or generalized motor deficit account is one that considers a profile of motor strengths and weaknesses in children with SLI that is related to the well-described language profile observed in these children. One of these accounts posits timing deficits as a major factor underlying both language and motor difficulties (Alcock, Passingham, Watkins, & Vargha-Khadem, 2000; Bishop, 2002; Corriveau & Goswami, 2009). Lashley (1951) proposed that neural mechanisms responsible for timing precision and serial order control underlie both language production and skilled movement. In line with this hypothesis, and consistent with findings in children with dyslexia (Wolff, Melngailis, Obregon, & Bedrosian, 1995), Bishop (2002) suggested that a deficit in timing precision (i.e., the ability to keep time with relatively low levels of variability) underlies both the language and motor deficits seen in children with SLI. Timing deficits also may be predicted on the basis of prosodic difficulties apparent in children with SLI (Gerken & McGregor, 1998; Goffman, 1999, 2004; McGregor & Leonard, 1994).
Perhaps the most prominent hypothesis is the procedural deficit hypothesis, which proposes that procedural learning deficits underlie the specific profile of weaknesses seen in language and motor domains in children with SLI (Ullman & Pierpont, 2005). This hypothesis implicates timing as well as other skills that rely on procedural memory, such as sequencing, speed, and balance. The procedural deficit hypothesis is based on Ullman's (2001, 2004) declarative-procedural model, which posits that there are two distinct memory systems: the declarative memory system and the procedural memory system. The declarative memory system is responsible for learning, storing, and consciously recalling explicit knowledge, such as facts and events, as well as lexical knowledge (Ullman & Pullman, 2015). The medial temporal lobes, including the entorhinal, perirhinal, and parahippocampal cortices and the hippocampus, are the primary structures responsible for the development of the declarative memory system (Ullman & Pullman, 2015). In contrast, the procedural memory system is responsible for the acquisition and use of new cognitive, perceptual, and motor skills (Viskontas & Knowlton, 2003) and is associated with phonological, morphological, and syntactic rule learning (Lum et al., 2012). Procedural learning occurs without conscious awareness (Frensch & Rünger, 2003), in a gradual fashion over many presentations. Frontal/basal ganglia circuits, including Broca's area and the caudate nucleus, are particularly implicated in procedural learning, as well as the supramarginal gyrus, superior temporal sulcus, and cerebellum (Ullman & Pierpont, 2005).
Ullman and Pierpont (2005) propose that brain structures and pathways underlying the procedural memory system have developed abnormally in children with SLI. Therefore, the procedural deficit hypothesis predicts impairments in procedural speech, language, and motor tasks that require timed, speeded, and/or sequential movements. This hypothesis offers one possible account for the specific profile of language, motor, and other cognitive strengths and weaknesses observed in children with SLI. For example, the procedural memory system processes the acquisition and use of morphosyntax (Ullman et al., 1997), the hallmark of the language deficit seen in children with SLI. Children with SLI also exhibit performance decrements on nonlinguistic procedural tasks, such as statistical learning (e.g., Evans, Saffran, & Robe-Torres, 2009) and the serial reaction time task (e.g., Hsu & Bishop, 2014; Tomblin, Mainela-Arnold, & Zhang, 2007). In contrast, the acquisition of the lexicon, which relies more heavily on the declarative memory system (Ullman et al., 1997; Ullman & Pullman, 2015), is typically less affected in children with SLI (e.g., Lum & Bleses, 2012; Lum et al., 2012; Ullman & Pierpont, 2005). Thus, the procedural deficit hypothesis provides one framework for assessing common mechanisms that underlie motor and language deficits in children with SLI.
Unimanual Timing
The cerebellum, one of the structures responsible for the development of the procedural memory system, is crucial for the control of timing (Ivry & Keele, 1989; Spencer, Ivry, & Zelaznik, 2005; Spencer, Zelaznik, Diedrichsen, & Ivry, 2003). Tapping is a timing task that is produced with discrete movements. This is in contrast to timing tasks that require continuous movements (such as circle drawing). The quintessential unimanual tapping task, a discrete timing task that provides an index of timing precision, is well documented to reflect cerebellar timing processes (e.g., Spencer et al., 2003). Patients with cerebellar lesions show marked impairments in timing precision in the discrete tapping task, but not in the continuous circle drawing task (e.g., Schlerf, Spencer, Zelaznik, & Ivry, 2007; Spencer et al., 2003).
In the unimanual tapping task, the participant taps in time with an auditory metronome (referred to as the synchronization phase) at a given cycle duration. One cycle is defined as the amount of time between the onset of one presentation of a tone and the onset of the next presentation of a tone, or the time interval between taps or claps (e.g., 600 ms). When the metronome disengages, the participant continues tapping with the goal of maintaining the same cycle duration specified by the metronome (referred to as the continuation phase). The within-participant, within-trial variability of the interval time series during the continuation phase is calculated to provide an overall index of timing precision and, thus by inference, cerebellar functioning.
Variability is the crucial analysis in timing studies, but mean cycle duration is also reported as a measure of accuracy. Spontaneous tapping tempo, or an individual's natural tapping rate without an auditory aid, has a clear maturational trajectory. This developmental change may be influenced by increased attentiveness to the stimulus (Drake, Jones, & Baruch, 2000). Whereas 4- to 6-year-old children tap at a mean spontaneous tempo of approximately 400 ms, adults tap about every 600 ms on average (Drake et al., 2000). Furthermore, preschoolers are more accurate when tapping synchronously to faster tempos (Grieshaber, 1987), and tempo discrimination accuracy in perceptual tasks increases with age. Timing variability and accuracy are separate phenomena, with timing variability associated with cerebellar function and accuracy associated with maturation.
Unimanual Timing in Children With SLI
Even though numerous researchers have proposed a timing deficit in children with SLI, few empirical studies have directly assessed timing precision in these children. Corriveau and Goswami (2009) found that 7- to 11-year-old children with SLI were more variable compared with aged-matched peers when required to tap accurately to a 667 ms target during the continuation phase. There were no group differences in the 500 ms or 400 ms condition. In contrast, Zelaznik and Goffman (2010) found that 6- to 8-year-old children with SLI and typically developing peers were equivalent in their timing precision in four different unimanual timing tasks with a 600-ms target. The four tasks were index finger tapping, as well as timing with the whole hand (a motorically easier task that does not require the isolation of finger movement; Zelaznik & Goffman, 2010), timed circle drawing, and a modulated task that required alternation of large and small taps.
Specifying the Role of Procedural Learning in SLI
Although procedural learning deficits have been documented in children with SLI through tasks such as the serial reaction time task, performance on several nonsequential procedural motor tasks appears to be unaffected in these children. Two studies have assessed the functional integrity of the cerebellum in children with SLI through a delay eyeblink conditioning task (Hardiman, Hsu, & Bishop, 2013; Steinmetz & Rice, 2010). In this task, repeated pairings of a conditioned stimulus (such as a tone) with an unconditioned stimulus (a puff of air to the cornea) eventually elicits a conditioned eyeblink response just prior to the presentation of the unconditioned stimulus. This type of delay conditioning occurs without explicit awareness of the relationship between the tone and the puff of air and therefore is presumably a type of procedural learning (although some controversy exists regarding this point; see Lovibond, Liu, Weidemann, & Mitchell, 2011). Neither Hardiman and colleagues (2013) nor Steinmetz and Rice (2010) identified impaired procedural learning in children with SLI on this task. Children with SLI also demonstrate comparable performance to their typically developing, age-matched peers on the pursuit rotor task, a procedural learning task that does not require sequential processing (Hsu & Bishop, 2014). Based on these nonsequential procedural tasks that appear to be unaffected in children with SLI, we agree with Hardiman and colleagues that “procedural learning deficits in SLI are not general” (2013, p. 436). Timing is another domain that may show some components that are affected and some that are unaffected in children with SLI. In the current study, we aim to further specify the procedural deficit hypothesis by assessing timing that is discrete in contrast to timing that requires bimanual coordination.
Bimanual Clapping
Timing is one crucial component of language production. An additional core feature is the dynamic and coordinated orchestration of multiple effectors (Browman & Goldstein, 1992). An effector is a body part that acts on the environment in isolation, such as a finger, hand, or arm. Speech production requires the control of multiple articulatory, laryngeal, and respiratory effectors. The coordination of two hands may capture the basic nature of multieffector control. A simple model of coordinated action can be found in timed clapping. A requirement of timed clapping is that two effectors (i.e., the two hands) move in a coordinated manner toward an abstract spatial target at midline. Thus, we posit that bimanual timed clapping is similar to speech production in that two effectors must be moved in concert to achieve a spatial and temporal goal (i.e., in clapping, contact at midline in time with the metronome).
Bimanual movements are more complex and require greater information processing than unimanual movements (Serrien, Cassidy, & Brown, 2003). This makes sense given that interlimb coordination develops over a protracted period (Corbetta & Thelen, 1996). Unimanual and bimanual actions are also organized differently in the brain, with unimanual actions organized contralaterally and bimanual actions showing a strong left hemisphere dominance (Jäncke et al., 1998; Serrien et al., 2003; Viviani, Perani, Grassi, Bettinardi, & Fazio, 1998; but see Chen et al., 2005; Foltys et al., 2001).
In the dyslexia literature, several studies have reported timing control deficits in bimanual coordination tasks with half of the participants with dyslexia affected (Wolff, 1993; Wolff, Michel, Ovrut, & Drake, 1990; Wolff et al., 1995). Given that SLI and dyslexia are often comorbid (Snowling, 2012), and procedural learning deficits have been implicated in children with SLI, it is reasonable to hypothesize that bimanual coordination is impaired in children with SLI.
Compared with bimanual clapping, the unimanual tapping task requires fewer degrees of freedom for the single effector, requiring that children achieve only a temporal goal, but without orchestrated and coordinated control demands. It may be that a core deficit in SLI is not in basic timing but rather in the implementation of complex, sequenced, or coordinated movements. Just as procedural tasks that require sequencing are affected in children with SLI, we hypothesize that children with SLI will show impairments on a task that requires coordination across multiple effectors. Procedural learning tasks that do not require sequencing or coordination, such as the pursuit rotor task and unimanual timing, are predicted to be unaffected in children with SLI.
The Current Study
In this study, we focus on (a) timing precision in the archetypal timing task, tapping to a metronome and then continuing to time following removal of the metronome, and (b) timing precision in a task that requires bimanual coordination of two effectors, clapping to a metronome and then continuing to time following removal of the metronome. We are not aware of any studies that have investigated bimanual timing control in children with SLI; thus, the inclusion of this task in the current study is novel. We focus on 4- to 5-year-old children, a crucial age during which children with SLI can be reliably identified and demonstrate the hallmark overt tense and agreement errors (Leonard, 2014). One of two outcomes is possible: (a) timing precision is impaired in both unimanual tapping and bimanual clapping in children with SLI, because both tasks rely on timing, which is a central component of the procedural memory system, or (b) timing precision is only impaired in the bimanual clapping task in children with SLI, because bimanual clapping requires coordination demands. Based on the literature reviewed above, we propose that children with SLI will show deficits when coordinated and bimanual constraints are introduced in a clapping task, while basic cerebellar timing mechanisms revealed through a unimanual tapping task will be spared. We also include a standardized measure of fine and gross motor skill to verify the well-documented motor impairment in the SLI group.
Method
Participants
Forty-eight children ranging in age from 4;0 to 5;11 (years; months) served as participants. Twenty-seven children (14 male, 13 female) met exclusionary criteria for SLI (M age = 5;1, SD = 0;6) and 21 children (9 male, 12 female) were typically developing (M age = 4;11, SD = 0;6). All data related to performance on standardized tests are presented in Table 1. All children were required to be monolingual English speakers, have normal nonverbal intelligence as measured by the Columbia Mental Maturity Scale–Third Edition (Burgemeister, Blum, & Lorge, 1972), pass a hearing screening (pure tones presented bilaterally at 20 dB HL at 500, 1000, 2000, and 4000 Hz), and receive a total structural score within expected levels on the Robbins and Klee (1987) protocol. In addition, all children were required to score in the minimal-to-no symptoms of autism spectrum disorder range on the Childhood Autism Rating Scale–Second Edition (Schopler, Van Bourgondien, Wellman, & Love, 2010), and per parent report have no history of neurological dysfunction (such as seizures, epilepsy, or head injury). Children participated in a number of other assessments and tasks, not reported in this article, as part of a larger study on the relationship between language and motor development in children with SLI. Parent consent, child assent, and approval from the Institutional Review Board at Purdue University were obtained prior to participation.
Table 1.
Language, speech, and motor scores for the typically developing (TD) and specific language impairment (SLI) groups.
| Tests | TD (n = 21) |
SLI (n = 27) |
SLI M-TYP (n = 17) |
SLI M-IMP (n = 9) |
|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | |
| CMMS | 116.7 (9.1) | 104.4 (9.2) | 106.3 (10.3) | 100.3 (6.0) |
| TSS | 24.0 (0.0) | 23.4 (1.2) | 23.6 (0.8) | 22.9 (1.7) |
| CARS-2 | 15.1 (0.3) | 16.5 (1.5) | 16.2 (1.2) | 16.6 (1.9) |
| SPELT-P2/3 | 111.3 (11.1) | 76.7 (9.5) | 77.1 (9.4) | 74.8 (10.0) |
| BBToP CI | 100.1 (8.5) | 72.7 (10.3) | 71.3 (10.2) | 73.4 (9.9) |
| PPVT-4 | 116.9 (10.8) | 101.2 (12.9) | 103.0 (13.1) | 99.2 (13.0) |
| EVT-2 | 115.2 (11.6) | 97.8 (14.9) | 99.5 (8.7) | 94.8 (22.1) |
| MABC-2 Total | 10.7 (2.3) | 8.3 (3.2) | *9.8 (2.8) | *5.3 (0.9) |
| MABC-2 MD | 9.8 (2.2) | 7.5 (2.8) | *8.7 (2.4) | *5.1 (1.8) |
| MABC-2 AC | 10.2 (2.9) | 10.2 (2.4) | 10.6 (2.7) | 9.4 (1.8) |
| MABC-2 BAL | 12.1 (2.8) | 8.8 (3.5) | *10.5 (3.2) | *5.6 (0.5) |
Note. SLI M-TYP = specific language impairment and typical motor skills; SLI M-IMP = specific language impairment and motor impairment; CMMS = Columbia Mental Maturity Scale–Third Edition; TSS = Total Structural Score from the Robbins and Klee (1987) protocol; CARS-2 = Childhood Autism Rating Scale–Second Edition; SPELT-P2/3 = Structured Photographic Expressive Language Test–Preschool 2/Third Edition; BBToP CI = Bankson–Bernthal Test of Phonology Consonant Inventory, to calculate the mean and SD, scores <65 were calculated as 65 (12 of 27 children scored <65); PPVT-4 = Peabody Picture Vocabulary Test–Fourth Edition; EVT-2 = Expressive Vocabulary Test–Second Edition; MABC-2 = Movement Assessment Battery for Children–Second Edition; MABC-2 MD = Movement Assessment Battery for Children–Second Edition Manual Deterity; MABC-2 AC = Movement Assessment Battery for Children–Second Edition Aiming & Catching; MABC-2 BAL = Movement Assessment Battery for Children–Second Edition Balance.
Significant difference between SLI M-TYP and SLI M-IMP, p < .05.
The children with SLI met exclusionary criteria on the basis of Leonard (2014). These children participated in the Purdue University Department of Speech-Language and Hearing Sciences “Summer Fun” Research and Intervention Program. All of the children participating in this program met criteria for SLI and participated in studies directed by Laurence Leonard and Lisa Goffman. The series of diagnostic procedures conducted to verify SLI status are delineated below (Leonard et al., 2007). Children who met criteria for SLI demonstrated significantly impaired language abilities, as indicated by a standard score of 87 or below on the Structured Photographic Expressive Language Test–Preschool 2 (Dawson et al., 2005). They also received a finite verb morphology composite score (i.e., percent correct production of regular past –ed, present third person singular –s, and the copula and auxiliary forms of is, are, and am) greater than 1.25 SD below the mean, using normative data from the local Lafayette, IN, area (Goffman & Leonard, 2000; Leonard, Miller, & Gerber, 1999; M = 53.0, SD = 25.2). The Structured Photographic Expressive Language Test–Preschool 2 using a cutoff of 87 and the finite verb morphology composite score are sensitive and specific indicators of SLI status (Bedore & Leonard, 1998; Goffman & J. Leonard, 2000; Greenslade, Plante, & Vance, 2009).
Inclusionary criteria for the typically developing children included performance within the normal range on the Structured Photographic Expressive Language Test–Third Edition (Dawson et al., 2003), Bankson–Bernthal Test of Phonology (BBToP; Bankson & Bernthal, 1990), Peabody Picture Vocabulary Test–Fourth Edition (Dunn & Dunn, 2007), and Expressive Vocabulary Test–Second Edition (Williams, 2007).
The speech and vocabulary measures were also administered to the children with SLI, although performance on these tests was not used for classification. As is consistent with findings reported in numerous studies of young children diagnosed with SLI (Alt, Plante, & Creusere, 2004; Deevy, Weil, Leonard, & Goffman, 2010; Gray, 2006; Leonard, 2014), 22 out of 27 children in the SLI group scored greater than 1 SD below expected levels on the BBToP.
The Movement Assessment Battery for Children–Second Edition (MABC-2; Henderson, Sugden, & Barnett, 2007) was also administered to all participants to assess fine and gross motor skill. The MABC-2 is a standardized, norm-referenced assessment battery frequently used in clinical practice and research to classify motor skill development in children as typical or impaired. This test was administered according to the standard procedures described in the examiner's manual. Children completed the 3- to 6-year-old age band, which contains eight different tasks across three subtests. One child with SLI did not complete the MABC-2 due to time constraints. For the manual dexterity subtest, the child must place plastic coins into a piggy bank as fast as possible first with the dominant and then with the nondominant hand (posting coins), thread beads onto a string as fast as possible (threading beads), and follow a trail with one continuous line without crossing its boundaries (drawing trail). For the aiming and catching subtest, the examiner and child each stand on a mat 1.8 m apart facing each other. The examiner throws a bean bag, providing 10 opportunities for the child to catch it (catching beanbag). The child then stands on one mat and throws the bean bag to make contact with the other mat, for a total of 10 opportunities (throwing bean bags). For the balance subtest, the child balances on one leg for as long as possible and then balances on the other leg (balance), walks along a 4.5-m yellow line taped to the floor without stepping off and with both heels raised (walking heels raised), and hops from one mat to the next with both feet together for up to five jumps (jumping on mats). The standard scores from each of the eight tasks are summed to yield subtest standard scores and a Total Test Score. Each subtest and the Total Test Score has a mean of 10 and an SD of 3. Group results for the subtests and Total Test are reported in Table 1.
Experimental Timing Tasks
Children sat in a child-sized chair at a table 51 cm in height and the examiner instructed them to tap (unimanual timing task) or clap (bimanual clapping task) in time to tones presented by a metronome. On each trial, the metronome presented 16 synchronization tones at 600-ms intervals. The 600-ms interval consisted of a 10-ms tone followed by 590 ms of silence. When the metronome disengaged, children continued to tap or clap at the same rate for 32 continuation intervals. Timing variability during the continuation phase is the most widely used measure of timing precision (Wing & Kristofferson, 1973).
To create a child friendly task and ensure that children continued tapping or clapping for the 32 continuation intervals, the examiner silently counted until the child tapped or clapped for 32 intervals, then made an elephant puppet make a trumpeting sound to signal that the child should stop. Each participant completed a practice trial with the examiner, who modeled the correct pace, provided online feedback, and reinforced continuing to tap or clap until the elephant puppet trumpeted. All sessions were videotaped using a Panasonic HDC-HS700 camera.
For the tapping task, children used their dominant hand and tapped on the table in front of them. The examiner modeled and encouraged children to tap by lifting their arm from the shoulder. However, flexing from the wrist was also accepted on the basis of the preference of the child. The examiner instructed children to tap using a whole arm or hand movement because Zelaznik and Goffman (2010) found that differentiating movement of a single finger, as is done in the classical finger tapping task, was more difficult for young children. The clapping task was similar to the tapping task but involved the child clapping rather than tapping to the metronome. Children completed six clapping trials followed by six tapping trials.
Data Collection and Reduction
A Polhemus Liberty-8 magnetic motion capture system (Polhemus, Colchester, VT) was used to capture hand movements in three dimensions at a 240-Hz sampling rate. Sensors were taped to the top of the left and right hands and to each collarbone. The sensors on the collarbone provided a stable frame of reference but did not factor into any analyses. The Liberty magnet was connected to a Dell Optiplex 760 computer, and all tapping and clapping trials were collected through a locally developed Matlab routine. The Liberty system began collecting movement data when a second examiner manually started the trial in Matlab. A series of two beeps alerted the examiners that the trial had started, and the metronome then immediately began presenting the synchronization tones.
All data were low-pass filtered (Butterworth, 5th order) in a forward and backward direction at 15 Hz. Velocity was calculated using a three-point central difference technique. A locally developed Matlab routine was used to score kinematic records and to determine the time at which one tapping or clapping interval ended and the subsequent interval commenced. For tapping, the superior–inferior movement trajectory for the dominant hand was used in all analyses. For clapping, the medial–lateral dimension was utilized for each hand. The algorithms and scoring routines for tapping and clapping were identical except that tapping used one (dominant hand movement) receiver and clapping used two receivers (right and left hand movement). Because of the location of the Polhemus motion capture system, for the clapping task, when the right hand was moving toward the midline, velocity was positive, and when the left hand was moving toward the midline the velocity was negative. Our algorithm searched for local maxima (right hand) or minima (left hand) in velocity. It then searched for the first sample going forward in which velocity fell to below (right hand) or above (left hand) 3% of the largest amplitude movement.
These claps or taps were displayed in Matlab (see Figure 1) and we utilized an interactive graphical routine where records that contained hesitations or false alarms were corrected by hand. For clapping, the interval was determined for each hand as there could be slight differences in when each hand reached the low velocity criterion. We included all tapping and clapping intervals that occurred after 2 s following the offset of the metronome (i.e., the beginning of the continuation portion of the trial) to avoid including taps or claps the child may produce with hesitancies. A minimum of 14 continuation tapping or clapping intervals was required for the trial to be included in the analysis.
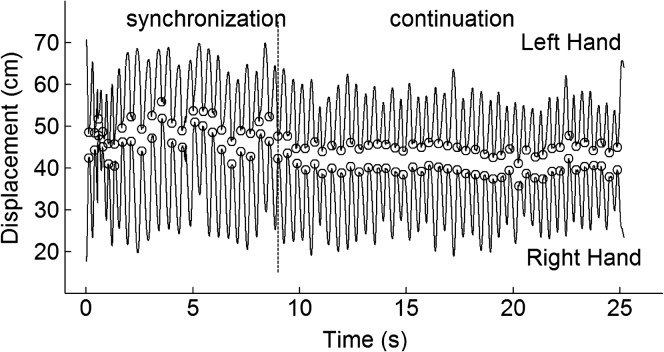
Figure 1.
Typical clapping trial. We show a displacement record for a clapping trial for a 5-year-old male participant. The dashed vertical line demarcates the point in time when the metronome disengaged. At time zero the hands start apart and then come together for a clap. The open circles denote when our algorithm detected the end (start) of a clapping interval.
Data Analyses
Two measures were the focus of investigation, mean cycle duration and cycle variability. The latter was calculated using the coefficient of variation, defined as the within-subject, within-trial standard deviation of the interval time series divided by the average intertap or interclap interval and converted to a percentage. Because variability due to increasing or decreasing rate during a trial is not of theoretical interest (Ivry & Hazeltine, 1995), the interval time-series was linearly detrended before computing the coefficient of variation. Linear detrending removes variance that is due to the participant drifting off the target rate and is used as a measure of timing precision that is not affected by changes in goal average frequency (see Zelaznik, Spencer, & Ivry, 2008). Any trial with a coefficient of variation greater than 50% was removed from further analysis, as this reflects off-task behavior. Cycle duration is reported in milliseconds.
Results are reported for children who produced at least three usable trials. This resulted in usable data for 26 children with SLI in the tapping task and all 27 in the clapping task. Data were analyzed from all 21 typically developing children in both tasks. For the tapping task, this yielded a mean of 4.7 trials for the SLI group (SE = .11) and 4.6 trials for the typically developing group (SE = .11). For the clapping task, the mean number of trials for the SLI group was 5.4 (SE = .18) and for the typically developing group was 4.3 (SE = .14). For the tapping task, a one-way analysis of variance was used with Group (SLI vs. typically developing) as the between-subjects variable. For the clapping task, a mixed design analysis of variance was used with group (SLI vs. typically developing) as the between-subjects variable and coefficient of variation/cycle duration of the dominant and nondominant hands as the within-subjects variables.
Results
Tapping
As shown on the left side of Figure 2, there were no group differences in timing variability in the unimanual tapping task, F(1, 45) = 1.20, p = .28. The right side of Figure 2 reveals that the cycle duration of the dominant hand was shorter for children with SLI compared with their typically developing peers, F(1, 45) = 8.01, p = .007, η2 = .151. In sum, timing variability did not differentiate the two groups of children. However, children with SLI tapped at a faster rate than did their typically developing peers.
Figure 2.
Mean coefficient of variation (%) and mean cycle duration (ms) for the typically developing (TD) and specific language impairment (SLI) groups for the tapping and clapping tasks. Error bars represent standard errors. D = dominant hand; N = nondominant hand.
Clapping
In the bimanual clapping task, as illustrated on the left side of Figure 2, children in the SLI group showed greater variability compared with their typically developing peers, F(1, 46) = 10.74, p = .002, η2 = .189. There was no hand effect, F(1, 46) = 1.61, p = .21, or interaction, F(1, 46) = 2.66, p = .11. As shown on the right side of Figure 2, there were no group differences in cycle duration, F(1, 46) = 1.85, p = .18, and no hand effect, F(1, 46) = 0.03, p = .85, or interaction, F(1, 46) = 1.02, p = .32. In the clapping task, which requires bimanual coordination, children with SLI demonstrated significantly greater timing variability compared with typically developing controls.
Movement Assessment Battery for Children–Second Edition
On the MABC-2, children with SLI demonstrated lower overall scores than their peers, F(1, 45) = 8.79, p = .005, η2 = .163. A repeated-measures analysis of the individual subtests (manual dexterity, balance, and aiming and catching) showed main effects of group, F(1, 45) = 9.34, p = .004, η2 = .172 and subtest, F(2, 44) = 8.31, p = .001, η2 = .286, as well as a group*subtest interaction, F(2, 44) = 6.176, p = .004, η2 = .219. Follow-up analyses revealed that children with SLI scored lower on manual dexterity, F(1, 45) = 9.34, p = .004, η2 = .172, and balance, F(1, 45) = 12.74, p < .001, η2 = .221, subtests. There were no group differences on the aiming and catching subtest, F(1, 45) = 0.003, p = .96. It is notable that, although 20 out of 21 of the children with typical development scored within expected levels on the MABC-2 (i.e., achieved a standard score within 1 SD of the mean), only 17 out of 26 children with SLI scored within expected levels. Therefore, nine children with SLI displayed a motor impairment on the basis of this measure. An additional four children with SLI showed relatively weak performance with a standard score of 7 (which is 1 SD below the mean).
Relationships Among Timing Tasks in All Participants
To further evaluate relationships across variability and accuracy in the clapping and tapping tasks, we conducted Pearson correlations and found that tapping variability and clapping variability were not significantly correlated with one another, r = .24. There was a significant correlation between cycle duration in the tapping and clapping task, r = .49. Children who produce small/large intervals in one task tend to produce small/large intervals on the other task.
Follow-up Analyses Based on Motor Performance in Children With SLI
As noted above, 17 of the children with SLI performed within the normal range on the MABC-2. Based on this observation, we further investigated the relationship between MABC-2 scores and timing precision in children with SLI by splitting children into two groups on the basis of their MABC-2 Total Test scores. A cutoff of 7, which corresponds to 1 SD below the mean and the 15th percentile, was used. Children who received a score of 7 or higher (n = 17) were considered to have SLI and typical motor skills, and children who received a score of 6 or lower (n = 9) were considered to have SLI and motor impairment. There were no age (SLI and typical motor skills: M = 59.6 months, SD = 6.8 months; SLI and motor impairment: M = 59.4 months, SD = 6.5 months) or sex (SLI and typical motor skills = seven girls, 10 boys; SLI and motor impairment = four girls, five boys) differences between the groups.
Table 1 shows the standardized test scores for the children with SLI and typical motor skills and the children with SLI and motor impairment. These two subgroups differed only the MABC-2 Total Test score and the manual dexterity and balance subtests; no differences were observed in language, speech, or nonverbal reasoning performance.
Movement Assessment Battery for Children–Second Edition
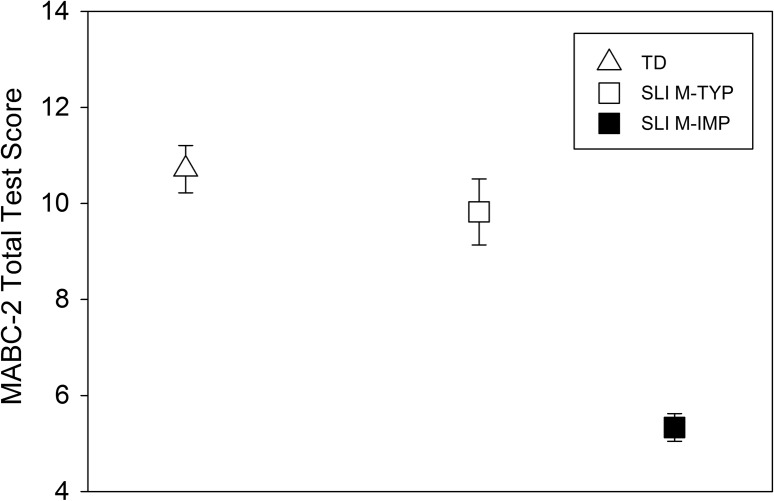
In Figure 3, we present the mean MABC-2 Total Test scores for children with SLI and typical motor skills, children with SLI and motor impairment, and typically developing peers. It was not surprising that, as the groups were selected on this variable, there was a significant group difference, F(2, 44) = 17.48, p < .001, η2 = .44. Post hoc analyses using the Tukey's honestly significant difference (HSD) procedure confirmed that children with SLI and motor impairment differed from the typically developing children, p < .001, and the children with SLI and typical motor skills, p < .001. Children with SLI and typical motor skills performed similarly to the typically developing children, p = .47.
Figure 3.
Mean Movement Assessment Battery for Children–Second Edition (MABC-2) Total Test scores for the typically developing (TD), specific language impairment and typical motor skills (SLI M-TYP), and specific language impairment and motor impairment (SLI M-IMP) groups. Error bars represent standard errors.
Tapping
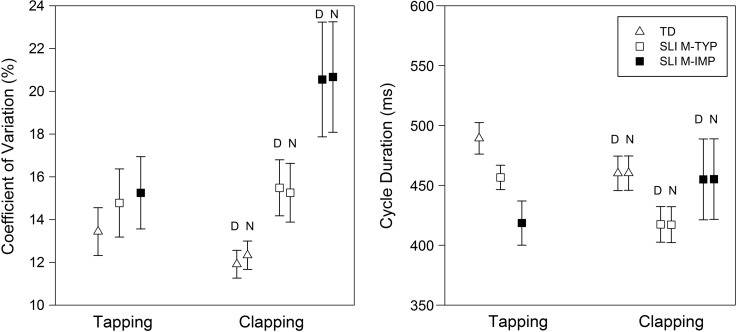
Tapping variability and cycle duration were examined considering children with SLI and typical motor skills and children with SLI and motor impairment as separate groups. The left side of Figure 4 depicts the coefficient of variation for children with SLI and typical motor skills, children with SLI and motor impairment, and typically developing children in the tapping task. There were no group differences in variability, F(2, 43) = 0.44, p = .65. As shown on the right side of Figure 4, there was a group difference in cycle duration, F(2, 43) = 5.80, p = .006, η2 = .21. Post hoc analysis using the Tukey's HSD procedure revealed that children with SLI and motor impairment tapped significantly faster than peers with typically developing language, p = .005. Children with SLI and typical motor skills group tapped at a similar rate to the typically developing children, p = .17, and the children with SLI and motor impairment, p = .21. In sum, children in all three groups controlled timing in the unimanual tapping task with similar amounts of variability. Children with SLI and motor impairment tapped significantly faster compared with their typically developing peers.
Figure 4.
Mean coefficient of variation (%) and mean cycle duration (ms) for the typically developing (TD), specific language impairment and typical motor skills (SLI M-TYP), and specific language impairment and motor impairment (SLI M-IMP) groups for the tapping and clapping tasks. Error bars represent standard errors. D = dominant hand; N = nondominant hand.
Clapping
The left side of Figure 4 shows the coefficient of variation for children with SLI and typical motor skills, children with SLI and motor impairment, and typically developing children in the clapping task. There was a group effect of variability, F(2, 42) = 5.20, p = .01, η2 = .20, but no hand effect, F(1, 42) = 0.84, p = .36, or interaction, F(2, 42) = 2.08, p = .14. A Tukey's HSD test revealed that children with SLI and motor impairment exhibited greater timing variability than their typically developing peers, p = .03. Children with SLI and typical motor skills performed similarly to typically developing children, p = .75, and children with SLI and motor impairment, p = .90. The right side of Figure 4 shows there were no group differences in cycle duration, F(2, 42) = 2.87, p = .07, and no hand effect, F(1, 42) = 0.004, p = .95, or interaction, F(2, 42) = 0.921, p = .41. Timing precision, not rate, is related to language and motor status in the bimanual clapping task.
Relationships Among Timing Tasks, Standardized Motor Performance, and Language Performance
An obvious follow-up question is whether motor performance variables influence timing precision in the children with SLI. To test this possibility, a Pearson correlation test was performed to compare timing precision in the clapping task to the manual dexterity and balance subtest scores on the MABC-2. Timing precision in the clapping task was not significantly correlated with manual dexterity, r = −.25, or balance, r = −.38.
Because a core question addressed in the present work is how subcomponents of language and motor performance relate to one another, Supplemental Materials S1 and S2 contains correlation matrices for children with SLI and for their typically developing peers. It is particularly striking that almost no within- or cross-domain relationships are significant for the children with SLI. In contrast, children with typical language development show relationships across all domains of speech and language.
Discussion
We found that children with SLI exhibited greater timing variability when coordinating two effectors in the bimanual clapping task compared with typically developing peers. We propose that timing precision in a bimanual task relies upon complex cognitive processes (Franz, Zelaznik, Swinnen, & Walter, 2001; Mechsner, Kerzel, Knoblich, & Prinz, 2001) that are impaired in children with SLI. This may be similar to the multieffector control demands required for language production. In the unimanual tapping task, there were no group differences in timing precision. Young children who produce the overt grammatical errors associated with the SLI diagnosis do not demonstrate a deficit in unimanual timing precision.
The results of this study allow us to assess several competing hypotheses concerning the relationship between language and motor deficits in children with SLI. First, we discuss how the current data contribute to claims that impaired timing underlies language and motor deficits (Alcock et al., 2000; Bishop, 2002; Ullman & Pierpont, 2005). Second, we consider the MABC-2 scores in the SLI group and the results of the post hoc analyses in relation to the general comorbidity hypothesis (Bishop, 2002; Hill, 2001). We present a working model to account for common characteristics of language and motor deficits seen in children with SLI.
Hypotheses Regarding Impaired Timing and Procedural Deficits in SLI
Several authors have claimed that timing is impaired in children with SLI (Alcock et al., 2000; Bishop, 2002; Corriveau & Goswami, 2009; Ullman & Pierpont, 2005). Results from the current study, along with prior findings (Zelaznik & Goffman, 2010), however, support that unimanual tapping is unaffected in children with SLI. It is important to note that timing variability in the present study was not tied to accuracy, as shifts in cycle duration over a trial were removed by detrending, so that timing variability can be considered separately from average rate or rate changes. This differs from the method used by Corriveau and Goswami (2009), in which variability was calculated in relation to whether the unpaced responses were similar to what would be expected if the metronome beeps were present. The classic paradigm employed in the current study reveals that discrete timing precision, associated with cerebellar damage (Spencer et al., 2003), is spared in children with SLI.
One central tenet of the procedural deficit hypothesis predicts timing deficits associated with cerebellar involvement in children with SLI, because the procedural memory system is mediated in part by the cerebellum. Thus, given the results of the present study, at least some components of the procedural deficit hypothesis require modification. Indeed, the control of timing is one of only a few dimensions of motor processing identified to date that is quite clearly spared in SLI. These results are consistent with other work demonstrating that the procedural deficit hypothesis does not fully account for difficulties experienced by children with SLI and suggest that a more nuanced account is required. Hsu and Bishop (2014) reported that aspects of procedural learning related to sequencing, as indexed by the serial reaction time task, are impaired, whereas performance on another procedural but nonsequential task, the pursuit rotor task, is not. The present findings are consistent with this more complex profile of performance in procedural memory tasks, in that discrete timing, the most basic cerebellar task, is not affected in SLI. However, we found that timing precision in a bimanual coordination task was impaired in children with SLI.
The present findings support that processes associated with bimanual and multieffector coordination are indeed impaired in children with SLI. In our earlier work, we hypothesized that coordination across effectors, as is required for language production, may be a locus of impairment in children with SLI. The current experiment explicitly assessed timing independent of complex and orchestrated spatiotemporal demands (unimanual tapping) as well as timing that incorporates these more complex demands (bimanual clapping). The control of timing during bimanual clapping requires that the two hands must be coordinated in time and space, and for children with SLI this increase in complexity leads to weaknesses in timing precision. It appears that only some specific components of procedural learning relate to SLI status. Some aspects of motor performance (unimanual timing, aiming, and catching) are unaffected in children with SLI. However, along with other aspects of procedural learning, such as the serial reaction time task (e.g., Hsu & Bishop, 2014; Tomblin et al., 2007) and statistical learning (e.g., Evans et al., 2009), children with SLI demonstrate difficulty with orchestrated and coordinative timing.
Hypotheses Regarding Language and Motor Relationships in SLI
In the present study, children with SLI performed more poorly on a standardized test of motor skill compared with typically developing peers. When performance on this standardized assessment, the MABC-2, is viewed in terms of typical versus disordered status, 9 (out of 26) of the children with SLI showed an overt motor deficit. Many children with SLI who performed within normal limits on the MABC-2, however, received scores that were equivalent to those of their typically developing peers.
On the surface, the finding that only 9 of the children with SLI scored below the expected range on the MABC-2 may appear consistent with a comorbidity hypothesis. A comorbidity hypothesis would predict that motor deficits are randomly rather than systematically associated with SLI. This is clearly not the case, because unimanual timing performance is unaffected in all children with SLI, regardless of motor status. To further accentuate this relationship, unimanual timing precision did not correspond with MABC-2 scores on the manual dexterity and balance subtests (the two subtests on which children with SLI showed impaired performance). Neither the presence of motor or language impairments disrupted children's ability to maintain timing in the unimanual tapping task; all of the children with SLI showed timing variability that was similar to that of their typically developing peers.
In the bimanual clapping task, as a group, children with SLI were significantly more variable in their control of timing compared to typically developing peers. But when children with SLI were separated according to motor performance on the MABC-2, only those with both SLI and motor impairment showed significantly impaired performance compared with typically developing peers. A stair step progression was observed, with children with SLI with motor impairment showing the weakest performance, those with SLI and typical motor performance at an intermediate level, and typically developing children showing the strongest performance. These results suggest that motor factors may also contribute to deficits in bimanual timing precision and coordination. We found it striking that even the children with SLI and significant motor deficits showed no impairments in single effector timing. It will be important to increase the sample size of children with co-occurring motor deficits in future work to fully understand the relative contributions of motor and language impairments on the procedural clapping task. In sum, timing precision is affected by the language and motor deficits associated with SLI in a bimanual clapping task but unaffected in a discrete unimanual tapping task.
A few interesting findings regarding timing accuracy also emerged. In general, all children tapped and clapped faster than the 600-ms target. This is consistent with research showing that 4- to 6-year-old children produce a mean cycle duration of approximately 400 ms during spontaneous tapping (Drake et al., 2000). It is important to note that in the unimanual tapping task, rate of tapping aligned with motor status, but not with language status; children with SLI and motor impairment demonstrated the fastest tapping rates in the unimanual task. Accuracy is tied to maturation as well as motor status. Further supporting this relationship, across all participants rate was significantly correlated between the tapping and clapping tasks, but variability was not.
A Shared Mechanisms Account of Language and Motor Relationships in SLI
Our long-term goal is to develop a model that accounts for language and motor deficits seen in children with SLI. The results from the present study support that language and motor processes do not develop separately. Rather, specific cognitive functions interact with language and motor processing. Deficits in cognitive functioning can lead to cascading impairments in both language and motor domains.
An important component of a shared mechanisms account is the specificity of those components of language and action that are impaired in children with SLI, and those that are unaffected. Impairments in grammatical morphology and bimanual timing precision are both implicated in SLI, whereas discrete timing is spared. The motor disorder observed in SLI appears far removed from low-level movement implementation, and to involve the processing and execution of coordinated and sequenced elements.
To understand mechanisms underlying SLI, it is important to consider what components of language and of motor processing pattern together. In the present findings, bimanual timing precision, representing coordinative processes, was related to language status as well as motor status. However, we found no correlations between language and motor measures in children with SLI—severity of language impairment was not related to the presence or absence of a general fine and gross motor impairment. In addition (see Supplemental Materials S1 and S2), children with SLI, unlike their typically developing peers, showed few correlations within the language domain or the motor domain. For example, vocabulary, speech sound accuracy, fine motor control, and balance are not obligatorily affected in children with SLI. Together, these findings support the view that children with SLI do not necessarily display global language or motor deficits, but rather they show a specific profile of language and motor weaknesses related to common cognitive factors.
Conclusion
SLI has classically been viewed as a modular deficit that is confined to the language domain. It is increasingly apparent that children with SLI have deficits outside of the language domain that are not simple comorbidities but are directly related to their language profiles. Further specification of how these broader cognitive deficits contribute to the unique profiles present in children with SLI will inform theories regarding typical and atypical language acquisition. It is also likely that by framing future research within this perspective we will improve our ability to identify and treat these children well before they are in the thick of their grammatical disorder at age 4 years.
Supplementary Material
Acknowledgments
This research was supported by the National Institute on Deafness and Other Communication Disorders Grant R01 DC04826 (awarded to Lisa Goffman). We thank Janna Berlin, Barb Brown, Allison Gladfelter, and Meredith Saletta for their assistance with many phases of this study.
Funding Statement
This research was supported by the National Institute on Deafness and Other Communication Disorders Grant R01 DC04826 (awarded to Lisa Goffman).
References
- Alcock K. J., Passingham R. E., Watkins K., & Vargha-Khadem F. (2000). Pitch and timing abilities in inherited speech and language impairment. Brain and Language, 75, 34–46. [DOI] [PubMed] [Google Scholar]
- Alt M., Plante E., & Creusere M. (2004). Semantic features in fast-mapping performance of preschoolers with specific language impairment versus preschoolers with normal language. Journal of Speech, Language, and Hearing Research, 47, 407–420. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). Washington, DC: Author. [Google Scholar]
- Archibald L. M., & Alloway T. P. (2008). Comparing language profiles: Children with specific language impairment and developmental coordination disorder. International Journal of Language & Communication Disorders, 43, 165–180. [DOI] [PubMed] [Google Scholar]
- Archibald L. M., & Gathercole S. E. (2006). Short-term and working memory in specific language impairment. International Journal of Language & Communication Disorders, 41, 675–693. [DOI] [PubMed] [Google Scholar]
- Archibald L. M., & Joanisse M. F. (2009). On the sensitivity and specificity of nonword repetition and sentence recall to language and memory impairments in children. Journal of Speech, Language, and Hearing Research, 52, 899–914. [DOI] [PubMed] [Google Scholar]
- Bankson N. W., & Bernthal J. E. (1990). Bankson–Bernthal Test of Phonology. Chicago, IL: Riverside Press. [Google Scholar]
- Bedore L. M., & Leonard L. B. (1998). Specific language impairment and grammatical morphology: A discriminant function analysis. Journal of Speech, Language, and Hearing Research, 41, 1185–1192. [DOI] [PubMed] [Google Scholar]
- Bishop D. V. (2002). Motor immaturity and specific speech and language impairment: Evidence for a common genetic basis. American Journal of Medical Genetics, 114, 56–63. [DOI] [PubMed] [Google Scholar]
- Bishop D. V., & Edmundson A. (1987). Specific language impairment as a maturational lag: Evidence from longitudinal data on language and motor development. Developmental Medicine & Child Neurology, 29, 442–459. [DOI] [PubMed] [Google Scholar]
- Browman C. P., & Goldstein L. (1992). Articulatory phonology: An overview. Haskins Laboratories Status Report on Speech Research, 112, 23–42. [Google Scholar]
- Brumbach A. C. D., & Goffman L. (2014). Interaction of language processing and motor skill in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 57, 158–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgemeister B., Blum L., & Lorge I. (1972). Columbia Mental Maturity Scale–Third Edition. New York, NY: Harcourt Brace Jovanovich. [Google Scholar]
- Chen J. T., Lin Y. Y., Shan D. E., Wu Z. A., Hallett M., & Liao K. K. (2005). Effect of transcranial magnetic stimulation on bimanual movements. Journal of Neurophysiology, 93, 53–63. [DOI] [PubMed] [Google Scholar]
- Corbetta D., & Thelen E. (1996). The developmental origins of bimanual coordination: A dynamic perspective. Journal of Experimental Psychology: Human Perception and Performance, 22, 502. [DOI] [PubMed] [Google Scholar]
- Corriveau K. H., & Goswami U. (2009). Rhythmic motor entrainment in children with speech and language impairments: Tapping to the beat. Cortex, 45, 119–130. [DOI] [PubMed] [Google Scholar]
- Dawson J. I., Stout C., Eyer J. A., Tattersall P., Fonkalsrud J., & Croley K. (2003). Structured Photographic Expressive Language Test—Third Edition. DeKalb, IL: Janelle Publications. [Google Scholar]
- Dawson J. I., Stout C., Eyer J. A., Tattersall P., Fonkalsrud J., & Croley K. (2005). Structured Photographic Expressive Language Test–Preschool: Second Edition. DeKalb, IL: Janelle Publications. [Google Scholar]
- Deevy P., Weil L. W., Leonard L. B., & Goffman L. (2010). Extending use of the NRT to preschool-age children with and without specific language impairment. Language, Speech, and Hearing Services in Schools, 41, 277–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodwell K., & Bavin E. L. (2008). Children with specific language impairment: An investigation of their narratives and memory. International Journal of Language & Communication Disorders, 43, 201–218. [DOI] [PubMed] [Google Scholar]
- Drake C., Jones M. R., & Baruch C. (2000). The development of rhythmic attending in auditory sequences: Attunement, referent period, focal attending. Cognition, 77, 251–288. [DOI] [PubMed] [Google Scholar]
- Dunn L. M., & Dunn D. M. (2007). Peabody Picture Vocabulary Test–Fourth Edition. San Antonio, TX: Pearson. [Google Scholar]
- Evans J. L., Saffran J. R., & Robe-Torres K. (2009). Statistical learning in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 52, 321–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltys H., Sparing R., Boroojerdi B., Krings T., Meister I. G., Mottaghy F. M., & Töpper R. (2001). Motor control in simple bimanual movements: A transcranial magnetic stimulation and reaction time study. Clinical Neurophysiology, 112, 265–274. [DOI] [PubMed] [Google Scholar]
- Franz E. A., Zelaznik H. N., Swinnen S., & Walter C. (2001). Spatial conceptual influences on the coordination of bimanual actions: When a dual task becomes a single task. Journal of Motor Behavior, 33, 103–112. [DOI] [PubMed] [Google Scholar]
- Frensch P. A., & Rünger D. (2003). Implicit learning. Current Directions in Psychological Science, 12, 13–18. [Google Scholar]
- Gerken L., & McGregor K. (1998). An overview of prosody and its role in normal and disordered child language. American Journal of Speech-Language Pathology, 7, 38–48. [Google Scholar]
- Goffman L. (1999). Prosodic influences on speech production in children with specific language impairment and speech deficits: Kinematic, acoustic, and transcription evidence. Journal of Speech, Language, and Hearing Research, 42, 1499–1517. [DOI] [PubMed] [Google Scholar]
- Goffman L. (2004). Kinematic differentiation of prosodic categories in normal and disordered language development. Journal of Speech, Language, and Hearing Research, 47, 1088–1102. [DOI] [PubMed] [Google Scholar]
- Goffman L., & Leonard J. (2000). Growth of language skills in preschool children with specific language impairment: Implications for assessment and intervention. American Journal of Speech-Language Pathology, 9, 151–161. [Google Scholar]
- Gray S. (2006). The relationship between phonological memory, receptive vocabulary, and fast mapping in young children with specific language impairment. Journal of Speech, Language, and Hearing Research, 49, 955–969. [DOI] [PubMed] [Google Scholar]
- Grieshaber K. (1987). Children's rhythmic tapping: A critical review of research. Bulletin of the Council for Research in Music Education, 90, 73–81. [Google Scholar]
- Greenslade K. J., Plante E., & Vance R. (2009). The diagnostic accuracy and construct validity of the Structured Photographic Expressive Language Test–Preschool. Language, Speech, and Hearing Services in Schools, 40, 150–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardiman M. J., Hsu H. J., & Bishop D. V. (2013). Children with specific language impairment are not impaired in the acquisition and retention of Pavlovian delay and trace conditioning of the eyeblink response. Brain and Language, 127, 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. E., Sugden D. A., & Barnett A. L. (2007). Movement Assessment Battery for Children–Second Edition. London, United Kingdom: Pearson Assessment. [Google Scholar]
- Hill E. L. (1998). A dyspraxic deficit in specific language impairment and developmental coordination disorder? Evidence from hand and arm movements. Developmental Medicine & Child Neurology, 40, 388–395. [DOI] [PubMed] [Google Scholar]
- Hill E. L. (2001). Non-specific nature of specific language impairment: A review of the literature with regard to concomitant motor impairments. International Journal of Language & Communication Disorders, 36, 149–171. [DOI] [PubMed] [Google Scholar]
- Hsu H. J., & Bishop D. V. (2014). Sequence-specific procedural learning deficits in children with specific language impairment. Developmental Science, 17, 352–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivry R. B., & Hazeltine R. E. (1995). Perception and production of temporal intervals across a range of durations: Evidence for a common timing mechanism. Journal of Experimental Psychology: Human Perception and Performance, 21, 3–18. [DOI] [PubMed] [Google Scholar]
- Ivry R. B., & Keele S. W. (1989). Timing functions of the cerebellum. Journal of Cognitive Neuroscience, 1, 136–152. [DOI] [PubMed] [Google Scholar]
- Jäncke L., Peters M., Schlaug G., Posse S., Steinmetz H., & Müller-Gärtner H. W. (1998). Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant and subdominant hand. Cognitive Brain Research, 6, 279–284. [DOI] [PubMed] [Google Scholar]
- Johnston J. (1994). Cognitive abilities of children with language impairment. In Watkins R. and Rice M. (Eds.), Specific language impairments in children (vol. 4, pp. 107–121). Baltimore, MD: Brookes. [Google Scholar]
- Johnston J. R., & Weismer S. E. (1983). Mental rotation abilities in language-disordered children. Journal of Speech, Language, and Hearing Research, 26, 397–403. [DOI] [PubMed] [Google Scholar]
- Lashley K. S. (1951). The problem of serial order in behavior. In Jeffress L. A. (Ed.), Cerebral mechanisms in behavior (pp. 112–136). New York, NY: Wiley. [Google Scholar]
- Leonard L. B. (2014). Children with specific language impairment. Cambridge, MA: MIT Press. [Google Scholar]
- Leonard L. B., Miller C., & Gerber E. (1999). Grammatical morphology and the lexicon in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 42, 678–689. [DOI] [PubMed] [Google Scholar]
- Leonard L. B., Weismer S. E., Miller C. A., Francis D. J., Tomblin J. B., & Kail R. V. (2007). Speed of processing, working memory, and language impairment in children. Journal of Speech, Language, and Hearing Research, 50, 408–428. [DOI] [PubMed] [Google Scholar]
- Locke J. L. (1997). A theory of neurolinguistic development. Brain and Language, 58, 265–326. [DOI] [PubMed] [Google Scholar]
- Lovibond P. F., Liu J. C., Weidemann G., & Mitchell C. J. (2011). Awareness is necessary for differential trace and delay eyeblink conditioning in humans. Biological Psychology, 87, 393–400. [DOI] [PubMed] [Google Scholar]
- Lum J. A., & Bleses D. (2012). Declarative and procedural memory in Danish speaking children with specific language impairment. Journal of Communication Disorders, 45, 46–58. [DOI] [PubMed] [Google Scholar]
- Lum J. A., Conti-Ramsden G., Page D., & Ullman M. T. (2012). Working, declarative and procedural memory in specific language impairment. Cortex, 48, 1138–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor K. K., & Leonard L. B. (1994). Subject pronoun and article omissions in the speech of children with specific language impairment: A phonological interpretation. Journal of Speech, Language, and Hearing Research, 37, 171–181. [DOI] [PubMed] [Google Scholar]
- McPhillips M., Finlay J., Bejerot S., & Hanley M. (2014). Motor deficits in children with autism spectrum disorder: A cross-syndrome study. Autism Research, 7, 664–676. [DOI] [PubMed] [Google Scholar]
- Mechsner F., Kerzel D., Knoblich G., & Prinz W. (2001). Perceptual basis of bimanual coordination. Nature, 414, 69–73. [DOI] [PubMed] [Google Scholar]
- Powell R. P., & Bishop D. V. M. (1992). Clumsiness and perceptual problems in children with specific language impairment. Developmental Medicine & Child Neurology, 34, 755–765. [DOI] [PubMed] [Google Scholar]
- Robbins J., & Klee T. (1987). Clinical assessment of oropharyngeal motor development in young children. Journal of Speech and Hearing Disorders, 52, 271–277. [DOI] [PubMed] [Google Scholar]
- Schlerf J. E., Spencer R. M. C., Zelaznik H. N., & Ivry R. B. (2007). Timing of rhythmic movements in patients with cerebellar degeneration. The Cerebellum, 6, 221–231. [DOI] [PubMed] [Google Scholar]
- Schopler E., Van Bourgondien M., Wellman J., & Love S. (2010). Childhood Autism Rating Scale–Second Edition (CARS2): Manual. Los Angeles, CA: Western Psychological Services. [Google Scholar]
- Schwartz M., & Regan V. (1996). Sequencing, timing, and rate relationships between language and motor skill in children with receptive language delay. Developmental Neuropsychology, 12, 255–270. [Google Scholar]
- Serrien D. J., Cassidy M. J., & Brown P. (2003). The importance of the dominant hemisphere in the organization of bimanual movements. Human Brain Mapping, 18, 296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowling M. J. (2012). Changing concepts of dyslexia: Nature, treatment and comorbidity. Journal of Child Psychology and Psychiatry and Allied Disciplines, 53, e1–e3. [DOI] [PubMed] [Google Scholar]
- Spencer R. M., Ivry R. B., & Zelaznik H. N. (2005). Role of the cerebellum in movements: Control of timing or movement transitions? Experimental Brain Research, 161, 383–396. [DOI] [PubMed] [Google Scholar]
- Spencer R. M., Zelaznik H. N., Diedrichsen J., & Ivry R. B. (2003. May 30). Disrupted timing of discontinuous but not continuous movements by cerebellar lesions. Science, 300, 1437–1439. [DOI] [PubMed] [Google Scholar]
- Steinmetz A. B., & Rice M. L. (2010). Cerebellar-dependent delay eyeblink conditioning in adolescents with specific language impairment. Journal of Neurodevelopmental Disorders, 2(4), 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomblin J. B., Mainela-Arnold E., & Zhang X. (2007). Procedural learning in adolescents with and without specific language impairment. Language Learning and Development, 3, 269–293. [Google Scholar]
- Ullman M. T. (2001). A neurocognitive perspective on language: The declarative/procedural model. Nature Reviews Neuroscience, 2, 717–726. [DOI] [PubMed] [Google Scholar]
- Ullman M. T. (2004). Contributions of memory circuits to language: The declarative/procedural model. Cognition, 92, 231–270. [DOI] [PubMed] [Google Scholar]
- Ullman M. T., Corkin S., Coppola M., Hickok G., Growdon J. H., Koroshetz W. J., & Pinker S. (1997). A neural dissociation within language: Evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience, 9, 266–276. [DOI] [PubMed] [Google Scholar]
- Ullman M. T., & Pierpont E. I. (2005). Specific language impairment is not specific to language: The procedural deficit hypothesis. Cortex, 41, 399–433. [DOI] [PubMed] [Google Scholar]
- Ullman M. T., & Pullman M. Y. (2015). A compensatory role for declarative memory in neurodevelopmental disorders. Neuroscience & Biobehavioral Reviews, 51, 205–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viskontas I. V., & Knowlton B. J. (2003). Procedural learning: Humans. In Byrne J. H. (Ed.), Learning and Memory–Second Edition (pp. 547–550). New York, NY: Macmillan Reference. [Google Scholar]
- Viviani P., Perani D., Grassi F., Bettinardi V., & Fazio F. (1998). Hemispheric asymmetries and bimanual asynchrony in left- and right-handers. Experimental Brain Research, 120, 531–536. [DOI] [PubMed] [Google Scholar]
- Weismer S. E., Evans J., & Hesketh L. J. (1999). An examination of verbal working memory capacity in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 42, 1249–1260. [DOI] [PubMed] [Google Scholar]
- Williams K. T. (2007). EVT-2: Expressive Vocabulary Test–Second Edition. London, United Kingdom: Pearson Assessment. [Google Scholar]
- Wing A. M., & Kristofferson A. B. (1973). Response delays and the timing of discrete motor responses. Perception & Psychophysics, 14(1), 5–12. [Google Scholar]
- Wolff P. H. (1993). Impaired Temporal Resolution in Developmental Dyslexia. Annals of the New York Academy of Sciences, 682, 87–103. [DOI] [PubMed] [Google Scholar]
- Wolff P. H., Melngailis I., Obregon M., & Bedrosian M. (1995). Family patterns of developmental dyslexia, part II: Behavioral phenotypes. American Journal of Medical Genetics, 60, 494–505. [DOI] [PubMed] [Google Scholar]
- Wolff P. H., Michel G. F., Ovrut M., & Drake C. (1990). Rate and timing precision of motor coordination in developmental dyslexia. Developmental Psychology, 26, 349. [Google Scholar]
- Zelaznik H. N., & Goffman L. (2010). Generalized motor abilities and timing behavior in children with specific language impairment. Journal of Speech, Language, and Hearing Research, 53, 383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelaznik H. N., Spencer R. M., & Ivry R. B. (2008). Behavioral analysis of human movement timing. In Grondin S. (Ed.). Psychology of time (pp. 233–260). Bingley, United Kingdom: Emerald Group. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.