Abstract
Background
Organophosphate insecticides (OPs) are used worldwide, yet despite nearly ubiquitous exposure in the general population, few have been studied outside the laboratory. Fetal brains undergo rapid growth and development, leaving them susceptible to long-term effects of neurotoxic OPs. The objective here was to investigate the extent to which prenatal exposure to OPs affects infant motor development.
Methods
30 OPs were measured in umbilical cord blood using gas chromatography tandem mass spectrometry in a cohort of Chinese infants. Motor function was assessed at 6-weeks and 9-months using Peabody Developmental Motor Scales 2nd edition (PDMS-2) (n=199). Outcomes included subtest scores: reflexes, stationary, locomotion, grasping, visual-motor integration (V-M), composite scores: gross (GM), fine (FM), total motor (TM), and standardized motor quotients: gross (GMQ), fine (FMQ), total motor (TMQ).
Results
Naled, methamidophos, trichlorfon, chlorpyrifos, and phorate were detected in ≥10% of samples. Prenatal naled and chlorpyrifos were associated with decreased 9-month motor function. Scores were 0.55, 0.85, and 0.90 points lower per 1 ng/mL increase in log-naled, for V-M (p=0.04), FM (p=0.04), and FMQ (p=0.08), respectively. For chlorpyrifos, scores were 0.50, 1.98, 0.80, 1.91, 3.49, 2.71, 6.29, 2.56, 2.04, and 2.59 points lower for exposed versus unexposed infants, for reflexes (p= 0.04), locomotion (p=0.02), grasping (p=0.05), V-M (p<0.001), GM (p=0.007), FM (p=0.002), TM (p<0.001), GMQ (p=0.01), FMQ (p=0.07), and TMQ (p=0.008), respectively. Girls appeared to be more sensitive to the negative effects of OPs on 9-month motor function than boys.
Conclusions
We found deficits in 9-month motor function in infants with prenatal exposure to naled and chlorpyrifos. Naled is being aerially sprayed to combat mosquitoes carrying Zika virus, yet this is the first non-occupational human study of its health effects. Delays in early-motor skill acquisition may be detrimental for downstream development and cognition.
Keywords: Organophosphate, Pesticide, Peabody, PDMS-2, Motor development, Neurodevelopment
1. INTRODUCTION
Synthetic pesticides are used extensively for pest management in a wide range of residential, occupational, and agricultural settings. China reports some of the highest pesticide usage rates in the world (Ding and Bao 2013; U.S.EPA 2011; Zhang et al. 2011), at up to 5 times the global average (Huang et al. 2001; Zhang et al. 2014). Organophosphate insecticides (OPs) account for more than a third of all insecticide use in China (Zhang et al. 2014). The primary route of OP exposure in the general population is via the diet, though exposure can also occur from ingestion of contaminated drinking water or dust, residential pest control applications, or topical treatments (Huang et al. 2001; NPIC 2010; U.S.CDC 2016). Additionally, warming temperatures have seen a surge in the transmission of mosquito-borne infectious diseases (Bai et al. 2013), likely leading to aerial OP spraying to combat disease spread.
OPs are neurotoxicants, and over the last couple of decades have emerged as a particular concern for developmental neurotoxicity. Developing fetal brains undergo rapid growth and maturation, leaving them susceptible to possible long-term effects of exposure (Garcia et al. 2005). Fetal susceptibility is further increased by the fact that OPs can cross the placenta (Bradman et al. 2003; Koutroulakis et al. 2014; Tzatzarakis et al. 2009). Associations have been reported between prenatal exposures to OPs and deficits in IQ (Bouchard et al. 2011; Engel et al. 2011; Rauh et al. 2011), and increases in autism spectrum (Shelton et al. 2014), attention deficit-hyperactivity (Marks et al. 2010; Rauh et al. 2006), and pervasive developmental disorder (Eskenazi et al. 2007; Rauh et al. 2006).
Despite a growing body of evidence regarding prenatal OP exposure and such neurodevelopmental endpoints, less is known about effects on early-life motor function. Motor skill acquisition in infancy provides a foundation for downstream cognitive and socio-emotional development in childhood (Clearfield 2004, 2011). Motor functions improve rapidly in infancy with increasing central nervous system maturation and serve as an early benchmark of healthy neurological development (Noritz and Murphy 2013). Delays in meeting early motor milestones may be indicative of a developmental disorder (De Felice et al. 2015; Noritz and Murphy 2013).
Epidemiological studies provide preliminary evidence that prenatal OP exposure may negatively affect infant or child motor function. Maternal urinary OP metabolites during pregnancy (total dialkyl phosphates [DAPs] (Young et al. 2005; Zhang et al. 2014), dimethylphosphates [DMPs] (Young et al. 2005), diethylphosphates [DEPs] (Engel et al. 2007; Young et al. 2005), and malathion dicarboxylic acid [MDA] (Engel et al. 2007)) have been associated with deficits in infant/newborn reflexes. Chlorpyrifos, measured directly in umbilical cord plasma, has been found to be inversely associated with psychomotor development in 3-year-olds (Rauh et al. 2006). Two studies of maternal occupational exposure to unspecified OPs during pregnancy found deficits in fine, but not gross, motor skills in infants (Handal et al. 2008) and reduced motor speed and coordination in 6- to 8-year-olds (Harari et al. 2010). With the exception of the Rauh, et al., 2006 study, which only looked at chlorpyrifos, the existing body of work is largely limited by the use of imprecise exposure assessments, such as maternal non-specific urinary metabolites or self-reported occupational exposure during pregnancy. These limited exposure assessments make it difficult to accurately define exposure and attribute observed effects to specific OPs, thus restricting the utility of the findings for regulatory considerations. Additionally, with the exception of a few well-studied OPs, such as chlorpyrifos, many of the OPs in use today have not been studied for neurodevelopmental effects in non-occupationally exposed populations. Therefore, the current study sought to investigate associations between prenatal exposure to multiple OP insecticides, measured directly in umbilical cord blood, and many of which have been understudied in humans, and gross and fine motor function in infancy.
2. METHODS
2.1 Population
Pregnant women with healthy, uncomplicated, singleton pregnancies (n=359) were recruited at 37–42 weeks gestation from Fuyang Maternal and Children’s hospital between 2008 and 2011 and enrolled into a longitudinal study of iron deficiency and infant neurodevelopment. Of the 359 participants, 237 had a sufficient volume of cord blood for pesticide analysis. Written informed consent was obtained, and the institutional review boards of the University of Michigan and Zhejiang University Children’s Hospital approved this study.
2.2 Cord blood pesticides
The protocol for the determination of pesticides in cord blood has been described elsewhere (Silver et al. 2016). Briefly, cord blood plasma samples were at analyzed the Institute of Toxicology at Nanjing Medical University using gas chromatography tandem mass spectrometry (GC-MS/MS). Methods were modified from previously published protocols (Perez et al. 2010; ThermoScientific). We analyzed for 24 organophosphate (OP) insecticides and 6 OP metabolites. Limits of detection (LODs) were determined by analyzing fortified serum on a signal-to-noise (S/N) ratio of three. Quality control samples were generated using serum samples with 0.675 and 1.35 ng/mL pesticide standards. Quality control samples and blanks were analyzed in parallel with study samples in each batch.
Individual OPs were treated as continuous when detection rates were ≥80% (values below the limit of detection [<LOD] were replaced with LOD/√2), three-level ordinal (<LOD/medium/high [median split for those above LOD]) when detection rates were 40–79%, and dichotomous (<LOD/detect) when detection rates were 10–39%. Naled (99.6% detected) was log-transformed prior to statistical analysis to account for a right-skewed distribution. Methamidophos and trichlorfon (64.6% and 51.0% detected) were converted to 3-level ordinal variables, while chlorpyrifos and phorate (36.7% and 16.9% detected) were treated as dichotomous. A “number of OP detects” variable was created by assigning OP measurements <LOD a value of 0, while detects were assigned a value of 1; these were summed to create an index of OP exposure for each infant(Wickerham et al. 2012).
2.3 Peabody Developmental Motor Scales 2nd edition (PDMS-2)
The Peabody Developmental Motor Scales (PDMS-2) (Folio and Fewell 1983, 2000) is a standardized test that assesses gross and fine motor abilities in children from birth through 5 years. PDMS-2 was administered here around 6 weeks and 9 months of age. The PDMS-2 has been proven to have excellent internal consistency (r = 0.89–0.97), test-retest reliability (r = 0.89–0.96), and inter-rater reliability (r =0.96–0.99) (Folio and Fewell 2000). For this study, PDMS-2 testing was performed by four examiners, with one serving as reference. After training, agreement between the reference and the other three examiners was 95% or higher. Inter- and intra-tester reliability measures were also monitored over the course of the study.
The gross motor function assessment is comprised of 4 multi-item subtests (reflexes, stationary, and locomotion) that measure interrelated motor abilities of large muscle systems (Folio and Fewell 1983, 2000). Gross motor subtest scores were summed to create a composite gross motor raw score (GM). The fine motor function assessment is comprised of two multi-item subtests (grasping and visual-motor integration [V-M]) that measure the development of fine muscle systems. Fine motor subtest scores were summed to create a composite fine motor raw score (FM). GM and FM were summed to create a composite total motor raw score (TM) to measure overall motor abilities. Additionally, raw subtest scores were converted to standard scores using PDMS-2 guidelines. Standard scores were then summed and converted to gross (GMQ), fine (FMQ), and total motor quotients (TMQ) according to PDMS-2 guidelines (Folio and Fewell 1983). PDMS-2 data was available for 199 infants.
2.4 Statistical analysis
Statistical analyses were conducted using SAS 9.3 (Cary, North Carolina). Percentile tables were created to determine the individual OP distributions within the sample. Descriptive statistics and frequencies were examined for all covariates of interest, including sex, age at PDMS-2 testing, cord ferritin, gestational age, birth weight, maternal education and occupation, family income, and season of PDMS-2 testing.
Linear mixed models (LMM) were used to evaluate associations between cord OP exposures and PDMS raw scores (subtest [reflexes, stationary, locomotion, grasping, V-M] and composite [GM, FM, TM]), as well as motor quotients (GMQ, FMQ, TMQ), at 6 weeks and 9 months. A number of covariates, including maternal education and occupation, income, and season in which motor testing took place, were considered, but ultimately excluded, from the final models. We previously reported that income and education were not associated with OP exposure in our sample, while season and naled and maternal occupation and methamidophos were significantly associated (Silver et al. 2016). Of these factors, only season of testing was associated with both OP exposure and PDMS-2 motor outcomes. Inclusion of season in the models did not significantly influence the results, therefore the most parsimonious model was chosen to maximize sample size. Final models were adjusted for sex, age at testing, and cord ferritin. For continuous exposures (number of OP detects, log-naled), the parameter of interest was the slope estimating change in 6-week or 9-month motor scores per 1 unit increase in OP. For categorical exposures (methamidophos, trichlorfon, chlorpyrifos, phorate), the parameter of interest was the difference in mean 6-week or 9-month motor score by category of OP exposure. We also completed a sensitivity analysis to determine if 9-month iron status was significantly affecting 9-month motor outcomes by including it in the model. Finally, to investigate differences in the effect of prenatal OP exposure on infant motor function by sex, we carried out additional LMM modeling stratified by infant sex.
3. RESULTS
All infants were born to term (≥37 weeks) and of healthy birth weight (≥2.5 kg). Pertinent sample characteristics are presented in Table 1. Additional household and parental characteristics of the study sample have been previously published (Silver et al. 2016). Infants with missing exposure or outcome data did not significantly differ from those with complete data (Table A.1). Levels of OP exposure for those with and without PDMS data are compared in Table A.2. There were no statistically significant differences in exposure between the two groups. Of the 30 OPs and OP metabolites, 18 were detectable in at least one cord blood sample. The mean (standard deviation) number of OPs detected per sample was 3.0 (1.6) and ranged from 1 to 8. Distributions of detectable OPs and their LODs are shown in Table 2.
Table 1.
Study sample characteristics
| Variable | N | Mean (SD) |
|---|---|---|
| Age (days) at 6 week testing | 231 | 43.0 (5.1) |
| Age (days) at 9 month testing | 218 | 283.3 (10.6) |
| Gestational age (weeks) | 233 | 39.6 (1.0) |
| Birth weight (kg) | 233 | 3.4 (0.4) |
| N (%) | ||
| Sex (male) | 233 | 121 (51.9) |
| Low cord ferritin (≤75 μg/L) | 218 | 42 (19.3) |
Table 2.
Distribution of OP insecticide concentrations in umbilical cord blood plasma samples (ng/mL) at delivery, Zhejiang Province, China (n=237)
| Organophosphate | LOD | N >LOD (%) | 50th | 75th | 90th | 95th | 99th | Max |
|---|---|---|---|---|---|---|---|---|
| Acephate | 0.10 | 7 (3.0) | ND | ND | ND | ND | 0.57 | 0.68 |
| Chlorpyrifos | 0.40 | 87 (36.7) | ND | 0.56 | 1.92 | 2.71 | 4.65 | 7.33 |
| Chlorpyrifos-methyl | 0.01 | 18 (7.6) | ND | ND | ND | 0.07 | 0.47 | 1.14 |
| Fensulfothion | 0.03 | 1 (0.4) | ND | ND | ND | ND | ND | 10.35 |
| Fosthiazate | 0.07 | 1 (0.4) | ND | ND | ND | ND | ND | 7.82 |
| Isofenphos-methyl | 0.13 | 2 (0.8) | ND | ND | ND | ND | ND | 14.70 |
| Methamidophos | 1.52 | 153 (64.6) | 6.11 | 28.10 | 59.60 | 113.71 | 231.96 | 496.86 |
| Methidathion | 0.07 | 1 (0.4) | ND | ND | ND | ND | ND | 9.23 |
| Mevinphos | 0.12 | 15 (6.3) | ND | ND | ND | 0.14 | 0.25 | 0.26 |
| Naled | 0.42 | 236 (99.6) | 1.51 | 4.33 | 9.17 | 12.22 | 22.13 | 29.23 |
| Omethoate | 1.35 | 19 (8.0) | ND | ND | ND | 1.83 | 4.37 | 9.34 |
| Phorate | 1.79 | 40 (16.9) | ND | ND | 3.10 | 5.14 | 7.02 | 12.82 |
| Terbufos | 0.33 | 3 (1.3) | ND | ND | ND | ND | 0.39 | 3.32 |
| Trichlorfon | 0.35 | 121 (51.0) | 0.46 | 1.69 | 3.65 | 5.52 | 10.56 | 11.30 |
| * Carbophenothion sulfone | 0.02 | 16 (6.8) | ND | ND | ND | 0.49 | 1.64 | 18.83 |
| * DEDTP | 0.06 | 2 (0.8) | ND | ND | ND | ND | ND | 0.69 |
| * DMTP | 1.35 | 1 (0.4) | ND | ND | ND | ND | ND | 9.24 |
| * TCPY | 2.32 | 1 (0.4) | ND | ND | ND | ND | ND | 13.52 |
Undetected:
Diazinon, Dicrotophos, Dimethoate, Formothion, Phosphamidon, Dimethylvinphos, Parathion-methyl, Malathion, Dichlorphos, Monocrotophos, *Phorate sulfone, *DMDTP
Denotes a metabolite
Abbreviations: ND, non-detect; DEDTP, diethyldithiophosphate; DMTP, dimethylthiophosphate, TCPY, 3,5,6-trichloro-2-pyridinol; DMDTP, dimethyldithiophosphate
There were no significant associations between any of the OPs measured and PDMS outcomes at 6 weeks (Table A.3). Adjusted LMM results for PDMS outcomes at 9 months are shown in Table 3. Log-naled was associated with deficits in fine motor function (Table 3). In adjusted analyses, 9-month raw scores were 0.55 and 0.85 points lower per 1 ng/mL increase in log-naled for V-M (p=0.04) and FM (p=0.04), respectively. 9-month FMQs were 0.90 points lower per 1 ng/mL increase in log-naled (p=0.08). High prenatal methamidophos exposure (compared to ND) was consistently associated with deficits in PDMS outcomes, though results were not statistically significant. Detectable chlorpyrifos was associated with lower scores for all PDMS subtest and composite scores at 9 months of age (Table 3). In adjusted analyses, 9-month raw scores were 0.50, 1.98, 0.80, 1.91, 3.49, 2.71, and 6.29 points lower for chlorpyrifos-exposed versus unexposed infants, for reflexes (p= 0.04), locomotion (p=0.02), grasping (p=0.05), V-M (p<0.001), GM (p=0.007), FM (p=0.002), and TM (p<0.001), respectively. 9-month motor quotients were also 2.56, 2.04, and 2.59 points lower in chlorpyrifos-exposed versus unexposed infants, for GMQ (p=0.01), FMQ (p=0.07), and TMQ (p=0.008), respectively.
Table 3.
Adjusteda change/difference in PDMS motor scores at 9 months by OP exposure
| OP Insecticide | Raw Subtest Scores | Composite Raw Scores | Motor Quotients | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Reflexes (n=182) |
Stationary (n=188) |
Locomotion (n=187) |
Grasping (n=191) |
V-M (n=192) |
GM (n=180) |
FM (n=191) |
TM (n=179) |
GMQ (n=180) |
FMQ (n=191) |
TMQ (n=179) |
|
| Continuous | β (95% CI) for OPb | ||||||||||
| # OP Detects | 0.03 (−0.10, 0.17) | 0.08 (−0.18, 0.33) | 0.04 (−0.44–0.53) | −0.08 (−0.32, 0.17) | −0.21 (−0.54, 0.13) | 0.12 (−0.64, 0.88) | −0.29 (−0.80, 0.23) | −0.14 (−1.21, 0.93) | 0.11 (−0.48, 0.69) | −0.23 (−0.89, 0.43) | −0.03 (−0.60, 0.53) |
| Log-Naled | 0.08 (−0.14, 0.30) | 0.07 (−0.33, 0.47) | 0.06 (−0.71, 0.83) | −0.30 (−0.68, 0.08) | −0.55 * (−1.07, −0.30) | 0.36 (−0.84, 1.55) | −0.85 * (−1.65, −0.06) | −0.53 (−2.20, 1.15) | 0.44 (−0.48, 1.36) | −0.90 † (−1.92, 0.12) | −0.10 (−0.99, 0.79) |
| 3-level (High/Med./ND) | Difference in least square means (95% CI)c | ||||||||||
| Methamidophos (High vs ND) | −0.12 (−0.66, 0.41) | −0.31 (−1.29, 0.67) | −1.03 (−2.91, 0.85) | −0.70 (−1.63, 0.23) | −0.55 (−1.83, 0.73) | −1.30 (−4.24, 1.65) | −1.25 (−3.21, 0.71) | −2.77 (−6.89, 1.36) | −1.32 (−3.51, 0.87) | −1.67 (−4.18, 0.85) | −0.62 (−2.90, 1.67) |
| Methamidophos (Med. vs ND) | 0.06 (−0.48, 0.59) | −0.13 (−1.10, 0.84) | 0.13 (−1.73, 1.99) | −0.13 (−1.06, 0.79) | −0.01 (−1.28, 1.26) | 0.41 (−2.53, 3.35) | −0.18 (−2.11, 1.77) | 0.46 (−3.64, 4.57) | 0.07 (−2.12, 2.25) | 0.25 (−2.25, 2.75) | −0.23 (−2.52, 2.06) |
| Trichlorfon (High vs ND) | 0.28 (−0.27, 0.83) | 0.59 (−0.40, 1.58) | 1.19 (−0.71, 3.09) | 0.08 (−0.87, 1.03) | −0.10 (−1.40, 1.21) | 2.12 (−0.88, 5.12) | −0.05 (−2.06, 1.96) | 2.39 (−1.84, 6.61) | 2.07 (−0.24, 4.38) | 0.70 (−1.87, 3.26) | 1.77 (−0.46, 4.00) |
| Trichlorfon (Med. vs ND) | 0.30 (−0.23, 0.84) | −0.17 (−1.14, 0.80) | −0.06 (−1.92, 1.81) | −0.54 (−1.46, 0.39) | 0.07 (−1.34, 1.21) | −0.28 (3.20, 2.64) | −0.60 (−2.55, 1.35) | −0.75 (−4.84, 3.33) | −0.26 (−2.53, 2.01) | −0.97 (−3.47, 1.54) | −0.50 (−2.67–1.67) |
| 2-level (Detect/ND) | Difference in least square means (95% CI)c | ||||||||||
| Chlorpyrifos (Detect vs ND) | −0.50 * (−0.96, 0.04) | −0.67 (−1.52, 0.18) | −1.98 * (−3.62, −0.35) | −0.80 † (−1.61, 0.01) | −1.91 *** (−3.01, −0.81) | −3.49 ** (−6.03, −0.95) | −2.71 ** (−4.40, −1.02) | −6.29 *** (−9.83, −2.75) | −2.56 * (−4.53, −0.59) | −2.04 † (−4.23, 0.15) | −2.59 ** (−4.49, −0.70) |
| Phorate (Detect vs ND) | 0.22 (−0.39, 0.82) | 0.70 (−0.40, 1.80) | 0.49 (−1.64, 2.61) | 0.66 (−0.40, 1.73) | −1.01 (−2.45, 0.44) | 1.28 (−2.00, 4.57) | −0.38 (−2.62, 1.86) | 1.07 (−3.59, 5.73) | 0.79 (−1.74, 3.33) | 0.51 (−2.35, 3.37) | 0.69 (−1.77, 3.16) |
Models adjusted for sex, age at testing, and cord ferritin
Estimated change in 9-month motor score per 1 unit increase in OP
Difference in mean 9-month motor score
High/Medium/ND cut-offs (ng/mL): methamidophos >18.2/1.5–18.2/ND; trichlorfon >1.7/0.4–1.7/ND; chlorpyrifos ≥0.04/ND; phorate ≥1.8/ND
Abbreviations: V-M, visual-motor integration; GM, gross motor score; FM, fine motor score; TM, total motor score; GMQ, gross motor quotient; FMQ, fine motor quotient, TMQ, total motor quotient
p<0.10,
p<0.05,
p<0.01,
p<0.001
The addition of 9-month iron status to the models did not substantially impact the results. Associations between naled and V-M, FM, and FMQ were slightly attenuated; β (p-value): −0.50 (p=0.05), −0.76 (p=0.05), and −0.87 (p=0.09), respectively. Associations between chlorpyrifos and PDMS-2 outcomes were somewhat strengthened, with the exception of V-M which was slightly attenuated; β (p-value): −0.48 (p=0.03), −0.73 (p=0.08), −2.23 (p=0.009), −0.98 (p=0.01), −1.87 (p=0.009), −3.77 (p=0.004), −2.84 (p<0.001), −6.71 (p<0.001), −3.05 (p=0.002), −2.47 (p=0.02), −3.12 (p=0.001), for reflexes, stationary, locomotion, grasping, V-M, GM, FM, TM, GMQ, FMQ, and TMQ, respectively.
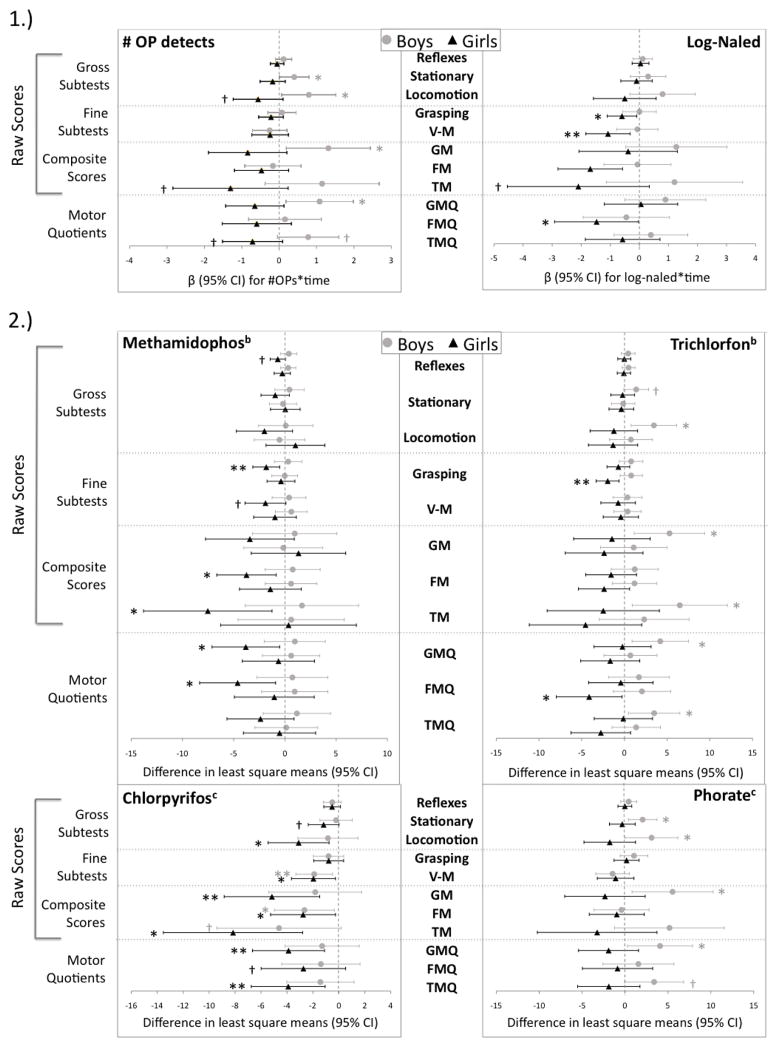
Sex-stratified LMM results are shown in Table A.4 (6 weeks) and Figure 1 (9 months). Overall, girls seemed to be more sensitive to negative effects of prenatal OP exposure on 9-month motor function than boys (Figure 1). For example, 9-month V-M scores were 1.69 points lower per 1 ng/mL increase in log-naled for girls (p=0.04) compared to only 0.06 points lower for boys (p=0.91). For girls, estimated changes in V-M, FM, FMQ, and TMQ at 9 months were negative for all OPs examined. Chlorpyrifos consistently yielded deficits in 9-month motor scores, regardless of sex, though results were stronger in girls for many PDMS outcomes (stationary, locomotion, GM, GMQ, FMQ, TMQ). 9-month raw V-M, FM, and TM, results were statistically significant for both sexes. Scores were 1.96 (p=0.02) and 1.90 (p=0.009) points lower for V-M, 2.76 (p=0.03) and 2.66 (p=0.03) points lower for FM, and 8.16 (p=0.003) and 4.61 (p=0.06) points lower for TM, for chlorpyrifos-exposed girls and boys, respectively, compared to unexposed infants of the same sex.
Figure 1.
Sex-stratified change/difference (95%) in PDMS motor scores at 9 months by OP exposurea
1.) Estimated change in 9-month motor score per 1 unit increase in OP exposure
2.) Difference in mean 9-month motor score by category of OP exposure
aModels adjusted for age at testing and cord ferritin
bCategories of OP exposure: high versus ND and medium versus ND
cCategories of OP exposure: exposed versus ND
High/Medium/ND cut-offs (ng/mL): methamidophos >18.2/1.5–18.2/ND; trichlorfon >1.7/0.4–1.7/ND; chlorpyrifos ≥0.04/ND; phorate ≥1.8/ND
†p<0.10, *p<0.05, **p<0.01
4. DISCUSSION
Prenatal naled and chlorpyrifos exposure was associated with decreased motor function in 9-month-old infants. For naled, negative effects were observed for fine motor outcomes, while chlorpyrifos was associated with deficits in both gross and fine motor function. Girls appeared to be more sensitive to the effects of prenatal OP exposure than boys. No significant findings were observed at 6-weeks. While PDMS-2 is indicated for use in children from birth to five years, test validity and reliability to tend to be low in infants less than 6 months of age, possibly contributing to the lack of findings at the early time point (Folio and Fewell 2000; Snider et al. 2009).
Of the OPs measured in this study, 7 have been previously reported in cord blood in U.S. or Chinese cohorts. A comparison of the high ends of the exposure distributions across these studies has been published elsewhere (Silver et al. 2016). For the current study, the 90th percentile concentration for chlorpyrifos (1.92 ng/mL) is several orders of magnitude higher than the maximums reported in the U.S. (0.002–0.065 ng/mL) (Neta et al. 2010; Whyatt et al. 2003; Yan et al. 2009; Young et al. 2005). Cord blood naled has not previously been reported.
Chlorpyrifos is employed widely in agricultural, land management, industrial, and vector control settings (NPIC 2010). However, concerns of developmental neurotoxicity have led more governments to restrict its use over the past couple of decades (U.S.EPA 2015). While chlorpyrifos has been highly studied as a neurotoxicant, relatively little has been published about naled. Naled’s primary use is controlling adult mosquito populations (U.S.EPA 2016). It has been employed for routine mosquito control in the U.S. and following hurricanes and floods (U.S.EPA 2016) and is currently being sprayed aerially in southern Florida (U.S.) as part of a campaign to combat the spread of Zika virus (Frieden et al. 2016). Both chlorpyrifos and naled are able to cross the placenta (Abdel-Rahman et al. 2002; EXTOXNET 1993).
The findings from the current study are consistent with previous literature. Similar to a study that found deficits in infant fine motor function following maternal occupational exposure to unspecified OPs during pregnancy (Handal et al. 2008), we observed deficits in raw FM at 9 months following prenatal exposure to all of the OPs analyzed in our study, with statistically significant results for naled and chlorpyrifos. Naled and chlorpyrifos also contributed to significant deficits in raw V-M, a fine motor subtest, as well as marginally significant deficits in FMQ. The only previous study to also use cord blood to assign exposure found associations between chlorpyrifos and psychomotor development in 3-year-olds (Rauh et al. 2006). We observed deficits in most 9-month PDMS measures following prenatal chlorpyrifos exposure, even with our relatively modest sample size. Two previous studies reported associations between maternal total urinary DEPs during pregnancy and infant/newborn reflexes (Engel et al. 2007; Young et al. 2005); 2 of the 3 DEPs used for the total DEP measurement (Engel et al. 2007; Young et al. 2005) are non-specific metabolites of chlorpyrifos (Bradman et al. 2011). We similarly observed significant deficits in 9-month reflexes in infants with prenatal exposure to chlorpyrifos.
While nearly all studies of prenatal OP exposure and motor-related functions control for sex in their analyses, few report sex-specific results (Eskenazi et al. 2007; Rauh et al. 2006; Zhang et al. 2014). The effect of prenatal OP exposure on psychomotor development did not differ by sex in two studies (Eskenazi et al. 2007; Rauh et al. 2006). However, a study that found inverse associations between reflexes and maternal urinary total DAPs during pregnancy reported that associations were slightly stronger for girls (Zhang et al. 2014). Interestingly, when total DMPs and total DEPs were examined separately, the authors found that DMPs were significantly associated with deficits in reflexes in girls, while DEPs were significantly associated with deficits in reflexes in boys (Zhang et al. 2014). We observed stronger negative effects of prenatal OP exposure on 9-month motor function in girls for nearly all of the motor outcomes examined in this study.
The mechanism of acute toxicity elicited by high exposures to OPs is well understood. OPs inhibit acetylcholinesterase (AChE), the enzyme responsible for terminating the neurotransmitter acetylcholine’s activity. Without functional AChE, acetylcholine builds up in the synapse, leading to hyperstimulation of the cholinergic receptors at neuronal and neuromuscular junctions (Abdollahi and Karami-Mohajeri 2012; Eddleston et al. 2008; Kamanyire and Karalliedde 2004). Cholinergic toxicity, as a result of acute chlorpyrifos poisoning, can result in motor dysfunction such as incoordination, loss of reflexes, muscle twitching, tremors, and paralysis (Kamanyire and Karalliedde 2004).
However, low dose exposure levels, typical of those seen in non-occupational settings like this study, do not usually elicit cholinergic toxicity or acetylcholinesterase inhibition, yet effects on motor function are still observed. Low-dose prenatal or neonatal chlorpyrifos administered to laboratory rodents has been associated with deficits in motor-related outcomes. One study reported significant effects on neonatal motor patterns and delayed motor maturation in mice, following prenatal exposure to chlorpyrifos (De Felice et al. 2015). Another similarly found motor abnormalities in developing rats following neonatal chlorpyrifos exposure (Dam et al. 2000). Interestingly, these findings were sex-specific. Deficits in coordination were observed almost immediately following chlorpyrifos administration in female rats only, while a delayed effect on locomotion was seen in male rats only. Both of these effects were largely independent of cholinesterase inhibition (Dam et al. 2000). Laboratory animal data concerning the neuromotor effects of naled are scarce. California EPA government reports note that naled at high exposure at levels can elicit gait alterations, tremors, reduced grasp, and hypoactivity in adult rats of both sexes (CalEPA 2004), while an additional study reported sporadic tremors in high-dose female, but not male, rats (ACGIH 2013). We were unable to find any published toxicology studies of gestational or neonatal naled exposure and motor-related outcomes in animal models.
The mechanisms by which OPs elicit these low-dose effects on motor function are unclear. An in-depth examination is beyond the scope of this discussion, but rodent models have yielded some plausible mechanisms that deserve mention. Briefly, low-dose prenatal chlorpyrifos has been found to have long-lasting effects on monoaminergic pathways of the brain (Aldridge et al. 2004; Torres-Altoro et al. 2011). Specifically, it has been shown to perturb the development of serotonin (5HT) receptor circuits in the developing rat brain, leading to dysfunction of 5HT systems and behavioral abnormalities (Slotkin and Seidler 2007a). Chlorpyrifos has also been found to induce aberrant striatal dopamine neurotransmission, possibly via the dysregulation of cyclin-dependent kinase 5 (Cdk5) activity (Torres-Altoro et al. 2011). Monoaminergic pathways play an important role in the maturation of spinal locomotor networks (De Felice et al. 2015) and in the mediation of motor control (Philibin et al. 2011). Disruption of the timing of their development, as result of prenatal OP exposure, could have potentially negative consequences on early-life motor function. An alternative hypothesis is that OPs may affect motor function via the disruption of glial cell development and function in the brain (Garcia et al. 2001; Garcia et al. 2002; Garcia et al. 2003; Zurich et al. 2004). Rodent studies have shown that low-level chlopyrifos and diazinon exposure during the onset of myelination elicits deficits in expression of genes involved in oligodendrocyte function and myelination processes (Garcia et al. 2001; Garcia et al. 2002; Garcia et al. 2003; Slotkin and Seidler 2007b). Gains in motor function and mobility during infancy correspond to increases in corticospinal tract myelination, a process that begins in late pregnancy (Carlson 2014; Dambska and Wisniewski 1999). Therefore, prenatal OP exposure during the onset of corticospinal tract myelination has the potential to disrupt motor-related outcomes in infancy.
Our study is limited in several ways. The OPs measured here were non-persistent. Having measures of exposure at only a single time point (birth) limited our ability to address the temporal variability of OP exposure during pregnancy and infancy; thus, we may have missed some exposure at sensitive developmental stages (Eskenazi et al. 2007). Since we measured a large number of pesticides with widely varying properties, our methods were not optimized solely for OP detection (Silver et al. 2016). This likely resulted in higher detection limits and greater numbers of non-detects for some OPs, compared to a more targeted approach. In general, OP levels in blood tend to be low anyway, due to short half-lives (<48 hours), which also likely contributed to large number of non-detects (Barr et al. 1999). This necessitated the use of crude exposure categories (<LOD/detect or <LOD/medium/high) for many of the OPs examined and limited the scope of our statistical analyses. We did not have information about parental interaction or play with the infants, while it may not directly confound the relationship between OP exposure and motor function, it is almost certainly associated with the outcome and could have added precision to the estimates. Finally, the findings here may not be generalizable to infants born in other parts of China or elsewhere around the world, especially considering that all the infants included in this study were carried to term and otherwise healthy. Low birth weight or pre-term infants are more likely to have delayed or impaired motor development (Snider et al. 2009) and may potentially be more susceptible to effects of prenatal pesticide exposures. The effects of prenatal OPs on infant motor function should be assessed in these vulnerable populations.
Despite its limitations, this study has a number of strengths. It used specific measurements of OP parent compounds in umbilical cord blood to assign prenatal exposure, rather than non-specific metabolites in maternal urine, thus providing direct evidence of fetal exposure (Barr et al. 1999; Munoz-Quezada et al. 2013). OP levels in cord blood may also be more likely to reflect the available dose, since the measured OPs have not yet been eliminated from the infant’s body (Needham et al. 1995). Of the previously published studies of prenatal OPs and motor function, only one directly measured concentrations of the parent pesticide in cord blood to assign prenatal exposure (Rauh et al. 2006); other studies used non-specific urinary metabolites during pregnancy (Engel et al. 2007; Engel et al. 2011; Eskenazi et al. 2007; Eskenazi et al. 2010; Young et al. 2005; Zhang et al. 2014) or maternal occupation (Handal et al. 2008; Harari et al. 2010) to assign exposure. This study also examined a large number of OPs (18 detected out of 30 analyzed), many of which have not been previously investigated for neurodevelopmental effects in infants. For example, to our knowledge, this is the first non-occupational human study of the health effects of naled. Additionally, we assessed motor development at two time points (6 weeks and 9 months), using the PDMS-2, which is sensitive to changes in both gross and fine motor function. The PDMS-2, which has been called a “gold standard” for predicting motor development (Liao et al. 2012), offers an advantage over the motor assessments used in previous studies, which have largely used the motor portions of larger tests of overall development such as the Bayley (Engel et al. 2011; Eskenazi et al. 2007; Eskenazi et al. 2010; Rauh et al. 2006) or Brazleton (Engel et al. 2007; Young et al. 2005). Therefore, the longitudinal design, as well as the use of the PDMS-2, gives a more comprehensive view of overall motor function in infancy than previous studies.
5. CONCLUSIONS
Prenatal naled and chlorpyrifos exposure was significantly associated with decreased motor function in Chinese infants. The clinical significance of these small, yet significant, deficits in infant motor development are unknown. Both chlorpyrifos and naled are used around the world. These results warrant further exploration of the effects of commonly used OPs on motor development. Proper motor skill acquisition in infancy is essential to downstream neurodevelopment and cognitive processes.
HIGHLIGHTS.
Chinese infants prenatally exposed to organophosphate insecticides were tested for motor function at 6-weeks and 9-months
Prenatal naled and chlorpyrifos exposure was associated with statistically significant deficits in 9-month motor outcomes.
Prenatal organophosphate exposure may affect infant motor skill development.
Acknowledgments
The authors are grateful to the families who participated in the study and the doctors and nurses at Zhejiang University Children’s Hospital for their dedication and assistance with the project.
FUNDING
This study was supported by R01ES021465 from the National Institute of Environmental Health Sciences (NIEHS), P01HD39386 from the National Institute of Child Health and Development (NICHD), and 81273085 from the National Natural Science Foundation of China (NNSFC).
ABBREVIATIONS
- OP
organophosphate
- PDMS-2
Peabody developmental motor scales- 2nd edition
- V-M
visual-motor integration subtest
- GM
gross motor score
- FM
fine motor score
- TM
total motor score
- GMQ
gross motor quotient
- FMQ
fine motor quotient
- TMQ
total motor quotient
Footnotes
CONFLICT OF INTEREST
All authors declare they have no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Rahman AA, Blumenthal GM, Abou-Donia SA, Ali FA, Abdel-Monem AE, Abou-Donia MB. Pharmacokinetic profile and placental transfer of a single intravenous injection of [(14)c]chlorpyrifos in pregnant rats. Arch Toxicol. 2002;76:452–459. doi: 10.1007/s00204-002-0366-2. [DOI] [PubMed] [Google Scholar]
- Abdollahi M, Karami-Mohajeri S. A comprehensive review on experimental and clinical findings in intermediate syndrome caused by organophosphate poisoning. Toxicol Appl Pharmacol. 2012;258:309–314. doi: 10.1016/j.taap.2011.11.014. [DOI] [PubMed] [Google Scholar]
- Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: Critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Conference of Governmental Industrial Hygienists. Documentation of the tlvs and beis with other world wide occupational exposure values. Cincinnati, Ohio: American Conference of Governmental Industrial Hygienists; 2013. [Google Scholar]
- Bai L, Morton LC, Liu Q. Climate change and mosquito-borne diseases in china: A review. Global Health. 2013;9:10. doi: 10.1186/1744-8603-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Barr JR, Driskell WJ, Hill RH, Jr, Ashley DL, Needham LL, et al. Strategies for biological monitoring of exposure for contemporary-use pesticides. Toxicol Ind Health. 1999;15:168–179. doi: 10.1191/074823399678846556. [DOI] [PubMed] [Google Scholar]
- Bouchard MF, Chevrier J, Harley KG, Kogut K, Vedar M, Calderon N, et al. Prenatal exposure to organophosphate pesticides and iq in 7-year-old children. Environ Health Perspect. 2011;119:1189–1195. doi: 10.1289/ehp.1003185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Barr DB, Claus Henn BG, Drumheller T, Curry C, Eskenazi B. Measurement of pesticides and other toxicants in amniotic fluid as a potential biomarker of prenatal exposure: A validation study. Environ Health Perspect. 2003;111:1779–1782. doi: 10.1289/ehp.6259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradman A, Castorina R, Barr DB, Chevrier J, Harnly ME, Eisen EA, et al. Determinants of organophosphorus pesticide urinary metabolite levels in young children living in an agricultural community. Int J Environ Res Public Health. 2011;8:1061–1083. doi: 10.3390/ijerph8041061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- California Environmental Protection Agency. Summary of toxicology data for naled, chemical code no.418. 2004. [Google Scholar]
- Carlson BM. Human embryology and developmental biology. 5. Elsevier; 2014. [Google Scholar]
- Clearfield MW. The role of crawling and walking experience in infant spatial memory. J Exp Child Psychol. 2004;89:214–241. doi: 10.1016/j.jecp.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Clearfield MW. Learning to walk changes infants’ social interactions. Infant Behav Dev. 2011;34:15–25. doi: 10.1016/j.infbeh.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Dam K, Seidler FJ, Slotkin TA. Chlorpyrifos exposure during a critical neonatal period elicits gender-selective deficits in the development of coordination skills and locomotor activity. Brain Res Dev Brain Res. 2000;121:179–187. doi: 10.1016/s0165-3806(00)00044-4. [DOI] [PubMed] [Google Scholar]
- Dambska M, Wisniewski KE. Normal and pathologic development of the human brain and spinal cord. London, England: John LIbby & Co. Ltd; 1999. [Google Scholar]
- De Felice A, Scattoni ML, Ricceri L, Calamandrei G. Prenatal exposure to a common organophosphate insecticide delays motor development in a mouse model of idiopathic autism. PLoS One. 2015;10:e0121663. doi: 10.1371/journal.pone.0121663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding G, Bao Y. Revisiting pesticide exposure and children’s health: Focus on china. Sci Total Environ. 2013;472C:289–295. doi: 10.1016/j.scitotenv.2013.11.067. [DOI] [PubMed] [Google Scholar]
- Eddleston M, Buckley NA, Eyer P, Dawson AH. Management of acute organophosphorus pesticide poisoning. Lancet. 2008;371:597–607. doi: 10.1016/S0140-6736(07)61202-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel SM, Berkowitz GS, Barr DB, Teitelbaum SL, Siskind J, Meisel SJ, et al. Prenatal organophosphate metabolite and organochlorine levels and performance on the brazelton neonatal behavioral assessment scale in a multiethnic pregnancy cohort. Am J Epidemiol. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Engel SM, Wetmur J, Chen J, Zhu C, Barr DB, Canfield RL, et al. Prenatal exposure to organophosphates, paraoxonase 1, and cognitive development in childhood. Environ Health Perspect. 2011;119:1182–1188. doi: 10.1289/ehp.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and neurodevelopment in young mexican-american children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Huen K, Marks A, Harley KG, Bradman A, Barr DB, et al. Pon1 and neurodevelopment in children from the chamacos study exposed to organophosphate pesticides in utero. Environ Health Perspect. 2010;118:1775–1781. doi: 10.1289/ehp.1002234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extension Toxicology Network. [accessed May 16 2017];Naled pesticide information profile. 1993 Available: http://pmep.cce.cornell.edu/profiles/extoxnet/metiram-propoxur/naled-ext.html.
- Folio MR, Fewell RR. Peabody developmental motor scales and actvity cards. Chicago, IL: Riverside; 1983. [Google Scholar]
- Folio MR, Fewell RR. Peabody developmental motor scales examiner’s manual. 2. Austin, TX: Pro-Ed; 2000. [Google Scholar]
- Frieden TR, Schuchat A, Petersen LR. Zika virus 6 months later. JAMA. 2016;316:1443–1444. doi: 10.1001/jama.2016.11941. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Crumpton TL, Slotkin TA. Does the developmental neurotoxicity of chlorpyrifos involve glial targets? Macromolecule synthesis, adenylyl cyclase signaling, nuclear transcription factors, and formation of reactive oxygen in c6 glioma cells. Brain Res. 2001;891:54–68. doi: 10.1016/s0006-8993(00)03189-9. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Qiao D, Slotkin TA. Chlorpyrifos targets developing glia: Effects on glial fibrillary acidic protein. Brain Res Dev Brain Res. 2002;133:151–161. doi: 10.1016/s0165-3806(02)00283-3. [DOI] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity elicited by prenatal or postnatal chlorpyrifos exposure: Effects on neurospecific proteins indicate changing vulnerabilities. Environ Health Perspect. 2003;111:297–303. doi: 10.1289/ehp.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SJ, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: Targeting glial cells. Environ Toxicol Pharmacol. 2005;19:455–461. doi: 10.1016/j.etap.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Handal AJ, Harlow SD, Breilh J, Lozoff B. Occupational exposure to pesticides during pregnancy and neurobehavioral development of infants and toddlers. Epidemiology. 2008;19:851–859. doi: 10.1097/EDE.0b013e318187cc5d. [DOI] [PubMed] [Google Scholar]
- Harari R, Julvez J, Murata K, Barr D, Bellinger DC, Debes F, et al. Neurobehavioral deficits and increased blood pressure in school-age children prenatally exposed to pesticides. Environ Health Perspect. 2010;118:890–896. doi: 10.1289/ehp.0901582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Qiao F, Zhang L, Rozelle S. Farm pesticide, rice production, and human health. Singapore: 2001. [Google Scholar]
- Kamanyire R, Karalliedde L. Organophosphate toxicity and occupational exposure. Occup Med (Lond) 2004;54:69–75. doi: 10.1093/occmed/kqh018. [DOI] [PubMed] [Google Scholar]
- Koutroulakis D, Sifakis S, Tzatzarakis MN, Alegakis AK, Theodoropoulou E, Kavvalakis MP, et al. Dialkyl phosphates in amniotic fluid as a biomarker of fetal exposure to organophosphates in crete, greece; association with fetal growth. Reprod Toxicol. 2014;46:98–105. doi: 10.1016/j.reprotox.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Liao W, Wen EY, Li C, Chang Q, Lv KL, Yang W, et al. Predicting neurodevelopmental outcomes for at-risk infants: Reliability and predictive validity using a chinese version of the infanib at 3, 7 and 10 months. BMC Pediatr. 2012;12:72. doi: 10.1186/1471-2431-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, Kogut K, Barr DB, Johnson C, et al. Organophosphate pesticide exposure and attention in young mexican-american children: The chamacos study. Environ Health Perspect. 2010;118:1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Quezada MT, Lucero BA, Barr DB, Steenland K, Levy K, Ryan PB, et al. Neurodevelopmental effects in children associated with exposure to organophosphate pesticides: A systematic review. Neurotoxicology. 2013;39:158–168. doi: 10.1016/j.neuro.2013.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Pesticide Information Center. [accessed May 16 2017];Chlorpyrifos general fact sheet. 2010 Available: www.npic.orst.edu/factsheets/chlorpgen.html.
- Needham LL, Ashley DL, Patterson DG., Jr Case studies of the use of biomarkers to assess exposures. Toxicol Lett. 1995;82–83:373–378. doi: 10.1016/0378-4274(95)03568-0. [DOI] [PubMed] [Google Scholar]
- Neta G, Goldman LR, Barr D, Sjodin A, Apelberg BJ, Witter FR, et al. Distribution and determinants of pesticide mixtures in cord serum using principal component analysis. Environ Sci Technol. 2010;44:5641–5648. doi: 10.1021/es1009778. [DOI] [PubMed] [Google Scholar]
- Noritz GH, Murphy NA. Motor delays: Early identification and evaluation. Pediatrics. 2013;131:e2016–2027. doi: 10.1542/peds.2013-1056. [DOI] [PubMed] [Google Scholar]
- Perez JJ, Williams MK, Weerasekera G, Smith K, Whyatt RM, Needham LL, et al. Measurement of pyrethroid, organophosphorus, and carbamate insecticides in human plasma using isotope dilution gas chromatography-high resolution mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:2554–2562. doi: 10.1016/j.jchromb.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philibin SD, Hernandez A, Self DW, Bibb JA. Striatal signal transduction and drug addiction. Front Neuroanat. 2011;5:60. doi: 10.3389/fnana.2011.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh V, Arunajadai S, Horton M, Perera F, Hoepner L, Barr DB, et al. Seven-year neurodevelopmental scores and prenatal exposure to chlorpyrifos, a common agricultural pesticide. Environ Health Perspect. 2011;119:1196–1201. doi: 10.1289/ehp.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, Andrews HF, Hoepner L, Barr DB, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:e1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton JF, Geraghty EM, Tancredi DJ, Delwiche LD, Schmidt RJ, Ritz B, et al. Neurodevelopmental disorders and prenatal residential proximity to agricultural pesticides: The charge study. Environ Health Perspect. 2014;122:1103–1109. doi: 10.1289/ehp.1307044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver MK, Shao J, Chen M, Xia Y, Lozoff B, Meeker JD. Distribution and predictors of pesticides in the umbilical cord blood of chinese newborns. Int J Environ Res Public Health. 2016:13. doi: 10.3390/ijerph13010094. [DOI] [PMC free article] [PubMed]
- Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: Critical periods for regional and sex-selective effects. Reprod Toxicol. 2007a;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Comparative developmental neurotoxicity of organophosphates in vivo: Transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Brain Res Bull. 2007b;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider L, Majnemer A, Mazer B, Campbell S, Bos AF. Prediction of motor and functional outcomes in infants born preterm assessed at term. Pediatr Phys Ther. 2009;21:2–11. doi: 10.1097/PEP.0b013e3181957bdc. [DOI] [PubMed] [Google Scholar]
- ThermoScientific. [accessed May 16, 2017];Rapid analysis of pesticides in difficult matrices using gc/ms/ms. Available: http://www.analiticaweb.com.br/newsletter/09/51880_RapidAnalysisPesticides.pdf.
- Torres-Altoro MI, Mathur BN, Drerup JM, Thomas R, Lovinger DM, O’Callaghan JP, et al. Organophosphates dysregulate dopamine signaling, glutamatergic neurotransmission, and induce neuronal injury markers in striatum. J Neurochem. 2011;119:303–313. doi: 10.1111/j.1471-4159.2011.07428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzatzarakis M, Koutroulakis D, Sifakis S, Kavalakis M, Tutudaki M, Mantas N, et al. Monitoring of the non-specific metabolites of organophosphate pesticide in amniotic fluid of pregnant women in the region of crete. Toxicol Lett. 2009;189:S156. [Google Scholar]
- U.S. Centers for Disease Control and Prevention. [accessed May 16 2017];Parasites- lice- head lice- treatment. 2016 Available: www.cdc.gov/parasites/lice/head/treatment.html.
- U.S. Environmental Protection Agency. [accessed May 16, 2017];Pesticide industry sales and usage: 2006 and 2007 market estimates. 2011 Available: http://www.panna.org/sites/default/files/EPA%20market_estimates2007.pdf.
- U.S. Environmental Protection Agency. [accessed May 16 2017];Epa revised chlorpyrifos assessment shows risk to workers. 2015 Available: https://www.epa.gov/newsreleases/epa-revised-chlorpyrifos-assessment-shows-risk-workers.
- U.S. Environmental Protection Agency. [accessed May 16 2017];Naled for mosquito control. 2016 Available: https://www.epa.gov/mosquitocontrol/naled-mosquito-control.
- Whyatt RM, Barr DB, Camann DE, Kinney PL, Barr JR, Andrews HF, et al. Contemporary-use pesticides in personal air samples during pregnancy and blood samples at delivery among urban minority mothers and newborns. Environ Health Perspect. 2003;111:749–756. doi: 10.1289/ehp.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerham EL, Lozoff B, Shao J, Kaciroti N, Xia Y, Meeker JD. Reduced birth weight in relation to pesticide mixtures detected in cord blood of full-term infants. Environ Int. 2012;47:80–85. doi: 10.1016/j.envint.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X, Lashley S, Smulian JC, Ananth CV, Barr DB, Ledoux TA, et al. Pesticide concentrations in matrices collected in the perinatal period in a population of pregnant women and newborns in new jersey, USA. Human and Ecological Risk Assessment: An International Journal. 2009;15:948–967. [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, et al. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26:199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Zhang W, Jiang F, JFO Global pesticide consumption and pollution: With china as a focus. Proceedings of the International Academy of Ecology and Environmental Sciences. 2011;1:125–144. [Google Scholar]
- Zhang Y, Han S, Liang D, Shi X, Wang F, Liu W, et al. Prenatal exposure to organophosphate pesticides and neurobehavioral development of neonates: A birth cohort study in shenyang, china. PLoS One. 2014;9:e88491. doi: 10.1371/journal.pone.0088491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurich MG, Honegger P, Schilter B, Costa LG, Monnet-Tschudi F. Involvement of glial cells in the neurotoxicity of parathion and chlorpyrifos. Toxicol Appl Pharmacol. 2004;201:97–104. doi: 10.1016/j.taap.2004.05.003. [DOI] [PubMed] [Google Scholar]