Abstract
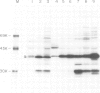
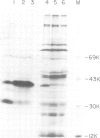
Biosynthesis of RAS1 and RAS2 proteins of Saccharomyces cerevisiae involves processing, fatty acid acylation and transport to plasma membranes. We now report the isolation of a mutant, termed dpr1, defective in these biosynthetic events. The dpr1 cells are temperature sensitive for growth and display sterile phenotype specific to a cells. The following observations were made using cells overproducing the RAS2 protein. (i) In the dpr1 cells, the RAS2 proteins remain as precursors and accumulate in the cytoplasm. (ii) The level of the RAS2 proteins in the plasma membrane of the dpr1 cells is much lower than that in the plasma membrane of wild-type cells. (iii) Fatty acid acylation appears to take place in the dpr1 cells. These results suggest that the major effect of the dpr1 mutation is in the processing of the precursor proteins, but not in their fatty acid acylation. Mutants such as dpr1 should be invaluable for further elucidation of the mechanisms of biosynthesis and transport of the RAS proteins, and presumably also a factor.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Fujiyama A., Tamanoi F. Processing and fatty acid acylation of RAS1 and RAS2 proteins in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1266–1270. doi: 10.1073/pnas.83.5.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs J. B., Sigal I. S., Poe M., Scolnick E. M. Intrinsic GTPase activity distinguishes normal and oncogenic ras p21 molecules. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5704–5708. doi: 10.1073/pnas.81.18.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Covalent binding of lipid to protein. Diglyceride and amide-linked fatty acid at the N-terminal end of the murein-lipoprotein of the Escherichia coli outer membrane. Eur J Biochem. 1973 Apr;34(2):284–296. doi: 10.1111/j.1432-1033.1973.tb02757.x. [DOI] [PubMed] [Google Scholar]
- Jensen R., Sprague G. F., Jr, Herskowitz I. Regulation of yeast mating-type interconversion: feedback control of HO gene expression by the mating-type locus. Proc Natl Acad Sci U S A. 1983 May;80(10):3035–3039. doi: 10.1073/pnas.80.10.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegren G., Hwang Y. L., Oshima Y., Lindegren C. C. Genetical mutants induced by ethyl methanesulfonate in Saccharomyces. Can J Genet Cytol. 1965 Sep;7(3):491–499. doi: 10.1139/g65-064. [DOI] [PubMed] [Google Scholar]
- McGrath J. P., Capon D. J., Goeddel D. V., Levinson A. D. Comparative biochemical properties of normal and activated human ras p21 protein. Nature. 1984 Aug 23;310(5979):644–649. doi: 10.1038/310644a0. [DOI] [PubMed] [Google Scholar]
- Sakagami Y., Yoshida M., Isogai A., Suzuki A. Peptidal Sex Hormones Inducing Conjugation Tube Formation in Compatible Mating-Type Cells of Tremella mesenterica. Science. 1981 Jun 26;212(4502):1525–1527. doi: 10.1126/science.212.4502.1525. [DOI] [PubMed] [Google Scholar]
- Scolnick E. M., Papageorge A. G., Shih T. Y. Guanine nucleotide-binding activity as an assay for src protein of rat-derived murine sarcoma viruses. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5355–5359. doi: 10.1073/pnas.76.10.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sefton B. M., Trowbridge I. S., Cooper J. A., Scolnick E. M. The transforming proteins of Rous sarcoma virus, Harvey sarcoma virus and Abelson virus contain tightly bound lipid. Cell. 1982 Dec;31(2 Pt 1):465–474. doi: 10.1016/0092-8674(82)90139-8. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Weeks M. O., Gruss P., Dhar R., Oroszlan S., Scolnick E. M. Identification of a precursor in the biosynthesis of the p21 transforming protein of harvey murine sarcoma virus. J Virol. 1982 Apr;42(1):253–261. doi: 10.1128/jvi.42.1.253-261.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet R. W., Yokoyama S., Kamata T., Feramisco J. R., Rosenberg M., Gross M. The product of ras is a GTPase and the T24 oncogenic mutant is deficient in this activity. Nature. 1984 Sep 20;311(5983):273–275. doi: 10.1038/311273a0. [DOI] [PubMed] [Google Scholar]
- Tamanoi F., Walsh M., Kataoka T., Wigler M. A product of yeast RAS2 gene is a guanine nucleotide binding protein. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6924–6928. doi: 10.1073/pnas.81.22.6924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temeles G. L., DeFeo-Jones D., Tatchell K., Ellinger M. S., Scolnick E. M. Expression and characterization of ras mRNAs from Saccharomyces cerevisiae. Mol Cell Biol. 1984 Nov;4(11):2298–2305. doi: 10.1128/mcb.4.11.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Towler D., Glaser L. Protein fatty acid acylation: enzymatic synthesis of an N-myristoylglycyl peptide. Proc Natl Acad Sci U S A. 1986 May;83(9):2812–2816. doi: 10.1073/pnas.83.9.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks M. O., Hager G. L., Lowe R., Scolnick E. M. Development and analysis of a transformation-defective mutant of Harvey murine sarcoma tk virus and its gene product. J Virol. 1985 May;54(2):586–597. doi: 10.1128/jvi.54.2.586-597.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D., Schlesinger M. J. Fatty acid-acylated proteins in secretory mutants of Saccharomyces cerevisiae. Mol Cell Biol. 1984 Apr;4(4):688–694. doi: 10.1128/mcb.4.4.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I., Shih T. Y., Scolnick E. M. Localization of the src gene product of the Harvey strain of MSV to plasma membrane of transformed cells by electron microscopic immunocytochemistry. Cell. 1980 Apr;19(4):1005–1014. doi: 10.1016/0092-8674(80)90091-4. [DOI] [PubMed] [Google Scholar]
- Willumsen B. M., Christensen A., Hubbert N. L., Papageorge A. G., Lowy D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature. 1984 Aug 16;310(5978):583–586. doi: 10.1038/310583a0. [DOI] [PubMed] [Google Scholar]