Abstract
Background
Psoriasis, a chronic inflammatory disease associated with an accelerated risk of MI, provides an ideal human model to study inflammatory atherogenesis in vivo. We hypothesized that the increased cardiovascular risk observed in psoriasis would be partially attributable to an elevated subclinical coronary artery disease (CAD) burden composed of non-calcified plaques with high-risk features. However, inadequate efforts have been made to directly measure CAD in this vulnerable population. As such, we sought to compare total (TB) and non-calcified (NCB) coronary plaque burden, and high-risk plaque (HRP) prevalence, between psoriasis patients (n=105), hyperlipidemic patients eligible for statin therapy under NCEP-ATP III guidelines (n=100) who were ~10 years older, and non-psoriasis healthy volunteers (HV) (n=25).
Methods
Patients underwent coronary computed-tomography angiography (CCTA) for TB and NCB quantification, and HRP identification, defined as low-attenuation (<30 HU), positive remodeling (>1.10), and spotty calcification. A consecutive sample of the first 50 psoriasis patients were scanned again at 1 year following therapy.
Results
Despite being younger and at lower traditional risk than hyperlipidemic patients, psoriasis patients had increased NCB (mean±S.D.:1.18±0.33 vs 1.11±0.32, p=0.02), and similar HRP prevalence (p=0.58). Furthermore, compared to HV, psoriasis patients had increased TB (1.22±0.31 vs 1.04±0.22, p=0.001), NCB (1.18±0.33 vs 1.03±0.21, p=0.004), and HRP prevalence beyond traditional risk (OR=6.0, 95% CI: 1.1–31.7; p=0.03). Finally, amongst psoriasis patients followed for 1-year, improvement in psoriasis severity associated with improvement in TB (β=0.45, 0.23–0.67; p<0.001) and NCB (β=0.53, 0.32–0.74; p<0.001) beyond traditional risk factors.
Conclusions
Psoriasis patients had greater NCB and increased HRP prevalence than HV. Additionally, psoriasis patients had elevated NCB and equivalent HRP prevalence as older, hyperlipidemic patients. Finally, modulation of target organ inflammation (eg. skin) associated with an improvement in NCB at 1 year, suggesting that control of remote sites of inflammation may translate into reduced CAD risk.
Keywords: high-risk plaque, non-calcified burden, coronary computed tomography angiography, psoriasis, inflammation
INTRODUCTION
Atherosclerosis is an inflammatory disorder.1 Psoriasis, a chronic inflammatory skin disease associated with increased cardiovascular risk, provides a clinical human model to study inflammatory atherogenesis. Psoriasis patients experience myocardial infarction at younger ages than non-psoriasis patients.2, 3 This accelerated cardiovascular risk is most evident in psoriatic adults < 50 years of age,3 is not accurately captured by traditional risk assessment,4 and is likely attributable to an increased burden of subclinical coronary artery disease (CAD) in psoriasis patients.5, 6 However, to date, inadequate efforts have been made to directly measure CAD in this vulnerable population prior to having cardiovascular events.
Coronary CT Angiography (CCTA) provides a high quality, non-invasive imaging modality to assess7 and quantify both total (TB), and non-calcified (NCB) burden of coronary plaque.8 Additionally, it provides a reliable tool to identify rupture prone, high-risk coronary atherosclerotic plaques (HRP),9, 10 which have been associated with prospective cardiovascular events.9 Indeed, lipid rich plaques contain higher density of macrophage content11 and are more inflammatory with higher expression of inflammatory cytokines. Other inflammatory disease states including HIV disease have been associated with increased non-calcified plaque and high-risk plaque features.12, 13
Psoriasis patients are younger when they experience their first cardiovascular event3 and as such have lower Framingham Risk Scores.14 Currently, statins are the most validated drugs for primary and secondary cardiovascular prevention.15 However, given the impact of age on statin recommendations, psoriasis patients usually do not meet criteria for statins. Before release of the recent ACC/AHA guidelines for statin recommendations,16 NCEP ATP III guidelines17 were used to decide eligibility for statin therapy in patients who were deemed at risk for future cardiovascular events. This group of patients represents a comparator of interest for psoriasis patients to understand prevalence of vascular disease since a statin eligible group of hyperlipidemic patients represents a common, already identified group at risk for future cardiovascular events.
Given the accelerated risk of myocardial infarction seen in psoriasis, the goal of our study was to compare coronary plaque burden, both TB and NCB, and the presence of HRP assessed by CT angiography between psoriasis patients and a comparator cohort pre-selected to be at increased traditional risk—namely, hyperlipidemic patients eligible for statin therapy under NCEP ATP III guidelines, matched to be >10 years older than psoriasis patients.18, 19 Additionally, we followed a consecutive sample of psoriasis patients (n=50) to investigate how modulation of skin disease severity over a 1-year follow-up period associated with longitudinal changes in coronary plaque burden. We hypothesized that: 1) psoriasis patients would have greater NCB than hyperlipidemic patients and non-psoriasis healthy volunteers (HV); 2) psoriasis patients would have greater burden of HRP than HV, and at least an equivalent burden as hyperlipidemic patients; and 3) patients who experienced a longitudinal improvement in the severity of skin disease, would not have progression in coronary burden, compared to patients with poorly controlled skin disease. Such investigation may provide unique insight into the elevated cardiovascular risk associated with this common inflammatory disorder and inform the natural history of inflammatory atherogenesis.
METHODS
Our study included three cohorts of participants, all recruited at the National Institutes of Health Clinical Center, namely, psoriasis patients (n=105), a hyperlipidemic cohort (eligible for statin therapy by NCEP ATP III guidelines and without psoriasis) (n=100), and healthy volunteers who did not have psoriasis, or any other underlying inflammatory disease, matched by age and sex to the psoriasis patients, henceforth the HV cohort (n=25). The psoriasis and HV cohorts were recruited starting from January 1, 2013 till October 31, 2015, while the hyperlipidemic cohort was recruited from September 30, 2010 to January 6, 2015. All patients provided written informed consent. The study protocols were approved by the institutional review board at the National Institutes of Health. All study protocols are in compliance with the Declaration of Helsinki.
Psoriasis patients were > 18 years of age, and required to have a diagnostic confirmation of plaque psoriasis by a dermatologist. Psoriasis patients’ exclusion criteria included estimated glomerular filtration rate < 30 mL/min/1.73m2, pregnancy, and lactating women. Hyperlipidemic patients were > 55 years old, and were candidates for lipid lowering therapy under NCEP ATP III guidelines. Hyperlipidemic patients were matched to the psoriasis patients by age > 10 years. Exclusion criteria for hyperlipidemic patients included estimated glomerular filtration rate < 45 mL/min/m2, pregnancy, and lactation. The detailed inclusion and exclusion criteria for the healthy volunteers have already been published in our previous work.20 Psoriasis patients were stratified by decades of age, and since >90% were within the age range of 35–65, 4–5 HV were selected for each 5-year age-range of psoriasis patients between 35 and 65. Furthermore, since two-third of psoriasis patients were males, we attempted to include similar proportion of males under the HV group.
All patients underwent fasting blood draws for the assessment of lipid panel including total, HDL and LDL cholesterol, glucose and high-sensitivity CRP levels. A dermatologist or a physician evaluated all psoriasis patients for the assessment of psoriasis skin disease severity measured as psoriasis area severity index (PASI) score. Systemic-biologic treatment was defined as the following drugs: methotrexate, anti-TNF, anti-IL12/IL23, and anti-IL17. Plasma inflammatory biomarkers were assessed (Mesoscale Diagnostics, Rockville, MD) based on previously published methods.20 Cholesterol efflux capacity measurement was performed using a validated ex vivo assay of J774 cholesterol-loaded macrophages as previously published.18
All patients underwent coronary computed tomography angiography (CCTA) on the same day as blood draw, using the same CT scanner (320-dectector row Aquilion ONE ViSION, Toshiba, Japan). All guidelines set forth by the NIH Radiation Exposure Committee were followed. Scans were performed with retrospective gating at 120 kV, tube current of 750–850 mA, with a gantry rotation time of ≤420ms. Image acquisition characteristics included slice thickness of 0.75mm and pitch 0.2–0.4. Coronary plaque burden adjusted for luminal attenuation was evaluated across each of the main coronary arteries using the dedicated software QAngio CT (Medis, The Netherlands) by previously described methods by a single, blinded reader.18, 21 Manual adjustment of inner lumen and outer vessel wall delineations were performed if needed. TB and NCB plaque volume indices, assessed as mm2, were calculated by dividing total vessel plaque volume by total vessel length and were attenuated for luminal intensity measures for accuracy. Test-retest reliability was analyzed using intraclass correlation coefficient testing, and demonstrated very good intra-examiner reliability (ICC=95%). Additionally, interclass correlation coefficient testing showed high ICC between two experienced readers (ICC = 92%) in a subset of patients. All coronary segments with a diameter > 2mm were then analyzed by a single-reader blinded to the patients’ coronary plaque burdens for the presence of HRP features, defined as positive remodeling (remodeling index > 1.10), low-attenuation (<30 HU), or spotty calcification.22 Positive remodeling was assessed visually in multiplanar reconstructed images in both short-axis and long-axis views of the vessel. Measurements of the outer vessel diameter were performed at the reader’s discretion, and remodeling index > 1.10 was defined as a positively remodeled plaque based on validated findings from the previously published literature.9 If low CT attenuation was visually observed within a non-calcified plaque, 3 region-of-interest measurements (>0.5mm2) were placed. Mean CT attenuation within these regions-of-interest <30 HU was deemed low-attenuation plaque.9, 10 Finally, spotty calcification was defined by the presence of calcified plaque (HU > 350)23 with a diameter < 3mm in any direction, length of the calcification (measured in longitudinal direction of the vessel) less than 1.5 times the vessel diameter, and width (measured in the perpendicular direction to the vessel) less than two-thirds of the vessel diameter.22 The patient was classified as having a high-risk plaque if any of these plaque features were present. Test-retest reliability for HRP evaluation was also assessed by two experienced readers and demonstrated similar results (ICC = 92% for intra-examiner and ICC= 90% for interexaminer).
Finally, a consecutive sample of the first 50 recruited psoriasis patients was followed for one year (Supplemental Figure 1). These patients underwent repeat blood tests, clinical assessment for skin disease severity, medication usage, and CCTA scans with the same scanner for a repeat quantification of coronary plaque burdens by the same reader, at one year follow-up visits.
Statistical Analyses
Psoriasis and HV cohorts were matched by age and sex. Hyperlipidemic cohort was separately recruited. Skewness and kurtosis measures were considered to assess normality. Continuous data were summarized as mean ± S.D. for parametric variables and as median with interquartile range for non-parametric variables. Categorical variables were reported as percentages. Student t-test, for parametric data, and Mann-Whitney U test, for non-parametric data, were performed for continuous variable comparisons and Pearson’s chi-square tests were performed for categorical variables. Univariable logistic regression analyses were performed to assess crude associations between the prevalence of HRP and study variables. Moreover, to compare the prevalence of HRP in psoriasis and hyperlipidemic to that in HV, we utilized multivariable logistic regression analyses. In these models, we adjusted for covariates including traditional cardiovascular risk factors such as age, sex, Atherosclerotic cardiovascular disease 10-year risk (ASCVD),24 BMI, glucose and statins. Residual plots were generated subsequent to the regression models confirming that the data were normally distributed. Odds ratios with 95% confidence intervals and corresponding p values were reported for all the models. Furthermore, for patients with longitudinal follow-up, we assessed continuous variables either by paired t-test (parametric data) or Wilcoxon signed-rank test (non-parametric data). Categorical variables were assessed over 1 year by chi-square test. Multivariable linear regression analyses were performed to assess the relationship between change in coronary plaque burden and change in PASI score. Standardized β co-efficient values were reported for these analyses. Standardized β co-efficient represents the number of standard deviation change in coronary plaque burden per one standard deviation change in PASI score.
We hypothesized a 20% difference in NCB between psoriasis patients and controls. Thus, our sample size had more than 90% power to detect this difference significantly. We used previously published values of NCB for psoriasis patients18 and healthy volunteers.25 STATA-12 (College Station, Texas) was utilized for all statistical analysis. P values < 0.05 were considered significant.
RESULTS
Characteristics of the study groups
We summarize the demographic and clinical characteristics of our study cohorts in Table 1. A total of 230 individuals participated in the study. Psoriasis patients were middle-aged (age, mean ± SD: 50.4±12.6) and had moderate-to-severe skin disease (PASI Score, med. [IQR]: 5.7 [3–10]), while the mean age in hyperlipidemic patients was 61.2 years (SD 3.5) and that in HV was 47.7 (SD 9.8). There was a male predominance (psoriasis: 61%, hyperlipidemic: 54%, HV: 76%) among all three groups. Psoriasis patients had a greater mean body-mass index (psoriasis:29.9±6.2 vs hyperlipidemic:27.5±5.0, p=0.001), while there was a higher prevalence of dyslipidemia and a greater use of lipid-lowering therapies in the hyperlipidemic group (psoriasis: 33% vs hyperlipidemic: 81%). Importantly, the hyperlipidemic group was at significantly greater cardiovascular risk than psoriasis patients by both Framingham risk score (median [IQR]; psoriasis: 4 [1–6] vs. hyperlipidemic: 6 [2–9], p=0.001) and ASCVD 10-year risk (4.4 [1.8–9.3] vs 7.0 [4.2–11.2], p<0.001). Interestingly, HV were at similar cardiovascular risk as psoriasis patients (ASCVD 10-year risk- HV: 4.2 [2.4–6.3] vs psoriasis: 4.4 [1.8–9.3], p=0.73), likely owing to the young age of both samples.
Table 1.
Comparison of baseline characteristics of the study groups
| Variable* | Psoriasis | Hyperlipidemic | HV† | p§
|
|
|---|---|---|---|---|---|
| (N=105) | (N=100) | (N=25) | Psoriasis vs HLD‡ | Psoriasis vs HV† | |
|
| |||||
| Demographic and Clinical History | |||||
|
| |||||
| Age, years | 50.2±12.3 | 61.2±3.5 | 47.7±10.2 | matched | matched |
| Male, % | 65 (62%) | 54 (54%) | 19 (76%) | matched | matched |
| Hypertension, % | 32 (30%) | 41 (41%) | 7 (28%) | 0.12 | 0.81 |
| Dyslipidemia, % | 52 (50%) | 100 (100%) | 11 (44%) | <0.001 | 0.62 |
| Diabetes Mellitus, % | 12 (11%) | 3 (3%) | 4 (16%) | 0.02 | 0.53 |
| Current smoking, % | 8 (8%) | 6 (6%) | 1 (4%) | 0.67 | 0.52 |
|
| |||||
| Clinical parameters: | |||||
|
| |||||
| Body Mass Index, kg/m2 | 29.9±6.2 | 27.5±5.0 | 27.5±5.4 | 0.001 | 0.03 |
| Systolic BP, mm Hg | 124.7±15.4 | 128.3±14.6 | 110.4±11.5 | 0.07 | <0.001 |
| Diastolic BP, mm Hg | 73.4±11.3 | 74.3±9.4 | 69.4±9.2 | 0.26 | 0.09 |
| Framingham 10-year risk | 4 (1–6) | 6 (2–9) | 2 (1–6) | 0.001 | 0.36 |
| Atherosclerotic cardiovascular disease 10-year risk | 4.4 (1.8–9.3) | 7.0 (4.2–11.2) | 4.2 (2.4–6.3) | <0.001 | 0.73 |
| Statin Therapy | 35 (33%) | 81 (81%) | 7 (28%) | <0.001 | 0.61 |
|
| |||||
| Lipid profile: | |||||
|
| |||||
| Total cholesterol, mg/dL | 181.4±38.9 | 188.7±48.1 | 185.5±40.1 | 0.34 | 0.28 |
| Low-density lipoprotein cholesterol, mg/dL | 100.8±32.8 | 103.9±41.3 | 104.1±33.5 | 0.28 | 0.33 |
| High-density lipoprotein cholesterol, mg/dL | 55.7±18.5 | 60.5±24.2 | 54.8±19.2 | 0.21 | 0.41 |
| Triglycerides, mg/dL | 101 (76–141) | 107 (71–153) | 95 (79–161) | 0.94 | 0.73 |
| Glucose, mg/dL | 101.2±18.5 | 99.2±23.2 | 97.5±14.5 | 0.24 | 0.17 |
| High-sensitivity C-reactive protein, mg/L | 1.8 (0.7–3.9) | 0.9 (0.4–2.4) | 1.0 (0.7–1.8) | 0.01 | 0.16 |
|
| |||||
| Psoriasis Characteristics | |||||
|
| |||||
| Psoriasis Area Severity Index Score | 5.7 (2.8–10.1) | – | – | – | – |
| Systemic/Biologic Treatment, % | 39 (37%) | – | – | – | – |
All values in table 2 are represented as mean ± SD or median (IQR) for continuous variables and as n (%) for categorical variables.
HV: healthy volunteers,
HLD: Hyperlipidemic.
P values were calculated by Student’s t-test or Mann-Whitney U test for continuous variables and by Pearson’s chi-square test for categorical variables.
Coronary plaque burden quantification and plaque characterization in the study groups
Quantitative coronary plaque burden evaluation was performed in 3380 of 3450 (98%) coronary segments available. Such analysis revealed that the psoriasis group had similar TB (psoriasis:1.22±0.31 vs hyperlipidemic:1.18±0.34, p=0.16), but significantly greater NCB (1.18±0.32 vs 1.11±0.33, p=0.02) than hyperlipidemic patients despite being one decade younger and at lower cardiovascular risk by traditional validated 10-year risk scores (Table 2). Additionally, despite similar dense-calcified burden (0.04±0.10 vs. 0.05±0.08) psoriasis patients had lesser coronary artery calcium assessed as mean Agatston score compared to hyperlipidemic patients (CAC score median [IQR]; psoriasis: 0 [0–68] vs hyperlipidemic: 38 [0–184], p=0.006). The psoriasis group also had significantly greater TB (1.22±0.31 vs 1.04±0.22, p=0.001) and NCB (1.18±0.32 vs 1.03±0.21, p=0.004) than the HV group. Furthermore, consistent with prior findings, we observed a significant association between PASI Score and NCB (β=0.16, 95% CI 0.13–0.20; p=0.004) and psoriasis disease duration and NCB (β=0.17, 0.15–0.18; p=0.028). On characterization of coronary plaque morphology, it was noted that psoriasis patients had a similar prevalence of HRP as hyperlipidemic patients (psoriasis: 34% vs hyperlipidemic: 38%, p=0.58), as well as similar mean number of HRP (0.58±1.14 vs 0.67±1.08, p=0.33). However, psoriasis patients had significantly greater prevalence of HRP, (psoriasis: 34% vs HV: 8%, p=0.01), and increased mean number of HRP (0.58±1.14 vs 0.08±0.28, p=0.01), compared to the HV group. This difference was predominantly driven by positive remodeling, and spotty calcifications (Supplemental Table 1). A representative HRP is shown in Figure 1. This figure demonstrates a baseline HRP in a psoriasis patient who during longitudinal follow-up had an acute myocardial infarction, and was found to have a culprit lesion on catheterization corresponding to the previously identified HRP in the right posterior descending artery (RPDA).
Table 2.
Characterization of coronary artery disease in the study groups
| Variable* | Psoriasis | Hyperlipidemic | HV† | p§
|
|
|---|---|---|---|---|---|
| (N=105) | (N=100) | (N=25) | Psoriasis vs HLD‡ | Psoriasis vs HV† | |
|
| |||||
| Coronary Artery Calcium Score | |||||
|
| |||||
| Coronary Artery Calcium (CAC) Score | 0 (0–68) | 38 (0–184) | 0 (0–2) | 0.006 | 0.07 |
| ln(CAC+1) | 0 (0–4.2) | 3.7 (0–5.2) | 0 (0–1.1) | 0.006 | 0.07 |
| CAC Score distribution | |||||
| 0 | 54 (51.5%) | 33 (33%) | 18 (72%) | 0.035 | 0.24 |
| 1–100 | 26 (25%) | 33 (33%) | 5 (20%) | ||
| 101–400 | 13 (12%) | 23 (23%) | 1 (4%) | ||
| >400 | 12 (11.5%) | 11 (11%) | 1 (4%) | ||
|
| |||||
| Coronary plaque burden: | |||||
|
| |||||
| Total Burden (× 100), mm2 | 1.22±0.31 | 1.18±0.34 | 1.04±0.22 | 0.16 | 0.001 |
| Non-Calcified Burden (× 100), mm2 | 1.18±0.32 | 1.11±0.33 | 1.03±0.21 | 0.02 | 0.004 |
| Dense-Calcified Burden (×100), mm2 | 0.04±0.06 | 0.05±0.05 | 0.01±0.01 | 0.15 | 0.006 |
|
| |||||
| Coronary Plaque Characterization | |||||
|
| |||||
| Presence of High-Risk Plaque, | 36 (34%) | 38 (38%) | 2 (8%) | 0.58 | 0.01 |
| N (%) (95% CI) | (25%–44%) | (29%–48%) | (1%–26%) | ||
| Distribution of High-Risk Plaque | |||||
| 0 High-Risk Plaques | 69 (66%) | 62 (62%) | 23 (92%) | 0.45 | 0.03 |
| 1 High-Risk Plaques | 20 (19%) | 20 (20%) | 2 (8%) | ||
| 2 High-Risk Plaques | 8 (7.5%) | 10 (10%) | 0 (0%) | ||
| 3≥ High-Risk Plaques | 8 (7.5%) | 8 (8%) | 0 (0%) | ||
| Average Number of High-Risk Plaques | 0.58±0.11 | 0.67±0.11 | 0.08±0.05 | 0.36 | 0.01 |
All values in table 2 are represented as mean ± SEM or median (IQR) for continuous variables and as n (%) for categorical variables.
HV: healthy volunteers,
HLD: Hyperlipidemic
P values were calculated by Student’s t-test or Mann-Whitney U test for continuous variables and by Pearson’s chi-square test for categorical variables.
Figure 1. High-risk plaque in a psoriasis patient that was subsequently found to be culprit for a ST-elevation myocardial infarction during follow-up.
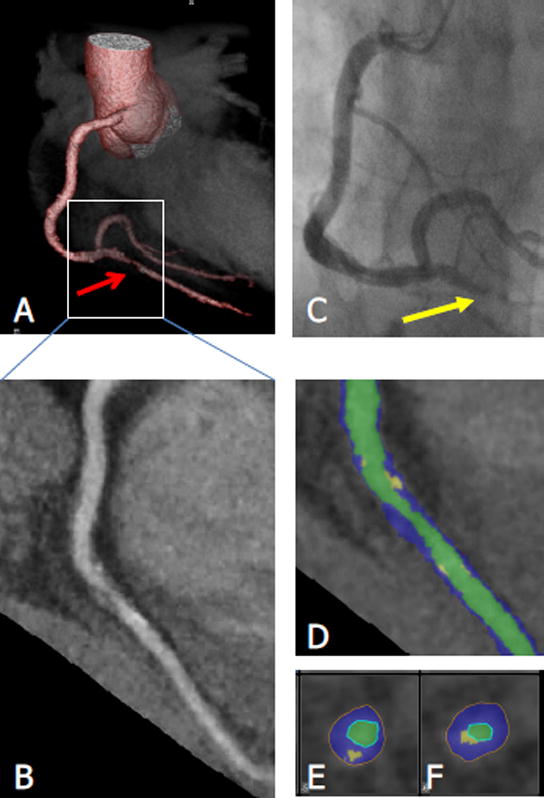
This figure demonstrates baseline high-risk plaque characteristics in a psoriasis patient, who during longitudinal follow-up was found to have an acute ST-Elevation Myocardial Infarction, with catheterization identified culprit lesion corresponding to the previously identified RPDA high-risk plaque (HRP).
A – 3-Dimensional Reconstruction of baseline CCTA, demonstrating stenosed vessel in RPDA. B- Planar reconstruction of vessel demonstrating HRP. C – Clinical catheterization identified culprit lesion (yellow arrow). D: Color coded curved multiplanar reconstruction. Green: Vessel lumen, blue: Non-calcified plaque component, yellow: Calcified plaque component. E and F: Cross sectional images. The plaque is only partially calcified, showing spotty calcification and some positive remodeling. Stenosis was graded as moderate-severe.
Greater non-calcified burden is associated with increased prevalence of high-risk plaque in psoriasis patients
To better understand the driving factors of coronary artery disease in psoriasis, we compared the demographic and clinical characteristics of psoriasis patients by stratifying the psoriasis cohort based on median value of NCB (Table 3). Psoriasis patients with greater NCB had a greater prevalence of male sex (79.3% vs. 44.2%, p<0.001) and hypertension (39.6% vs. 21.1%, p=0.04), as well as an increased body-mass index (32.5±6.3 vs. 27.1±3.9, p<0.001) and elevated cardiovascular 10-year risk (ASCVD 10-year risk: 4.9 [2.5–10.2] vs 2.7 [1.2–7.8], p=0.02). Patients with NCB below the cohort median had higher concentrations of HDL-cholesterol (61.4±21.0 vs. 50.2±14.4, p=0.001), and significantly higher usage of systemic-biologic psoriasis therapies (46.1% vs. 26.4%, p=0.04). Finally, as expected, patients with greater NCB had a significantly higher prevalence of HRP (49% vs 19.2%, p=0.001).
Table 3.
Comparison of Psoriasis Patients Stratified by Non-Calcified Burden
| Variable* | Lower NCB‡ than median (N=52) |
Higher NCB‡ than median (N=53) |
P† |
|---|---|---|---|
|
| |||
| Demographic and Clinical History | |||
|
| |||
| Age, years | 49.6±11.8 | 50.7±12.9 | 0.63 |
| Male, % | 23 (44.2%) | 42 (79.3%) | <0.001 |
| Hypertension, % | 11 (21.1%) | 21 (39.6%) | 0.04 |
| Dyslipidemia, % | 21 (40.4%) | 31 (58.5%) | 0.06 |
| Diabetes Mellitus, % | 7 (13.2%) | 5 (9.4%) | 0.52 |
| Current smoking, % | 5 (9.6%) | 3 (5.7%) | 0.45 |
|
| |||
| Clinical parameters: | |||
|
| |||
| Body Mass Index, kg/m2 | 27.1±3.9 | 32.5±6.3 | <0.001 |
| Systolic BP, mm Hg | 119.8±11.8 | 129.5±15.9 | <0.001 |
| Diastolic BP, mm Hg | 70.3±7.6 | 76.5±12.5 | 0.002 |
| Framingham 10-year risk score | 3 (1–6) | 4 (2–7) | 0.11 |
| Atherosclerotic cardiovascular disease (ASCVD) 10-year risk | 2.7 (1.2–7.8) | 4.9 (2.5–10.2) | 0.02 |
| Statin Therapy | 11 (21.1%) | 24 (45.2%) | 0.01 |
|
| |||
| Lipid profile: | |||
|
| |||
| Total cholesterol, mg/dL | 188.2±36.5 | 174.8±39.7 | 0.04 |
| Low-density lipoprotein cholesterol, mg/dL | 102.6±32.7 | 99.0±32.4 | 0.58 |
| High-density lipoprotein cholesterol, mg/dL | 61.4±21.0 | 50.2±14.4 | 0.001 |
| Triglycerides, mg/dL | 92.5 (67.5–134.5) | 108.0 (84–174) | 0.15 |
| Glucose, mg/dL | 99.5±21.1 | 102.9±13.9 | 0.34 |
|
| |||
| Psoriasis Characteristics | |||
|
| |||
| Psoriasis Area Severity Index Score | 4.8 (2.4–8.4) | 6.1 (3.2–12.2) | 0.28 |
| Systemic/Biologic Treatment, % | 24 (46.1%) | 14 (26.4%) | 0.04 |
|
| |||
| Coronary plaque burden: | |||
|
| |||
| Total Burden (× 100), mm2 | 0.90±0.22 | 1.54±0.43 | <0.001 |
| Non-Calcified Burden (× 100), mm2 | 0.85±0.22 | 1.50±0.43 | <0.001 |
| Dense-Calcified Burden (×100), mm2 | 0.05±0.07 | 0.04±0.07 | 0.69 |
|
| |||
| Coronary Plaque Characterization | |||
|
| |||
| Presence of High-Risk Plaque, % | 10 (19.2%) | 26 (49.0%) | 0.001 |
| Average Number of High-Risk Plaques | 0.43±1.23 | 0.74±1.08 | 0.08 |
All values in table 3 are represented as mean ± SD or median (IQR) for continuous variables and as n (%) for categorical variables.
P values were calculated by Student’s t-test or Mann-Whitney U test for continuous variables and by Pearson’s chi-square test for categorical variables.
NCB: non-calcified burden
Association between prevalence of high-risk plaque and study variables
We then conducted univariate logistic regression analyses to examine the relationships between traditional cardiometabolic risk factors, and the presence of HRP (Supplemental Table 2). Amongst both the psoriasis and hyperlipidemic patients, male sex [OR (95% CI), p value; psoriasis: 4.10 (1.56–10.76), <0.004; hyperlipidemic: 5.65 (1.49–21.41), p=0.01], systolic blood pressure [psoriasis: 1.04 (1.01–1.06), p=0.008; hyperlipidemic 1.04 (1.01–1.08), p=0.03], and Framingham Risk Score [psoriasis: 1.13 (1.02–1.25), p=0.02; hyperlipidemic: 1.19 (1.107–1.33), p=0.001] were associated with the presence of HRP. Amongst psoriasis patients, history of hypertension [3.04 (1.37–6.78), p=0.007)], history of hyperlipidemia [2.73 (1.24–6.01), p=0.01], and statin use [2.55 (1.16–5.61), p=0.02], were additionally associated with the presence of HRP.
Psoriasis had a strong association with high-risk plaques, to a similar extent as seen in high-risk hyperlipidemic patients
To evaluate the extent of HRP in the psoriasis and hyperlipidemic groups, as compared to HV, we subsequently conducted multivariable logistic regression analyses (Table 4). Both psoriasis and hyperlipidemic patients had elevated prevalence of HRP as compared to HV in unadjusted analyses [psoriasis: 6.0 (1.3–26.9), p=0.019; hyperlipidemic: 7.1 (1.6–31.6), p=0.011]. Amongst psoriasis, this association persisted beyond adjustment for ASCVD 10-year risk, LDL, BMI, glucose, and statin use [psoriasis: 5.9 (1.2–28.7), p=0.029], however in hyperlipidemic patients this relationship attenuated in significance upon adjustment for BMI [2.9 (0.6–14.6), p=0.195].
Table 4.
Logistic multivariable regression analyses demonstrate higher odds of high-risk plaque prevalence in psoriasis and hyperlipidemic patients compared to healthy volunteers beyond traditional cardiovascular risk factors.
| Model | Psoriasis | Hyperlipidemic | ||
|---|---|---|---|---|
|
| ||||
| Odds ratio (95% CI) | p | Odds ratio (95% CI) | p | |
|
| ||||
| Unadjusted | 6.0 (1.3–26.9) | 0.019 | 7.1 (1.6–31.6) | 0.011 |
| Adjusted for ASCVD* score | 6.7 (1.4–31.7) | 0.013 | 5.9 (1.2–28.0) | 0.025 |
| Adjusted for ASCVD score, LDL, BMI, glucose, hsCRP and statins | 6.0 (1.1–31.7) | 0.034 | 3.6 (0.6–20.5) | 0.195 |
Results are reported as logistic regression co-efficient and as odds ratio with 95% confidence interval along with p values.
ASCVD: Atherosclerotic cardiovascular disease 10-year risk, hsCRP: high-sensitivity C-reactive protein, BMI: body mass index
Reduction in skin inflammation was associated with improvement in inflammatory cytokine profile and non-calcified plaque burden beyond traditional cardiovascular risk-factors at 1-year
Finally, to understand the potential role of psoriasis skin disease severity in progression of coronary artery disease, we followed the first 50 psoriasis participants in our cohort for 1 year. Demographic and clinical characteristics of these patients at baseline and 1-year follow up, stratified by an improvement versus worsening in skin inflammation, are presented in (Table 5). Furthermore, the specific biologic treatment modalities that the patients received are elaborated in (Supplemental Table 3). Of note, when PASI score improved (ΔPASI −27%; p<0.001) (N=33) there was a significant improvement in TB (β=0.45, 95% CI: 0.23–0.67; p<0.001) and NCB (β=0.53, 0.32–0.74; p<0.001) (Table 6), after adjustment for age, sex, ASCVD 10-year risk, BMI, lipid lowering therapy, and systemic/biologic psoriasis treatment. Conversely, in patients whose PASI score worsened, there was an increase in NCB (Baseline: 1.18±0.42 vs 1-year 1.26±0.51, p=0.03). Insulin resistance and cholesterol efflux capacity did not change from baseline to 1-year follow up measurements. However, patients with improvement in psoriasis severity did have an improvement in the plasma levels of inflammatory proteins including tumor necrosis factor-α (Baseline: 2.67 (2.00–4.47) vs 1-year 2.17 (1.76–2.48), p=0.005), interleukin 1-β (0.07 (0.07–0.40) vs 0.04 (0.00–0.38), p<0.0001), and monocyte chemotactic protein-1 (96.95 (60.89–128.55) vs 64.99 (48.83–82.67), p<0.0001). However, adjustment for these biomarkers did not change the association between reduction in PASI score and improvement in coronary plaque burden (Table 6). Finally, in patients who did not have improvement in their skin disease, we did not observe a reduction in these proteins (Table 5).
Table 5.
Demographic and clinical characteristics of psoriasis patients at baseline and 1-year follow up, as stratified by improvement in psoriasis severity (N=50)
| Parameter* | No improvement in PASI (N=17) | Improvement in PASI (N=33) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | 1-year f/u | p† | Baseline | 1-year f/u | p† | |
|
| ||||||
| Demographic and Clinical Characteristics | ||||||
|
| ||||||
| Age, years | 52.2±13.6 | 53.3±13.6 | N/A | 52.2±10.9 | 53.3±10.9 | N/A |
| Males, N (%) | 11 (65%) | – | 19 (58%) | – | ||
| Hypertension, N (%) | 6 (35%) | 5 (29%) | 0.56 | 11 (33%) | 8 (24%) | 0.08 |
| Type 2 Diabetes, N (%) | 2 (12%) | 1 (6%) | 0.32 | 5 (15%) | 4 (12%) | 0.32 |
| Hyperlipidemia, N (%) | 13 (76.5%) | 10 (59%) | 0.18 | 21 (64%) | 21 (64%) | 1 |
| Current Smoker, N (%) | 2 (12%) | 3 (18%) | 0.33 | 3 (9%) | 1 (3%) | 0.16 |
| Body mass index, kg/m2 | 29.7±5.8 | 30.3±5.0 | 0.18 | 30.1±5.2 | 29.8±5.2 | 0.11 |
| Lipid Treatment, N (%) | 9 (53%) | 10 (58%) | 0.25 | 13 (39%) | 14 (42%) | 0.32 |
|
| ||||||
| Clinical and Lab Values | ||||||
|
| ||||||
| Systolic BP, mm Hg | 124.4±11.5 | 115.5±13.6 | 0.01 | 125.3±12.1 | 116.5±13.8 | <0.001 |
| Diastolic BP, mm Hg | 71.1±7.8 | 67.9±7.4 | 0.08 | 74.6±9.2 | 71.2±8.0 | 0.02 |
| Total Cholesterol, mg/dL | 178.2±33.8 | 166.7±35.9 | 0.07 | 178.2±33.3 | 180.7±39.1 | 0.37 |
| HDL Cholesterol, mg/dL | 49.9±15.3 | 56.1±21.9 | 0.04 | 53.7±15.5 | 57±20.7 | 0.06 |
| LDL Cholesterol, mg/dL | 100.3±35.1 | 84.4±39.2 | 0.03 | 99.4±27.0 | 97.8±6.6 | 0.41 |
| Triglycerides, mg/dL | 108 (85–131) | 105 (79–150) | 0.17 | 111 (81–140) | 123 (84–165) | 0.28 |
| Framingham 10-year risk | 4 (2–7) | 5 (1–9) | 0.57 | 4 (3–7) | 2 (1–7) | 0.08 |
|
| ||||||
| Psoriasis Details | ||||||
|
| ||||||
| Disease Duration, years | 20 (8–33) | 21 (9–34) | N/A | 20 (7–32) | 21 (8–33) | N/A |
| PASI§ Score | 3.2 (1.4–5.1) | 4.9 (3.1–6.0) | <0.0001 | 5.6 (4–12.3) | 3.1 (1.9–4.8) | <0.0001 |
| Body Surface Area affected, % | 1.3 (0.4–4.9) | 3.9 (1–14) | <0.0001 | 4 (2.7–13.7) | 1.2 (0.8–2.0) | <0.0001 |
| Systemic and/or Biologic Therapy, N (%) | 8 (47%) | 10 (59%) | 0.16 | 15 (45.5%) | 21 (64%) | <0.01 |
|
| ||||||
| Cardiometabolic Risk Markers | ||||||
|
| ||||||
| HOMA-IR‡ | 2.9 (1.8–7.5) | 3.1 (2.0–5.5) | 0.36 | 3.5 (1.5–5.2) | 3.3 (1.6–5.2) | 0.14 |
| Cholesterol efflux capacity | 0.92±0.21 | 0.89±0.12 | 0.22 | 0.94±0.17 | 0.95±0.02 | 0.37 |
|
| ||||||
| Inflammatory Proteins‖ | ||||||
|
| ||||||
| Monocyte chemotactic protein-1 | 106.55 (69.54–134.63) | 99.12 (83.44–375.75) | 0.19 | 96.95 (60.89–128.55) | 64.99 (48.83–82.67) | <0.0001 |
| Tumor necrosis factor-α | 3.05 (2.05–4.02) | 3.46 (2.36–6.94) | 0.37 | 2.67 (2.00–4.47) | 2.17 (1.76–2.48) | 0.005 |
| Interleukin-1β | 0.07 (0.07–0.07) | 0.10 (0.07–0.13) | 0.001 | 0.07 (0.07–0.40) | 0.04 (0.00–0.38) | <0.0001 |
|
| ||||||
| Coronary Plaque Burden, adjusted for luminal attenuation | ||||||
|
| ||||||
| Total Burden (× 100), mm2 | 1.26±0.25 | 1.32±0.33 | 0.02 | 1.26±0.29 | 1.17±0.29 | 0.02 |
| Dense Calcified Burden (× 100), mm2 | 0.06±0.01 | 0.06±0.01 | 0.151 | 0.07±0.01 | 0.05±0.001 | 0.06 |
| Non-Calcified Burden (× 100), mm2 | 1.18±0.25 | 1.26±0.29 | 0.03 | 1.20±0.29 | 1.12±0.29 | 0.03 |
Values are reported as mean ± SD or median (IQR) for continuous variables and as n (%) for categorical variables.
P values were calculated by paired t-test or Wilcoxon signed rank test for continuous variables and by Pearson chi-squared test for categorical variables.
HOMA-IR: homeostatic model assessment of insulin resistance
PASI: psoriasis area severity index.
levels are expressed as ng/dL.
Table 6.
Adjusted analyses demonstrate a direct association between change in PASI score and change in total and non-calcified coronary plaque burden.
| TOTAL CORONARY PLAQUE BURDEN | |||
|---|---|---|---|
|
| |||
| Model | All patients (N=50) |
No Improvement (N=17) |
Improvement (N=33) |
|
| |||
| Unadjusted | 0.28 (0.12–0.43) (0.001) | −0.12 (−0.48–0.24) (0.41) | 0.25 (0.06–0.44) (0.013) |
| Adjusted for age and sex | 0.28 (0.12–0.44) (0.001) | −0.10 (−0.45–0.26) (0.58) | 0.21 (0.02–0.41) (0.034) |
| Adjusted for ASCVD score* | 0.37 (0.20–0.52) (<0.001) | −0.06 (−0.41–0.30) (0.73) | 0.42 (0.23–0.62) (<0.001) |
| Adjusted for ASCVD score, BMI, statins | 0.38 (0.21–0.53) (<0.001) | 0.07 (−0.29–0.42) (0.71) | 0.54 (0.31–0.77) (<0.001) |
| Adjusted for ASCVD score, BMI, statins, cholesterol efflux capacity | 0.39 (0.21–0.56) (<0.001) | 0.15 (−0.20–0.51) (0.71) | 0.45 (0.25–0.66) (0.005) |
| Adjusted for ASCVD score, BMI, statins, cholesterol efflux capacity, systemic of biologic treatment | 0.34 (0.21–0.48) (<0.001) | 0.24 (−0.12–0.59) (0.78) | 0.45 (0.23−0.67) (0.003) |
| Adjusted for ASCVD score, BMI, statins, cholesterol efflux capacity, systemic of biologic treatment, MCP1, IL1β, TNF-α | 0.37 (0.21–0.53) (<0.001) | 0.23 (−0.12–0.59) (0.27) | 0.36 (0.16–0.57) (0.001) |
| NON-CALCIFIED CORONARY PLAQUE BURDEN | |||
| Unadjusted | 0.29 (0.13–0.46) (<0.001) | −0.13 (−0.49–0.23) (0.38) | 0.28 (0.09–0.47) (0.005) |
| Adjusted for age and sex | 0.30 (0.14–0.46) (<0.001) | −0.11 (−0.47–0.25) (0.50) | 0.29 (0.06–0.51) (0.011) |
| Adjusted for ASCVD score* | 0.37 (0.21–0.54) (<0.001) | −0.08 (−0.45–0.28) (0.62) | 0.52 (0.30–0.74) (<0.001) |
| Adjusted for ASCVD score, BMI, statins | 0.42 (0.24–0.59) (<0.001) | 0.04 (−0.32–0.40) (0.80) | 0.59 (0.36–0.83) (<0.001) |
| Adjusted for ASCVD score, BMI, statins, cholesterol efflux capacity | 0.41 (0.24–0.58) (<0.001) | 0.13 (−0.23–0.49) (0.47) | 0.55 (0.33–0.77) (<0.001) |
| Adjusted for ASCVD score, BMI, statins, cholesterol efflux capacity, systemic or biologic treatment | 0.39 (0.23–0.56) (<0.001) | 0.22 (−0.15–0.58) (0.88) | 0.53 (0.32–0.74) (<0.001) |
| Adjusted for ASCVD score, BMI, statins, cholesterol efflux capacity, systemic of biologic treatment, MCP-1, IL1β, TNF-α | 0.40 (0.24–0.56) (<0.001) | 0.23 (−0.14–0.58) (0.29) | 0.44 (0.25–0.63) (<0.001) |
Results reported as standardized β value (95% CI) (p value).
Standardized β co-efficient for each model represents the number of standard deviation change in total or non-calcified burden per one standard deviation change in PASI score
ASCVD: Atherosclerotic cardiovascular disease 10-year risk, MCP-1: Monocyte chemotactic protein-1, IL1β: Interleukin-1β, TNF-α: Tumor necrosis factor-α
DISCUSSION
In this study we demonstrate several important, novel findings: 1) Psoriasis patients have increased coronary NCB, assessed by CCTA, as compared to a cohort of hyperlipidemic patients with a mean age > 10 years older; 2) Prevalence of HRP was similar between psoriasis and hyperlipidemic patients, and was approximately 6 times greater than that observed in HV despite psoriasis patients being at significantly lower traditional cardiovascular risk than hyperlipidemic patients, and equivalent risk as HV; 3) Psoriasis patients with increased NCB had a significantly higher prevalence of HRP, as compared to those with lower NCB; 4) Among consecutive psoriasis patients followed for 1 year, reduction in skin disease severity was associated with an improvement in NCB. Collectively, these findings provide unique insight into the epidemiologic association of psoriasis and increased cardiovascular risk, and support a role for a reduction of remote systemic inflammation in modulating CAD.
Inflammation is increasingly recognized as a driver of atherosclerotic cardiovascular diseases.1 Inflammatory mediators are known to play a critical role in each stage of atherogenesis, from initial endothelial cell dysfunction26 to ultimate atherosclerotic plaque rupture.27 Conversely, anti-inflammatory therapies, including statins, are commonly used for primary as well as secondary cardiovascular prevention.16 Psoriasis, a chronic inflammatory immune disease associated with increased cardiovascular risk, thus provides a useful clinical model to study the role of systemic inflammation in atherogenesis. Evidence suggests psoriasis is linked to baseline cardiometabolic dysfunction, including high insulin-resistance,28 poor HDL function,18 and obesity.29 Psoriasis patients are at significantly elevated relative risk for myocardial infarction.3 However, the increased risk noted in this common inflammatory condition is not captured by traditional risk assessment,4 and persists beyond adjustment for these factors.
CCTA is a reliable, non-invasive imaging modality that allows for quantification of total and non-calcified coronary plaque volume and characterization of coronary plaque content. Prior work demonstrates that TB and NCB correlate with traditional measures of cardiovascular risk30 and predict prospective cardiovascular events in patients without inflammatory conditions.25 We demonstrated that psoriasis and hyperlipidemic patients had increased TB as compared to HV. Furthermore, psoriasis patients had equivalent TB as hyperlipidemic patients, but greater NCB than both HV and hyperlipidemic patients, despite being 10 years younger, and at lower traditional cardiovascular risk, than hyperlipidemic patients. As non-calcified coronary plaques are frequently identified as culprit lesions in myocardial infarction,31 our results are consistent with the elevated cardiovascular event rate noted in young psoriasis patients2, 3 and provide an important advance in the understanding of the epidemiological association. Furthermore, these findings that inflammation may drive this NCB are in line with a recent study demonstrating that HIV is associated with higher NCB compared to healthy volunteers.13, 32
Recent literature has identified particular coronary plaque characteristics associated with plaque rupture and future cardiovascular events.9, 10 Our study indicates that psoriasis and hyperlipidemic patients have increased prevalence of HRP as compared to HV, but similar prevalence of HRP as compared to one another. The increased prevalence of HRP in psoriasis was independent of traditional cardiovascular risk factors, suggesting a link between this inflammatory condition and HRP. The prevalence of HRP amongst hyperlipidemic patients was comparable to the prevalence seen in a prior study, which defined HRP as spotty-calcification, low-attenuation, positive remodeling, and napkin ring sign positivity.10 Such prevalence is greater than that seen in a recent landmark study assessing cardiovascular outcome prediction by HRP;9 however this study defined HRP as positive remodeling and low-attenuation alone. Our findings that younger psoriasis patients with lower traditional cardiovascular risk have equal prevalence of HRP as compared to higher-risk hyperlipidemic patients is thus compelling. Indeed, prior studies have demonstrated that increased vascular inflammation by FDG PET/CT associates with HRP in HIV,12 and that HRP themselves have increased inflammatory PET uptake.33 Given the known association between psoriasis and elevated vascular inflammation,20 our findings are thus consistent with the hypothesized relationship between inflammation and rupture prone plaque formation.
As higher NCB associated with an increased prevalence of HRP, we followed a consecutive sample of psoriasis patients in the cohort to understand how modulation of skin inflammation associated with the longitudinal progression of NCB. In this consecutive sample, psoriasis patients who experienced a reduction in skin-inflammation were noted to have an improvement in NCB at 1-year follow up, independent of traditional cardiovascular risk. These results echo prior work which has revealed an association between psoriasis skin inflammation and cardiovascular risk,3 and suggests that anti-inflammatory treatment may mitigate such risk.34, 35, 36
As inflammation is a critical component of atherosclerosis pathophysiology, chronic inflammation in psoriasis patients plays a role in the shared mechanisms driving accelerated atherosclerosis. Emerging concepts suggest that inflammatory cytokines such as tumor necrosis factor-α and interleukins, which are well known elements in pathogenesis of atherosclerosis1, are also involved in the causal pathways of psoriasis.37 Therefore, novel treatment modalities targeting these pro-inflammatory cytokines may have a favorable impact beyond skin clearance in psoriasis patients and may mitigate the risk of cardiovascular disease. Interestingly, in our study, levels of MCP-1, TNF-α and IL-1β, decreased in blood at one-year in those who improved their skin disease severity, which was also associated with an improvement in total and non-calcified coronary plaque. However, adjustment of these biomarkers in our final statistical model did not attenuate the association between a decrease in PASI score and a decrease in coronary plaque burden. This suggests that these proteins may not be the primary drivers of our observation of the association between reduction in skin diseases severity and improvement in coronary plaque burden. Our findings do support future efforts to broadly characterize immune cells and their protein products to dissect potential shared mechanistic links between psoriasis improvement and improvement in coronary plaque.
A recent publication described a higher presence of calcified, mixed, and non-calcified plaque, compared to healthy controls, in psoriatic arthritis patients.38 While this publication provided important qualitative description of coronary plaque in psoriasis, our study extends these findings by providing quantitative analyses of coronary plaque by volume. To our knowledge, this is the first study to perform segmental quantification of coronary plaque burden in psoriasis and to compare it to an older, at risk group as well as an age-matched healthy group. Furthermore, our study characterized high risk features of coronary plaque in an effort to understand the increased risk of acute myocardial infarction observed in psoriasis patients. Finally, longitudinal examination over one-year in our study demonstrated that successful reduction of psoriatic skin disease was associated with an improvement in early, non-calcified coronary plaque burden. These findings in particular are of great interest since they suggest that early, non-calcified plaque may be reversible with careful anti-inflammatory therapy. Whether aggressive cardiovascular risk factor management will further improve non-calcified coronary plaque burden is unknown. Future studies should focus on aggressive cardiovascular risk reduction in conjunction with anti-psoriatic therapy since larger benefits may be observed with more aggressive lipid, glucose and blood pressure management.
Our study is limited by the small sample size; however, this is the largest systematic characterization to date in psoriasis and in hyperlipidemic patients eligible for statin therapy. Moreover, a single blinded reader interpreted the coronary CT angiography scans for all the patients, however, a good intra-examiner as well as inter-examiner reliability was present among a subset of patients whose scans were read by two separate readers. Furthermore, we had a disproportionately small cohort of HV, however, this group provided important comparator data in the context of the hyperlipidemic group. Additionally, we had follow-up data available for a subset of patients for a short duration of follow-up of only one year, which is relatively short compared to published literature on coronary plaque natural history. However, we followed a consecutive sample to minimize any selection bias in longitudinal follow up. Moreover, our consecutive sample of patients followed for improvement in skin disease to understand whether NCB improved was not stratified by treatment, nor was it randomized. Therefore, our study results should be interpreted with caution. Given the observational study design, the possibility of residual confounding cannot be excluded, though we attempted to adjust for sources of bias with the available data. Finally, our study relied on a surrogate marker of coronary artery disease (CCTA) as the primary outcome instead of hard cardiovascular events. However, our goal was to understand the natural history prior to events in these groups. Future studies should focus on characterization of the natural history of high-risk plaque over longer periods of time and with specific therapies for psoriasis in larger sample sizes. Furthermore, a dedicated study of healthy volunteers enrolled for longitudinal assessment of high-risk plaque should be performed to provide context to the natural history of such plaques in diseased populations. Ongoing studies are investigating the effect of anti-inflammatory therapies on vascular inflammation by FDG PET-CT in psoriasis (NCT02187172, NCT02690701) but none in coronary plaque burden at this time. Similar randomized control trials should be performed for the longitudinal characterization of high-risk coronary plaque in psoriasis. Finally, large epidemiologic, prospective event trials should be performed to investigate the proposed association of systemic inflammation and increased cardiovascular events.
CONCLUSION
In conclusion, psoriasis patients have an increased volume of non-calcified plaque burden compared to hyperlipidemic patients who are at higher cardiovascular risk by traditional scores. Additionally, we observed an equivalent prevalence of high-risk plaque between psoriasis and an older, hyperlipidemic patient comparator group eligible for statin therapy. Furthermore, modulation of remote skin inflammation associated with an improvement of non-calcified plaque burden in psoriasis patients at 1-year follow-up, suggesting control of remote inflammation may translate into reduction in coronary artery disease risk. We suggest that based on our findings, screening for cardiovascular risk factors in patients with psoriasis, especially when it is severe, is warranted.
Supplementary Material
Clinical Perspective.
What is new?
In a deeply phenotyped cohort, psoriasis was associated with an increased presence of non-calcified coronary plaque and a similar prevalence of high-risk plaque, as compared to an older, hyperlipidemic population.
In a consecutive sample of psoriasis patients, improvement in skin disease severity associated with an improvement in coronary plaque burden at 1-year follow up.
What are the clinical implications?
Psoriasis patients have similar coronary artery disease risk as hyperlipidemic patients one decade older.
As such, this young, vulnerable population should be screened earlier for cardiovascular disease, and should be educated about their elevated risk.
Further investigations should focus on the longitudinal impact of psoriasis treatment on high-risk plaque morphology, as well as on the extent of cardiovascular risk mitigation in randomized trials.
Acknowledgments
JBL, AAJ, and NNM had full access to all data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
Study concept and design: NNM, JBL and AAJ designed the study
Acquisition of data: JBL, AAJ, AC, TMA, AKD, JAR, TS, JC, AR, HLT acquired the data
Analysis and interpretation of data: JBL, AAJ, NNM
Drafting of manuscript: JBL, AAJ, NNM
Critical revision of manuscript: All authors
Sources of Funding:
This study was supported by the National Heart, Lung, and Blood Institute Intramural Research Program (HL006193-02), and the National Institutes of Health (grants R01-HL-69905 and N01-HC95159 through N01-HC95168).
Footnotes
Disclosures:
None
References
- 1.Libby P, Ridker PM, Hansson GK, Leducq Transatlantic Network on A Inflammation in atherosclerosis: from pathophysiology to practice. J Am Coll Cardiol. 2009;54:2129–38. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta NN, Azfar RS, Shin DB, Neimann AL, Troxel AB, Gelfand JM. Patients with severe psoriasis are at increased risk of cardiovascular mortality: cohort study using the General Practice Research Database. Eur Heart J. 2010;31:1000–1006. doi: 10.1093/eurheartj/ehp567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296:1735–1741. doi: 10.1001/jama.296.14.1735. [DOI] [PubMed] [Google Scholar]
- 4.Mehta NN, Krishnamoorthy P, Yu Y, Khan O, Raper A, Van Voorhees A, Troxel AB, Gelfand JM. The impact of psoriasis on 10-year Framingham risk. J Am Acad Dermatology. 2012;67:796–798. doi: 10.1016/j.jaad.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjuler KF, Bottcher M, Vestergaard C, Deleuran M, Raaby L, Botker HE, Iversen L, Kragballe K. Increased Prevalence of Coronary Artery Disease in Severe Psoriasis and Severe Atopic Dermatitis. Am J Med. 2015;128:1325–1334 e2. doi: 10.1016/j.amjmed.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 6.Mansouri B, Kivelevitch D, Natarajan B, Joshi AA, Ryan C, Benjegerdes K, Schussler JM, Rader DJ, Reilly MP, Menter A, Mehta NN. Comparison of Coronary Artery Calcium Scores Between Patients With Psoriasis and Type 2 Diabetes. JAMA Dermatol. 2016;152:1244–1253. doi: 10.1001/jamadermatol.2016.2907. [DOI] [PubMed] [Google Scholar]
- 7.Maurovich-Horvat P, Ferencik M, Voros S, Merkely B, Hoffmann U. Comprehensive plaque assessment by coronary CT angiography. Nat Rev Cardiol. 2014;11:390–402. doi: 10.1038/nrcardio.2014.60. [DOI] [PubMed] [Google Scholar]
- 8.Kwan AC, May HT, Cater G, Sibley CT, Rosen BD, Lima JA, Rodriguez K, Lappe DL, Muhlestein JB, Anderson JL, Bluemke DA. Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology. 2014;272:690–699. doi: 10.1148/radiol.14140611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motoyama S, Ito H, Sarai M, Kondo T, Kawai H, Nagahara Y, Harigaya H, Kan S, Anno H, Takahashi H, Naruse H, Ishii J, Hecht H, Shaw LJ, Ozaki Y, Narula J. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J Am Coll Cardiol. 2015;66:337–346. doi: 10.1016/j.jacc.2015.05.069. [DOI] [PubMed] [Google Scholar]
- 10.Puchner SB, Liu T, Mayrhofer T, Truong QA, Lee H, Fleg JL, Nagurney JT, Udelson JE, Hoffmann U, Ferencik M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: results from the ROMICAT-II trial. J Am Coll Cardiol. 2014;64:684–692. doi: 10.1016/j.jacc.2014.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yahagi K, Kolodgie FD, Otsuka F, Finn AV, Davis HR, Joner M, Virmani R. Pathophysiology of native coronary, vein graft, and in-stent atherosclerosis. Nat Rev Cardiol. 2016;13:79–98. doi: 10.1038/nrcardio.2015.164. [DOI] [PubMed] [Google Scholar]
- 12.Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, Wai B, Hoffmann U, Abbara S, Grinspoon S. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Def Synd. 2014;66:164–171. doi: 10.1097/QAI.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanni MV, Abbara S, Lo J, Wai B, Hark D, Marmarelis E, Grinspoon SK. Increased coronary atherosclerotic plaque vulnerability by coronary computed tomography angiography in HIV-infected men. AIDS. 2013;27:1263–1272. doi: 10.1097/QAD.0b013e32835eca9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta NN, Yu Y, Pinnelas R, Krishnamoorthy P, Shin DB, Troxel AB, Gelfand JM. Attributable risk estimate of severe psoriasis on major cardiovascular events. Am J Med. 2011;124:775.e1, 6. doi: 10.1016/j.amjmed.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins R, Reith C, Emberson J, Armitage J, Baigent C, Blackwell L, Blumenthal R, Danesh J, Smith GD, DeMets D, Evans S, Law M, MacMahon S, Martin S, Neal B, Poulter N, Preiss D, Ridker P, Roberts I, Rodgers A, Sandercock P, Schulz K, Sever P, Simes J, Smeeth L, Wald N, Yusuf S, Peto R. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet. 2016;388:2532–2561. doi: 10.1016/S0140-6736(16)31357-5. [DOI] [PubMed] [Google Scholar]
- 16.Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC., Jr Tomaselli GF and American College of Cardiology/American Heart Association Task Force on Practice G. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 17.National Cholesterol Education Program Expert Panel on Detection E and Treatment of High Blood Cholesterol in A. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 18.Salahuddin T, Natarajan B, Playford MP, Joshi AA, Teague H, Masmoudi Y, Selwaness M, Chen MY, Bluemke DA, Mehta NN. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur Heart J. 2015:2662–2665. doi: 10.1093/eurheartj/ehv339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, Polak JF, Lima JA, Bluemke DA. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: the Multi-Ethnic Study of Atherosclerosis (MESA) Radiology. 2014;271:381–389. doi: 10.1148/radiol.14131020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naik HB, Natarajan B, Stansky E, Ahlman MA, Teague H, Salahuddin T, Ng Q, Joshi AA, Krishnamoorthy P, Dave J, Rose SM, Doveikis J, Playford MP, Prussick RB, Ehrlich A, Kaplan MJ, Lockshin BN, Gelfand JM, Mehta NN. Severity of Psoriasis Associates With Aortic Vascular Inflammation Detected by FDG PET/CT and Neutrophil Activation in a Prospective Observational Study. Arterioscler Thromb Vasc Biol. 2015;35:2667–2676. doi: 10.1161/ATVBAHA.115.306460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi AA, Lerman JB, Aberra TM, Afshar M, Teague HL, Rodante JA, Krishnamoorthy P, Ng Q, Aridi TZ, Salahuddin T, Natarajan B, Lockshin BN, Ahlman MA, Chen MY, Rader DJ, Reilly MP, Remaley AT, Bluemke DA, Playford MP, Gelfand JM, Mehta NN. GlycA Is a Novel Biomarker of Inflammation and Subclinical Cardiovascular Disease in Psoriasis. Circ Res. 2016;119:1242–1253. doi: 10.1161/CIRCRESAHA.116.309637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Motoyama S, Kondo T, Sarai M, Sugiura A, Harigaya H, Sato T, Inoue K, Okumura M, Ishii J, Anno H, Virmani R, Ozaki Y, Hishida H, Narula J. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J Am Coll Cardiol. 2007;50:319–326. doi: 10.1016/j.jacc.2007.03.044. [DOI] [PubMed] [Google Scholar]
- 23.Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T, Sanda Y, Anno H, Kondo T, Wong ND, Narula J, Ozaki Y. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010;3:691–698. doi: 10.1016/j.jcmg.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 24.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, Robinson JG, Schwartz JS, Shero ST, Smith SC, Jr, Sorlie P, Stone NJ, Wilson PW, Jordan HS, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC, Jr, Tomaselli GF and American College of Cardiology/American Heart Association Task Force on Practice G 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49–73. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 25.Versteylen MO, Kietselaer BL, Dagnelie PC, Joosen IA, Dedic A, Raaijmakers RH, Wildberger JE, Nieman K, Crijns HJ, Niessen WJ, Daemen MJ, Hofstra L. Additive value of semiautomated quantification of coronary artery disease using cardiac computed tomographic angiography to predict future acute coronary syndrome. J Am Coll Cardiol. 2013;61:2296–2305. doi: 10.1016/j.jacc.2013.02.065. [DOI] [PubMed] [Google Scholar]
- 26.Szmitko PE, Wang CH, Weisel RD, de Almeida JR, Anderson TJ, Verma S. New markers of inflammation and endothelial cell activation: Part I. Circulation. 2003;108:1917–1923. doi: 10.1161/01.CIR.0000089190.95415.9F. [DOI] [PubMed] [Google Scholar]
- 27.Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278:483–493. doi: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Armstrong AW, Harskamp CT, Armstrong EJ. Psoriasis and the risk of diabetes mellitus: a systematic review and meta-analysis. JAMA Dermatol. 2013;149:84–91. doi: 10.1001/2013.jamadermatol.406. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong AW, Harskamp CT, Armstrong EJ. The association between psoriasis and obesity: a systematic review and meta-analysis of observational studies. Nutr Diabetes. 2012;2:e54. doi: 10.1038/nutd.2012.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriguez K, Kwan AC, Lai S, Lima JA, Vigneault D, Sandfort V, Pattanayak P, Ahlman MA, Mallek M, Sibley CT, Bluemke DA. Coronary Plaque Burden at Coronary CT Angiography in Asymptomatic Men and Women. Radiology. 2015:142551. doi: 10.1148/radiol.2015142551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S, Brady TJ. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655–1662. doi: 10.1016/j.jacc.2006.01.041. [DOI] [PubMed] [Google Scholar]
- 32.Fitch KV, Srinivasa S, Abbara S, Burdo TH, Williams KC, Eneh P, Lo J, Grinspoon SK. Noncalcified coronary atherosclerotic plaque and immune activation in HIV-infected women. J Infect Dis. 2013;208:1737–1746. doi: 10.1093/infdis/jit508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Figueroa AL, Subramanian SS, Cury RC, Truong QA, Gardecki JA, Tearney GJ, Hoffmann U, Brady TJ, Tawakol A. Distribution of inflammation within carotid atherosclerotic plaques with high-risk morphological features: a comparison between positron emission tomography activity, plaque morphology, and histopathology. Circ Cardiovasc Imaging. 2012;5:69–77. doi: 10.1161/CIRCIMAGING.110.959478. [DOI] [PubMed] [Google Scholar]
- 34.Ahlehoff O, Skov L, Gislason G, Lindhardsen J, Kristensen SL, Iversen L, Lasthein S, Gniadecki R, Dam TN, Torp-Pedersen C, Hansen PR. Cardiovascular disease event rates in patients with severe psoriasis treated with systemic anti-inflammatory drugs: a Danish real-world cohort study. J Intern Med. 2013;273:197–204. doi: 10.1111/j.1365-2796.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 35.Roubille C, Richer V, Starnino T, McCourt C, McFarlane A, Fleming P, Siu S, Kraft J, Lynde C, Pope J, Gulliver W, Keeling S, Dutz J, Bessette L, Bissonnette R, Haraoui B. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2015;74:480–489. doi: 10.1136/annrheumdis-2014-206624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hjuler KF, Bottcher M, Vestergaard C, Botker HE, Iversen L, Kragballe K. Association Between Changes in Coronary Artery Disease Progression and Treatment With Biologic Agents for Severe Psoriasis. JAMA Dermatol. 2016;152:1114–1121. doi: 10.1001/jamadermatol.2016.1984. [DOI] [PubMed] [Google Scholar]
- 37.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361:496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 38.Shen J, Wong KT, Cheng IT, Shang Q, Li EK, Wong P, Kun EW, Law MY, Yip R, Yim I, Ying S, Li M, Li TK, Wong CK, Zhu TY, Lee JJ, Chang M, Lee AP, Tam LS. Increased prevalence of coronary plaque in patients with psoriatic arthritis without prior diagnosis of coronary artery disease. Ann Rheum Dis. 2017 doi: 10.1136/annrheumdis-2016-210390. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


