Summary
Background
Diabetes is an independent risk factor for heart failure progression. Sacubitril/valsartan, a combination angiotensin receptor-neprilysin inhibitor, improves morbidity and mortality in patients with heart failure with reduced ejection fraction (HFrEF), compared with the angiotensin-converting enzyme inhibitor enalapril, and improves peripheral insulin sensitivity in obese hypertensive patients. We aimed to investigate the effect of sacubitril/valsartan versus enalapril on HbA1c and time to first-time initiation of insulin or oral antihyperglycaemic drugs in patients with diabetes and HFrEF.
Methods
In a post-hoc analysis of the PARADIGM-HF trial, we included 3778 patients with known diabetes or an HbA1c≥6·5% at screening out of 8399 patients with HFrEF who were randomly assigned to treatment with sacubitril/valsartan or enalapril. Of these patients, most (98%) had type 2 diabetes. We assessed changes in HbA1c, triglycerides, HDL cholesterol and BMI in a mixed effects longitudinal analysis model. Times to initiation of oral antihyperglycaemic drugs or insulin in subjects previously not treated with these agents were compared between treatment groups.
Findings
There were no significant differences in HbA1c concentrations between randomised groups at screening. During the first year of follow-up, HbA1c concentrations decreased by 0·16% (SD 1·40) in the enalapril group and 0·26% (SD 1·25) in the sacubitril/valsartan group (between-group reduction 0·13%, 95% CI 0·05–0·22, p=0·0023). HbA1c concentrations were persistently lower in the sacubitril/valsartan group than in the enalapril group over the 3-year follow-up (between-group reduction 0·14%, 95% CI 0·06–0·23, p=0·0055). New use of insulin was 29% lower in patients receiving sacubitril/valsartan (114 [7%] patients) compared with patients receiving enalapril (153 [10%]; hazard ratio 0·71, 95% CI 0·56–0·90, p=0·0052). Similarly, fewer patients were started on oral antihyperglycaemic therapy (0·77, 0·58–1·02, p=0·073) in the sacubitril/valsartan group.
Interpretation
Patients with diabetes and HFrEF enrolled in PARADIGM-HF who received sacubitril/valsartan had a greater long-term reduction in HbA1c than those receiving enalapril. These data suggest that sacubitril/valsartan might enhance glycaemic control in patients with diabetes and HFrEF.
Introduction
Heart failure and diabetes frequently coexist, with a prevalence of diabetes as high as 35–40% in patients with heart failure, independent of the degree of impairment in ejection fraction.1 Moreover, diabetes is considered to be a major comorbidity and strong independent risk factor for the progression of heart failure with either preserved or reduced ejection fraction,2 with an attendant elevated risk of both admission to hospital for heart failure and death, compared with patients without diabetes.3–5 The degree of risk has further been related to the level of glycaemic control in patients with heart failure,6 and more severe hyperglycaemia has been associated with worsening of cardiac structure and function.7,8
Compared with the angiotensin-converting enzyme inhibitor (ACEI) enalapril, sacubitril/valsartan (formerly known as LCZ696), an angiotensin receptor-neprilysin inhibitor (ARNI), improved morbidity and mortality in patients with heart failure and reduced ejection fraction (HFrEF) in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial, after a median follow-up of 27 months.5
Sacubitril/valsartan did not reduce the pre-specified exploratory outcome of new onset diabetes in comparison with enalapril, although the number of patients with new-onset diabetes during the course of the trial was very small.
The effect of neprilysin inhibitors on insulin sensitivity has been investigated in several studies. Sacubitril/valsartan improved peripheral insulin sensitivity in obese hypertensive patients,9 whereas omapatrilat, a dual angiotensin-converting-enzyme–neprilysin inhibitor, has potent insulin-sensitising effects experimentally, increasing myocardial glucose uptake in Zucker fatty rats,10 although there are no data on the effect of omapatrilat on insulin sensitivity and glycaemic control in humans. We therefore investigated the effect of sacubitril/valsartan versus enalapril on HbA1c in patients with diabetes and HFrEF enrolled in PARADIGM-HF, as well as the influence of these regimens on the initiation of oral antihyperglycaemic and insulin therapy in patients with diabetes.
Methods
Study design and participants
We did a post-hoc analysis of the PARADIGM-HF study, a multicentre, double-blind, parallel group, randomised active-controlled trial. Patients with HFrEF (left ventricular ejection fraction [LVEF] ≤40%) and elevated natriuretic peptides5 were randomly assigned to receive either sacubitril/valsartan 97 mg/103 mg twice a day or enalapril 10 mg twice a day. Full details of the trial design, entry criteria, and main results have been previously reported.5,11,12 New-onset of diabetes was a prespecified exploratory outcome in PARADIGM-HF and was diagnosed based on blood glucose levels meeting American Diabetes Association criteria or initiation of oral hypoglycaemic drugs, insulin sensitisers, insulin therapy, or HbA1c concentrations of 6·5% or more. The cases of new-onset of diabetes were adjudicated by an independent adjudication committee. The trial was approved by local ethics committees and all patients provided written informed consent.
In this study, the primary analysis was based on a subset of 3778 of the 8399 randomly assigned patients who reported a history of diabetes, had HbA1c concentrations of 6·5% or more, or both at screening.13 Among these patients, most (98%) had type 2 diabetes. Patients diagnosed with HbA1c of 6·5% or more at screening visit were considered as having diabetes from that date. The date of diabetes diagnosis in patients with a previous history of diabetes was assessed based on medical record review or self-report at the screening visit. Glycaemic control was assessed by measuring HbA1c concentration at screening, 1-year, 2-year, and 3-year visits. In order to further describe HbA1c changes from screening to the 1-year visit, we created categories of HbA1c (<6·5%, 6·5%–6·9%, 7%–7·9%, and ≥8%). Additionally, we analysed changes in triglycerides, HDL cholesterol, and body BMI during the course of the study. We further analysed time to oral antihyperglycaemic and insulin therapy initiation during follow-up. All assays included in this analysis were measured in a central laboratory.
HbA1c concentrations were measured by the BioRad D-10 Haemoglobin A1c Program as the percentage determination of HbA1c using ion-exchange high-performance liquid chromatography.14 The reportable range for this assay is 3·8%–18·5%, with any sample greater than 15% being suspected as having a haemoglobin variant. HDL cholesterol and triglycerides were measured with the Roche Boehringer Mannheim Diagnostics assay, by enzymatic in-vitro methodology.15,16 The measuring range of HDL cholesterol was 0·08–3·10 mmol/L and for triglycerides it was 0·05–11·4 mmol/L.
Statistical analysis
Descriptive data are presented as the mean (SD) for normally distributed variables and as median (IQR) for non-normally distributed variables. Categorical variables are expressed as proportions and were compared by the chi-square test. All continuous variables were compared using t-tests, with the exception of the duration of diabetes and triglycerides, which were compared using Wilcoxon rank-sum tests. Changes in HbA1c, triglycerides, HDL cholesterol, and BMI were assessed and compared at 1 year, 2 years, and 3 years after randomisation. Changes at annual visits were assessed via linear regression with adjustment for screening values. Overall changes were assessed in a mixed effects longitudinal analysis model. Time to initiation of oral antihyperglycaemic drugs and insulin in participants previously not treated with these drugs was compared using survival analysis techniques, including Kaplan-Meier estimates and Cox proportional hazards models. In order to assess whether the difference in time to initiation of insulin between groups could be attributed to more frequent hospital admission, we did a sensitivity analysis that censored patient follow-up at the time of the first post-randomisation hospital admission. Two-sided p values of <0·05 were considered significant. Analyses were done with Stata (version 14.1).
Role of the funding source
The funder of the study was involved in the PARADIGM-HF study design and protocol development, and data collection. The presented analysis was prepared jointly by all authors. The data analysis was done by the academic authors (SDS, BC, JPS) at Brigham and Women’s Hospital. The corresponding and coauthors had full access to the data in the study, contributed to the writing of the manuscript, and had final responsibility for the decision to submit for publication.
Results
Of the 8399 patients with HFrEF enrolled in PARADIGM-HF, 3778 (45%) patients were identified at screening as having diabetes based on their medical history (n=2896 [34%]) or a screening HbA1c concentration of 6·5% or higher without a reported diagnosis of diabetes (n=882 [11%]). Characteristics of patients with diabetes at screening are presented in table 1. The mean age of patients was 64 years (SD 11), and the majority were men (2970 [79%] of 3778) and white (2533 [67%] of 3778). The mean BMI was 29·03 kg/m2 (SD 5·78), and mean systolic and diastolic blood pressure were 129 mm Hg (SD 17) and 78 mm Hg (SD 11), respectively. Most patients were New York Heart Association (NYHA) functional class 2 (2321 [61%]) or 3 (1376 [36%]).17 2896 (77%) patients had a previous diagnosis of diabetes with a median duration of 3·5 years, and a screening mean HbA1c of 7·44% (SD 1·55). More than half of the patients (57%) used antihyperglycaemic therapy at screening, mostly metformin, sulfonylureas, and insulin. Among the participants with diabetes, there were no significant differences between the two treatment groups in baseline characteristics, except for triglycerides, which were lower in the sacubitril/valsartan group (table 1). Comparison of baseline characteristics of patients with diabetes and those without diabetes at screening is presented in the appendix (p 2).
Table 1.
Baseline characteristics of patients with diabetes overall and by treatment groups, at screening
| All patients with diabetes (n=3778) | Patients receiving enalapril (n=1874) | Patients receiving sacubitril/valsartan (n=1904) | |
|---|---|---|---|
| Age (years) | 64·1 (10·6) | 63·8 (10·4) | 64·4 (10·7) |
|
| |||
| Sex | |||
| Women | 808 (21%) | 416 (22%) | 392 (21%) |
| Men | 2970 (79%) | 1458 (78%) | 1512 (79%) |
|
| |||
| Race | |||
| White | 2533 (67%) | 1254 (67%) | 1279 (67%) |
| Black | 176 (5%) | 86 (45%) | 90 (5%) |
| Asian | 740 (20%) | 367 (20%) | 373 (20%) |
| Other | 329 (9%) | 167 (9%) | 162 (9%) |
|
| |||
| Previous history of diabetes | 2896 (77%) | 1450 (77%) | 1446 (76%) |
|
| |||
| Duration of diabetes (years) | 3·5 (0–10·3) | 3·7 (0–10·4) | 3·2 (0–10·2) |
|
| |||
| BMI (kg/m2) | 29·03 (5·78) | 29·01 (5·69) | 29·05 (5·87) |
|
| |||
| Systolic blood pressure (mm Hg) | 129 (17) | 129 (17) | 129 (17) |
|
| |||
| Diastolic blood pressure (mm Hg) | 78 (11) | 78 (11) | 78 (10) |
|
| |||
| NYHA functional class | |||
| 1 | 12 (<1%) | 6 (<1%) | 6 (<1%) |
| 2 | 2321 (61%) | 1157 (62%) | 1164 (61%) |
| 3 | 1376 (36%) | 679 (36%) | 697 (37%) |
| 4 | 64 (2%) | 31 (2%) | 33 (2%) |
|
| |||
| HbA1c (%) | 7·44 (1·55) | 7·48 (1·58) | 7·41 (1·51) |
|
| |||
| Total cholesterol (mmol/L) | 4·4 (1·2) | 4·4 (1·2) | 4·3 (1·2) |
|
| |||
| LDL cholesterol( mmol/L) | 2·4 (1·0) | 2·4 (1·0) | 2·4 (0·9) |
|
| |||
| HDL cholesterol (mmol/L) | 1·15 (0·34) | 1·16 (0·34) | 1·15 (0·33) |
|
| |||
| Triglycerides (mmol/L) | 1·51 (1·07–2·18) | 1·55 (1·10–2·23) | 1·47 (1·05–2·11) |
|
| |||
| Creatinine (μmol/L) | 100·3 (26·2) | 99·8 (26·2) | 100·9 (26·2) |
|
| |||
| eGFR (mL/min per 1·73m2) | 66·7 (19·3) | 67·1 (19·6) | 66·4 (19·0) |
|
| |||
| Medical history | |||
| Hypertension | 2922 (77%) | 1446 (77%) | 1476 (78%) |
| Atrial fibrillation | 1407 (37%) | 715 (38%) | 692 (36%) |
| Hospital admission for heart failure | 2474 (65%) | 1233 (66%) | 1241 (65%) |
| Myocardial infarction | 1781(47%) | 880 (47%) | 901 (47%) |
| Stroke | 336 (9%) | 176 (9%) | 160 (8%) |
|
| |||
| Treatment | |||
| ACE inhibitor | 2876 (76%) | 1416 (76%) | 1460 (77%) |
| Angiotensin-receptor blocker | 917 (24%) | 466 (25%) | 451 (24%) |
| Diuretic | 3181 (84%) | 1570 (84%) | 1611 (85%) |
| β-blocker | 3504 (93%) | 1738 (93%) | 1766 (93%) |
| Antihyperglycaemic drugs (total) | 2164 (57%) | 1093 (58%) | 1072 (56%) |
| Metformin | 868 (23%) | 416 (22%) | 452 (24%) |
| Sulfonylurea | 799 (21%) | 396 (21%) | 403 (21%) |
| Thiazolidinediones | 28 (1%) | 18 (1%) | 10 (1%) |
| Alpha-glucosidase inhibitors | 74 (2%) | 38 (2%) | 36 (2%) |
| Glinides | 43 (1%) | 18 (1%) | 25 (1%) |
| Dipeptidyl peptidase 4 inhibitors | 107 (3%) | 62 (3%) | 45 (2%) |
| GLP-1 receptor agonists | 11 (<1%) | 7 (<1%) | 4 (<1%) |
| Insulin | 715 (19%) | 375 (20%) | 340 (18%) |
Data are n (%), mean (SD), or median (IQR). There were no significant differences between groups at screening, except for triglycerides (p=0·016). eGFR=estimated glomerular filtration rate. ACE=angiotensin-converting-enzyme. GLP=glucagon-like peptide. HbA1c=glycated haemoglobin.
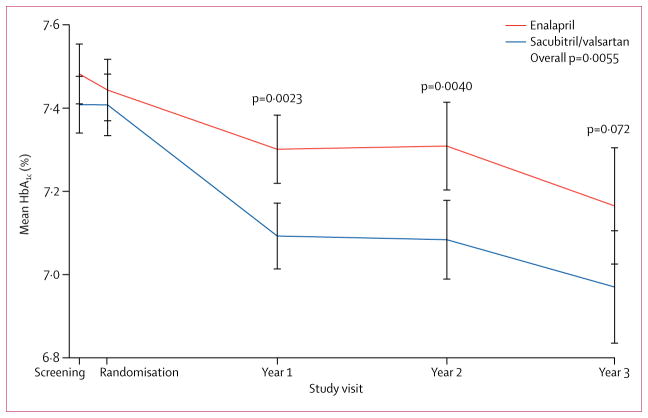
In PARADIGM-HF, there were 39 of 2741 (1%) cases of incident diabetes reported in the sacubitril/valsartan group and 44 of 2762 (2%) in the enalapril group among those with no known history of diabetes at screening (p=0·63). After excluding 912 patients who were identified as having HbA1c concentrations of 6·5% or greater (440 in the enalapril group and 472 in the sacubitril/valsartan group), there were 33 (1·4%) cases of incident diabetes in the sacubitril/valsartan group and 31 cases (1·3%) in the enalapril group (p=0·73). Among patients with diabetes at screening, there were no significant differences in HbA1c concentrations between randomised groups (table 2). During the first year of follow-up, HbA1c decreased by 0·16% (SD 1·40) in the enalapril group and 0·26% (SD 1·25) in the sacubitril/valsartan group (between-group reduction 0·13, 95% CI 0·05–0·22, p=0·0023 compared with baseline), and the estimated reduction was similar at years 2 and 3.
Table 2.
HbA1c concentrations (%) by treatment groups, over the course of four visits
| Enalapril (n=1874) | Sacubitril/valsartan (n=1904) | Adjusted for screening values | ||
|---|---|---|---|---|
| Difference (95% CI) | p value | |||
| Screening | 7·48 (1·58) | 7·41 (1·51) | ·· | ·· |
| 1-year | 7·30 (1·66) | 7·09 (1·60) | −0·13 (−0·22 to −0·05) | 0·0023 |
| 2-year | 7·31 (1·78) | 7·08 (1·61) | −0·17 (−0·28 to −0·05) | 0·0040 |
| 3-year | 7·16 (1·61) | 6·97 (1·58) | −0·15 (−0·32 to 0·01) | 0·072 |
| Overall* | ·· | ·· | −0·14 (−0·23 to −0·06) | 0·0055 |
Data mean (SD). Number of patients with measurements of HbA1c at screening=3765, 1-year=3160, 2-year=2219, and 3-year=1040.
Longitudinal model.
Over the full duration of follow-up, the decrease in HbA1c was significantly greater in patients receiving sacubitril/valsartan compared with those receiving enalapril (overall reduction 0·14%, 95% CI 0·06–0·23, p=0·0055; table 2, figure 1). The HbA1c reduction from sacubitril/valsartan was apparent only in patients identified as having diabetes at screening, with no treatment effect seen in patients without diabetes (appendix p 3). However, within the diabetes cohort, there was no significant relationship between screening HbA1c concentrations and the magnitude of the treatment effect. For patients with HbA1c concentrations of 8% or higher at screening, those in the sacubitril/valsartan group were more likely to change to a lower HbA1c category at the 1-year visit than those in the enalapril group (41% vs 33%, p=0·025; appendix, p 5). For patients with a 6·5%–6·9% or 7–7·9% concentration at screening, patients in the sacubitril/valsartan group were more likely to have moved to a lower HbA1c category and less likely to have moved to a higher HbA1c category compared to those receiving enalapril (net difference 11%, p=0·020 and 14%, p=0·017, respectively; appendix p 5). In a landmark analysis at 1 year considering the change in HbA1c and the screening value, we observed no significant relationship between change in HbA1c and the composite primary outcome of cardiovascular death or first hospital admission for heart failure in the entire cohort of patients with diabetes (hazard ratio [HR] 0·99, 95% CI 0·91–1·06 per HbA1c unit, p=0·70), suggesting that the potential benefit on heart failure outcomes and on HbA1c were independent of one another.
Figure 1.
Changes in mean HbA1c and confidence intervals by treatment group at screening, randomisation, 1-year, 2-year, and 3-year visits
In addition, the sacubitril/valsartan treatment effect HR for the primary PARADIGM-HF outcome in participants with diabetes was 0·84 (95% CI 0·74–0·95, p=0·0043)—similar to the overall treatment effect for the entire cohort (HR 0·80, 95% CI 0·73–0·87, p<0·001).5 As previously reported, we observed a marginal interaction between known diabetes status and the secondary endpoint of cardiovascular death, suggesting an attenuated benefit in patients with diabetes (pinteraction=0·052). Nevertheless, cardiovascular deaths were reduced in the sacubitril/valsartan group among patients with diabetes (appendix p 6).
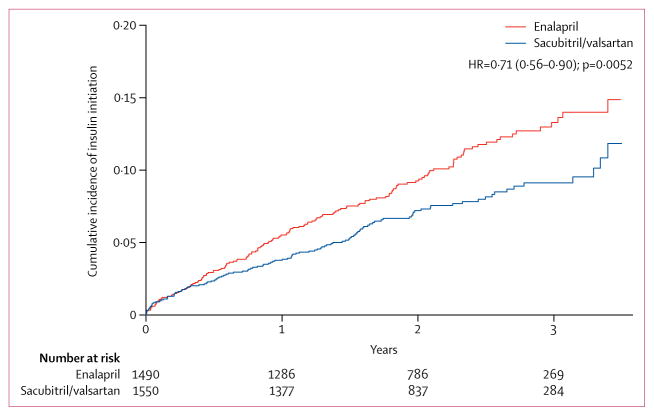
Among patients with diabetes who were insulin-naive at the time of randomisation, 153 (10%) patients in the enalapril group and 114 (7%) in the sacubitril/valsartan group were initiated on insulin therapy (HR 0·71, 95% CI 0·56–0·90, p=0·0052; table 3, figure 2). In a sensitivity analysis that censored patient follow-up at the time of the first post-randomisation hospital admission, the reduction in insulin initiation in the sacubitril/valsartan group (HR 0·69, 95% CI 0·50–0·97, p=0·031) remained consistent with the overall results. Similarly, fewer patients were started on oral antihyperglycaemic therapy (HR 0·77, 95% CI 0·58–1·02, p=0·073) in the sacubitril/valsartan group, although the difference did not reach statistical significance. Of those patients not identified as having diabetes at screening, the percentages of patients initiating antihyperglycaemic therapy post-randomisation were not significantly different between the enalapril and sacubitril/valsartan groups (insulin: 21 [0·9%] vs 15 [0·7%] p=0·35; oral antihyperglycaemic agents: 44 [1·9%] vs 50 [2·2%] p=0·46; either: 57 [2·4%] vs 63 [2·8%], p=0·49).
Table 3.
Cumulative incidence of new initiation of insulin therapy in patients with diabetes not on insulin at screening during the course of follow-up in PARADIGM-HF, by treatment assignment
| Enalapril (n=1490) | Sacubitril/valsartan (n=1550) | p value | |
|---|---|---|---|
| Overall | 153 (10%) | 114 (7%) | 0·0050 |
|
| |||
| Cumulative incidence over time | |||
| 1-year visit | 5·5% (4·4–6·8) | 3·8% (2·9–4·9) | 0·025 |
| 2-year visit | 9·3% (7·8–11·0) | 7·2% (5·9–8·7) | 0·057 |
| 3-year visit | 13·3% (11·2–15·7) | 9·1% (7·5–11·0) | 0·0037 |
|
| |||
| Incidence rate (per 100 person-years) | 5·0 (4·2–5·8) | 3·5 (2·9–4·2) | ·· |
|
| |||
| Hazard ratio | Reference | 0·71 (0·56–0·90) | 0·0052 |
Data are n (%) and 95% CI.
Figure 2.
Kaplan-Meier curve showing time to insulin initiation in the sacubitril/valsartan and enalapril groups, in patients previously not treated with insulin
During the trial follow-up, there were 97 hypoglycaemic events in patients with diabetes at screening, with 44 events occurring in patients receiving enalapril and 53 events in patients receiving sacubitril/valsartan (HR 1·18, 95% CI 0·79–1·76, p=0·42).
There was no consistent difference in triglyceride levels throughout the study. However, HDL cholesterol levels increased significantly by 0·02 mmol/L (95% CI 0·00–0·03) during the course of the trial in patients using sacubitril/valsartan compared with those using enalapril (p=0·043, table 4). BMI increased by an average of 0·28 kg/m2 (95% CI 0·14–0·41) over the course of follow-up in patients randomly assigned to sacubitril/valsartan, compared with those receiving enalapril (p<0·0001; table 4).
Table 4.
Changes in triglycerides and HDL-cholesterol concentrations, and body-mass index by treatment groups, over the course of four visits
| Enalapril (n=1874) | Sacubitril/valsartan (n=1904) | Adjusted for screening values | ||
|---|---|---|---|---|
|
| ||||
| Difference (95% CI) | p value | |||
| Triglycerides (mmol/L) | ||||
|
| ||||
| Screening | 1·88 (1·56) | 1·79 (1·15) | ·· | ·· |
| 1-year | 1·93 (1·46) | 1·95 (1·52) | 0·08 (−0·01 to 0·17) | 0·094 |
| 2-year | 2·04 (1·82) | 1·87 (1·22) | −0·16 (−0·27 to −0·04) | 0·0083 |
| 3-year | 1·92 (1·28) | 1·84 (1·57) | −0·06 (−0·22 to 0·10) | 0·46 |
| Overall* | ·· | ·· | −0·01 (−0·09 to 0·07) | 0·83 |
|
| ||||
| HDL cholesterol (mmol/L) | ||||
|
| ||||
| Screening | 1·16 (0·34) | 1·15 (0·33) | ·· | ·· |
| 1-year | 1·15 (0·35) | 1·16 (0·35) | 0·01 (−0·01 to 0·02) | 0·50 |
| 2-year | 1·15 (0·34) | 1·17 (0·36) | 0·03 (0·01 to 0·05) | 0·0073 |
| 3-year | 1·15 (0·33) | 1·21 (0·38) | 0·06 (0·02 to 0·09) | 0·0011 |
| Overall* | ·· | ·· | 0·02 (0·00 to 0·03) | 0·043 |
|
| ||||
| BMI (kg/m2) | ||||
|
| ||||
| Screening | 29·01 (5·69) | 29·05 (5·87) | ·· | ·· |
| 1-year | 29·23 (5·70) | 29·58 (5·83) | 0·26 (0·13 to 0·40) | 0·0001 |
| 2-year | 29·07 (5·77) | 29·68 (5·72) | 0·31 (0·12 to 0·49) | 0·0012 |
| 3-year | 29·57 (6·07) | 29·65 (5·67) | 0·27 (−0·02 to 0·57) | 0·065 |
| Overall* | ·· | ·· | 0·28 (0·14 to 0·41) | <0·0001 |
Data are mean (SD).
Longitudinal model.
Discussion
In this post-hoc analysis of patients with mostly type 2 diabetes and HFrEF from the PARADIGM-HF study, we found that treatment with sacubitril/valsartan was associated with greater reductions in HbA1c concentrations than treatment with enalapril. Sacubitril/valsartan was shown in PARADIGM-HF to reduce the risk of cardiovascular death, hospital admission for heart failure, and all-cause mortality compared with enalapril in patients with heart failure, and this benefit was observed in both those with and without diabetes at screening.5 The rates of these outcomes increased with increasing HbA1c at baseline6. In this post-hoc analysis of patients with diabetes and HFrEF from the PARADIGM-HF study, we found that treatment with sacubitril/valsartan was associated with greater reductions in HbA1c concentrations than treatment with enalapril. Moreover, during the 3-year course of the study, fewer participants in the sacubitril/valsartan group required initiation of insulin therapy for glycaemic control. These data suggest that in addition to the heart failure benefits previously shown, sacubitril/valsartan might have favourable metabolic effects in patients with heart failure and diabetes.
Sacubitril/valsartan blocks both the renin-angiotensin system and inhibits neprilysin, an enzyme expressed in a wide variety of tissues (endothelial, epithelial and smooth muscle cells, cardiac myocytes, and adipocytes)18,19 and which is responsible for the breakdown of a number of vasoactive peptides, including the biologically active natriuretic peptides, as well as bradykinin, angiotensin I and II, and glucagon-like peptide 1 (GLP-1).20 There are several potential mechanisms by which inhibition of neprilysin might lead to improvement in glycaemic control. Natriuretic peptides, which are increased by neprilysin inhibition, might have a crucial role in insulin sensitivity and metabolism. Neprilysin is known to promote lipid mobilisation from adipose tissue,21 increase postprandial lipid oxidation,22 promote adiponectin release,23 and enhance muscular oxidative capacity.24 Blood glucose concentrations have been shown to decrease after infusion of B-type natriuretic peptide (BNP).25
In the Atherosclerosis Risk in Communities study (n=7822) with a median follow-up of 12 years, higher concentrations of N-terminal proBNP were associated with a significantly decreased risk of diabetes, even after adjustment for traditional risk factors and fasting glucose.26 Augmentation of other neprilysin substrates by neprilysin inhibition might also play a part in glycaemic control. Bradykinin, a neprilysin substrate, can improve insulin sensitivity and attenuate lipolysis.27 Cyclic guanosine monophosphate, also increased by neprilysin inhibition, has known vasodilatory effects in skeletal muscle and facilitates lipolysis.20 Moreover, GLP-1, a neuropeptide of the incretin family and potent antihyperglycaemic hormone with a very short circulating half-life, is partially degraded by neprilysin.28 In high-fat-fed neprilysin deficient mice, improved glycaemic status was associated with elevated active GLP-1 concentrations, reduced plasma dipeptidyl peptidase 4 (DPP-4) activity and improved beta cell function, suggesting beneficial metabolic effects of neprilysin inhibition.29 Inhibition of the renin-angiotensin system has also improved glycaemic control,30,31 and angiotensin II promotes insulin resistance, while angiotensin-1-receptor blockade modestly improves insulin sensitivity.32 Nevertheless, the improvement of glucose metabolism by renin-angiotensin system inhibition alone is most likely to be modest.
Dual ACE-neprilysin inhibitors improve insulin sensitivity in preclinical studies. Inhibition of neprilysin with the dual ACE-neprilysin inhibitor omapatrilat improved whole-body insulin-mediated glucose disposal, induced profound insulin sensitisation, and increased myocardial glucose uptake in obese insulin-resistant Zucker rats.10 Also, both acute and long-term dual ACE-neprilysin inhibition with mixanpril improved whole-body insulin-mediated glucose disposal and insulin sensitivity in obese Zucker rats.33 In a study comparing the effects of sacubitril/valsartan and amlodipine on insulin resistance in 92 obese hypertensive patients treated for 8 weeks, those treated with sacubitril/valsartan showed a significant increase in insulin sensitivity using the hyperinsulinaemic-euglycaemic clamp technique.9
The magnitude of HbA1c reduction seen with sacubitril/valsartan in the patients with diabetes enrolled in PARADIGM-HF was smaller compared with that observed in studies of DPP-4 inhibitors,34–36 GLP-1 receptor agonists,37–39 and SGLT-2 inhibitors,40 in which investigators were also able to modify standard of care for diabetes at will. However, in all of these trials, these novel drugs were compared with placebo. By contrast, PARADIGM-HF was an active-controlled study comparing sacubitril/valsartan with an ACE inhibitor. Whether the magnitude of reduction in HbA1c would have been greater if the drug were compared with placebo is unknown. Moreover, our protocol allowed for individual physicians to adjust the doses of antihyperglycaemic therapy at will, and more patients in the enalapril group initiated use of oral antihyperglycaemic drugs including insulin, which would be expected to attenuate treatment differences. Because patients treated with sacubitril/valsartan had a greater increase in BMI, the reduction in HbA1c is unlikely to be due to weight loss. Of note, 23% of patients included in this cohort were diagnosed with a screening HbA1c concentration of 6·5% or more and did not have previous history of diabetes. This finding highlights the high prevalence of undiagnosed diabetes in patients with heart failure.
Several limitations of this analysis should be noted. Despite positive effects on glycaemic control shown in this analysis, sacubitril/valsartan did not reduce the pre-specified exploratory outcome of new-onset diabetes in comparison with enalapril, probably due to a very small number of new-onset diabetes cases (n=84) during the course of the trial. However, we believe that this endpoint might have been less sensitive to the effect of sacubitril/valsartan on glycaemic control for several reasons. First, this population would not be expected to be at especially high risk for developing diabetes, and indeed less than 2% of patients in the trial without diabetes at screening developed diabetes. Based on the limited number of events actually observed in the trial, the study would have been sufficiently powered only to detect reductions of approximately 50% or greater. Second, the determination of new-onset diabetes was clinical, and not based on periodic assessment. Although plasma glucose was later included in the routine biochemistry panel in PARADIGM-HF, this was not necessarily fasting by protocol, and we were less able to discern the effect of sacubitril/valsartan on this measure of glycaemic control. Nevertheless, HbA1c is considered to be a more stable and accurate measure of long-term glycaemia, correlating best with mean blood glucose over the previous 8–12 weeks, and also reflecting glycaemic changes during the day. We had no direct or indirect measures of insulin resistance in PARADIGM-HF. Whether sacubitril/valsartan would have a similar effect in patients with insulin resistance and earlier phases of glycaemic impairment remains unknown. Although we have start and stop data for antihyperglycaemic drugs, dosage was not assessed by protocol, therefore we were not able to determine dose changes in diabetes regimens. Finally, HbA1c assays can be confounded by haemoglobinopathies and therefore perform less accurately in ethnic minority populations. Nevertheless, any reduction in precision induced by the specific assay or the characteristics of the patient population would be expected to weaken the observed effects of therapy, which was randomised, and therefore not differentially affected by any of these factors.
In summary, we found that treatment with sacubitril/valsartan resulted in improved glycaemic control as shown by lower HbA1c concentrations compared with patients treated with enalapril for patients with diabetes and HFrEF. This beneficial metabolic effect is most likely secondary to the inhibition of neprilysin and consequent modulation of its circulating substrates. These post-hoc findings should be considered hypothesis-generating and should help inform clinicians who will be using sacubitril/valsartan in patients with heart failure, especially because doses of insulin or other antihyperglycaemic drugs might need to be adjusted if HbA1c concentrations decrease. Moreover, these data might encourage additional research into the beneficial metabolic properties of drugs of this class.
Research in context.
Evidence before this study
We searched PubMed for articles published up to Oct 11, 2016, using terms related to neprilysin inhibition, diabetes, and insulin resistance, with no language restrictions, to identify possible effects of this class of drugs on glycaemic control. There is evidence from both preclinical and human studies on the effect of neprilysin inhibitors on insulin sensitivity. Both omapatrilat and mixanpril, dual inhibitors of neprilysin and angiotensin-converting enzyme, improved insulin sensitivity in obese insulin-resistant Zucker rats, and sacubitril/valsartan (an angiotensin receptor neprilysin inhibitor) improved peripheral insulin sensitivity in obese hypertensive patients, compared with patients treated with amlodipine.
Added value of this study
To our knowledge, this is the first study investigating the effect of sacubitril/valsartan on glycaemic control. Our results showed that in patients with diabetes and heart failure with reduced ejection fraction, treatment with sacubitril/valsartan resulted in improved glycaemic control compared with enalapril. Also, fewer participants in the sacubitril/valsartan group required initiation of insulin therapy throughout the course of the study. Our findings suggest that sacubitril/valsartan, which has already been proven to reduce morbidity and mortality in heart failure, might provide additional metabolic benefits in patients with diabetes. Moreover, these data might suggest that patients with diabetes taking sacubitril/valsartan for treatment of heart failure might require dose adjustment of antihyperglycaemic therapy.
Implications of all the available evidence
Although our findings were post-hoc and hypothesis-generating, they should help to inform clinicians using sacubitril/valsartan in patients with heart failure of its potential beneficial effect on glycaemic control. Moreover, these data might encourage additional research into the beneficial metabolic properties of drugs of this class.
Acknowledgments
Funding Novartis.
Footnotes
Contributors
All authors contributed to the interpretation of the results, writing or revision of the manuscript, and approved the decision to submit the article for publication.
Declaration of interests
During the conduct of the PARADIGM-HF study, MP, MRZ, JLR, KS, ASD, JJVM, and SDS report either grant, personal, non-financial, or other support from Novartis, the sponsor of the study. ML and VCS report personal fees as employees of Novartis. Outside of the submitted work, MP reports personal fees from Amgen, BioControl, Cytokinetics, CardioKinetix, CardioMEMS, Cardiorentis, Janssen, Novartis, Pfizer, and Sanofi. MRZ reports grant support and personal fees from Bayer, CVRx, Medtronic, and Novartis; KS reports personal fees from Novartis, Amgen, and Servier. ASD reports personal fees from Novartis, Janssen, Sanofi, Merck, St. Jude Medical, AstraZeneca, and Relypsa. JJVM reports non-financial and other support from Novartis. JPS, BC, SBS, and EWS declare no competing interests.
References
- 1.McMurray JJ, Gerstein HC, Holman RR, Pfeffer MA. Heart failure: a cardiovascular outcome in diabetes that can no longer be ignored. Lancet Diabetes Endocrinol. 2014;2:843–51. doi: 10.1016/S2213-8587(14)70031-2. [DOI] [PubMed] [Google Scholar]
- 2.Pocock SJ, Wang D, Pfeffer MA, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. 2006;27:65–75. doi: 10.1093/eurheartj/ehi555. [DOI] [PubMed] [Google Scholar]
- 3.Badar AA, Perez-Moreno AC, Hawkins NM, et al. Clinical characteristics and outcomes of patients with angina and heart failure in the CHARM (Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity) programme. Eur J Heart Fail. 2015;17:196–204. doi: 10.1002/ejhf.221. [DOI] [PubMed] [Google Scholar]
- 4.Velazquez EJ, Pfeffer MA, McMurray JV, et al. for VALIANT Investigators. VALsartan In Acute myocardial iNfarcTion (VALIANT) trial: baseline characteristics in context. Eur J Heart Fail. 2003;5:537–44. doi: 10.1016/s1388-9842(03)00112-0. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Packer M, Desai AS, et al. for PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 6.Kristensen SL, Preiss D, Jhund PS, et al. for PARADIGM-HF Investigators and Committees. Risk Related to Pre-Diabetes Mellitus and Diabetes Mellitus in Heart Failure With Reduced Ejection Fraction: Insights From Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure Trial. Circ Heart Fail. 2016;9:e002560. doi: 10.1161/CIRCHEARTFAILURE.115.002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skali H, Shah A, Gupta DK, et al. Cardiac structure and function across the glycemic spectrum in elderly men and women free of prevalent heart disease: the Atherosclerosis Risk In the Community study. Circ Heart Fail. 2015;8:448–54. doi: 10.1161/CIRCHEARTFAILURE.114.001990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerstein HC, Swedberg K, Carlsson J, et al. CHARM Program Investigators. The hemoglobin A1c level as a progressive risk factor for cardiovascular death, hospitalization for heart failure, or death in patients with chronic heart failure: an analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and Morbidity (CHARM) program. Arch Intern Med. 2008;168:1699–704. doi: 10.1001/archinte.168.15.1699. [DOI] [PubMed] [Google Scholar]
- 9.Jordan J, Stinkens R, Jax T, et al. Improved Insulin Sensitivity with Angiotensin Receptor Neprilysin Inhibition in Individuals with Obesity and Hypertension. Clin Pharmacol Ther. 2017;101:254–63. doi: 10.1002/cpt.455. [DOI] [PubMed] [Google Scholar]
- 10.Wang CH, Leung N, Lapointe N, et al. Vasopeptidase inhibitor omapatrilat induces profound insulin sensitization and increases myocardial glucose uptake in Zucker fatty rats: Studies comparing a vasopeptidase inhibitor, angiotensin-converting enzyme inhibitor, and angiotensin II type I receptor blocker. Circulation. 2003;107:1923–29. doi: 10.1161/01.CIR.0000062646.09566.CC. [DOI] [PubMed] [Google Scholar]
- 11.McMurray JJ, Packer M, Desai AS, et al. for PARADIGM-HF. Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15:1062–73. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMurray JJ, Packer M, Desai AS, et al. for PARADIGM HF Committees Investigators. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF) Eur J Heart Fail. 2014;16:817–25. doi: 10.1002/ejhf.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamberlain JJ, Rhinehart AS, Shaefer CF, Jr, Neuman A. Diagnosis and management of diabetes: synopsis of the 2016 American Diabetes Association standards of medical care in diabetes. Ann Intern Med. 2016;164:542–52. doi: 10.7326/M15-3016. [DOI] [PubMed] [Google Scholar]
- 14.Mayer TK, Freedman ZR. Protein glycosylation in diabetes mellitus: a review of laboratory measurements and of their clinical utility. Clin Chim Acta. 1983;127:147–84. doi: 10.1016/s0009-8981(83)80002-3. [DOI] [PubMed] [Google Scholar]
- 15.Sugiuchi H, Uji Y, Okabe H, et al. Direct measurement of high-density lipoprotein cholesterol in serum with polyethylene glycol-modified enzymes and sulfated α-Cyclodextrin. Clin Chem. 1995;41:717–23. [PubMed] [Google Scholar]
- 16.Wahlefeld AW, Bergmeyer HU, editors. Methods of Enzymatic Analysis. 2. New York, NY: Academic Press Inc; 1974. p. 1831. English edn. [Google Scholar]
- 17.Criteria Committee, New York Heart Association. Nomenclature and criteria for diagnosis. 6. Boston: Little, Brown and Co; 1964. Diseases of the heart and blood vessels; p. 114. [Google Scholar]
- 18.Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–69. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 19.Turner AJ. Neprilysin. In: Barret AJ, Rawlings ND, Woessner JF, editors. Handbook of Proteolytic Enzymes. Amsterdam: Elsevier; 2004. pp. 419–26. [Google Scholar]
- 20.Kobalava Z, Kotovskaya Y, Averkov O, et al. Pharmacodynamic and pharmacokinetic profiles of sacubitril/valsartan (LCZ696) in patients with heart failure and reduced ejection fraction. Cardiovasc Ther. 2016;34:191–98. doi: 10.1111/1755-5922.12183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Birkenfeld AL, Boschmann M, Moro C, et al. Lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J ClinEndocrinol Metab. 2005;90:3622–28. doi: 10.1210/jc.2004-1953. [DOI] [PubMed] [Google Scholar]
- 22.Birkenfeld AL, Budziarek P, Boschmann M, et al. Atrial natriuretic peptide induces postprandial lipid oxidation in humans. Diabetes. 2008;57:3199–204. doi: 10.2337/db08-0649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coué M, Badin PM, Vila IK, et al. Defective natriuretic peptide receptor signaling in skeletal muscle links obesity to type 2 diabetes. Diabetes. 2015;64:4033–45. doi: 10.2337/db15-0305. [DOI] [PubMed] [Google Scholar]
- 24.Engeli S, Birkenfeld AL, Badin PM, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122:4675–79. doi: 10.1172/JCI64526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinisch BB, Vila G, Resl M, et al. B-type natriuretic peptide (BNP) affects the initial response to intravenous glucose: a randomised placebo-controlled cross-over study in healthy men. Diabetologia. 2012;55:1400–05. doi: 10.1007/s00125-011-2392-1. [DOI] [PubMed] [Google Scholar]
- 26.Lazo M, Young JH, Brancati FL, et al. NH2-terminal pro-brain natriuretic peptide and risk of diabetes. Diabetes. 2013;62:3189–93. doi: 10.2337/db13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mori MA, Sales VM, Motta FL, et al. Kinin B1 receptor in adipocytes regulates glucose tolerance and predisposition to obesity. PLoS One. 2012;7:e44782. doi: 10.1371/journal.pone.0044782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plamboeck A, Holst JJ, Carr RD, Deacon CF. Neutral endopeptidase 24.11 and dipeptidyl peptidase IV are both mediators of the degradation of glucagon-like peptide 1 in the anaesthetised pig. Diabetologia. 2005;48:1882–90. doi: 10.1007/s00125-005-1847-7. [DOI] [PubMed] [Google Scholar]
- 29.Willard JR, Barrow BM, Zraika S. Improved glycaemia in high-fat-fed neprilysin-deficient mice is associated with reduced DPP-4 activity and increased active GLP-1 levels. Diabetologia. 2016 doi: 10.1007/s00125-016-4172-4. published online Dec 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jing F, Mogi M, Horiuchi M. Role of renin-angiotensin-aldosterone system in adiposetissue dysfunction. Molecular Cell Endocrinol. 2013;378:23–28. doi: 10.1016/j.mce.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 31.Messerli FH, Weber MA, Brunner HR. Angiotensin II receptor inhibition: a new therapeutic principle. Arch Intern Med. 1996;156:1957–2019. [PubMed] [Google Scholar]
- 32.van der Zijl NJ, Moors CC, Goossens GH, Hermans MM, Blaak EE, Diamant M. Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: a randomized controlled trial. Diabetes Care. 2011;34:845–51. doi: 10.2337/dc10-2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arbin V, Claperon N, Fournié-Zaluski MC, Roques BP, Peyroux J. Effects of dual angiotensin-converting enzyme and neutral endopeptidase 24-11 chronic inhibition by mixanpril on insulin sensitivity in lean and obese Zucker rats. J Cardiovasc Pharmacol. 2003;41:254–64. doi: 10.1097/00005344-200302000-00015. [DOI] [PubMed] [Google Scholar]
- 34.White WB, Bakris GL, Bergenstal RM, et al. EXamination of cArdiovascular outcoMes with alogliptIN versus standard of carE in patients with type 2 diabetes mellitus and acute coronary syndrome (EXAMINE): a cardiovascular safety study of the dipeptidyl peptidase 4 inhibitor alogliptin in patients with type 2 diabetes with acute coronary syndrome. Am Heart J. 2011;162:620–26. doi: 10.1016/j.ahj.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 35.Scirica BM, Bhatt DL, Braunwald E, et al. for SAVOR-TIMI 53 Steering Committee and Investigators. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med. 2013;369:1317–26. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 36.Green JB, Bethel MA, Armstrong PW, et al. for TECOS Study Group. Effect of sitagliptin on cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2015;373:232–42. doi: 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 37.Pfeffer MA, Claggett B, Diaz R, et al. for ELIXA Investigators. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N Engl J Med. 2015;373:2247–57. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 38.Marso SP, Daniels GH, Brown-Frandsen K, et al. for LEADER Steering Committee, LEADER trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marso SP, Bain SC, Consoli A, et al. for SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 40.Zinman B, Wanner C, Lachin JM, et al. for EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–28. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]