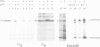Abstract
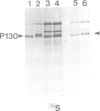
Site-directed mutagenesis of the Fujinami sarcoma virus (FSV) genome has suggested that Tyr 1073 of the P130gag--fps protein-tyrosine kinase is a regulatory site. To investigate directly the ability of tyrosine phosphorylation to affect P130gag--fps kinase activity, the phosphotyrosyl phosphatase inhibitor orthovanadate and partially purified phosphotyrosyl phosphatases were used to manipulate the stoichiometry of P130gag--fps phosphorylation. Phosphorylation of P130gag--fps at Tyr 1073 correlated with enhanced kinase activity. The thermolabile phosphorylation, kinase activity and transforming ability of P140gag--fps encoded by a temperature-sensitive (ts)FSV variant were restored at the non-permissive temperature for transformation by incubation of infected cells with orthovanadate. In this case tyrosine phosphorylation can apparently functionally reactivate a conditionally defective v-fps kinase activity. These data suggest that reversible autophosphorylation at a conserved tyrosine within the v-fps kinase domain is a positive regulator of enzymatic activity and biological function. Phenotypic suppression of the tsFSV genetic defect by orthovanadate emphasizes the potential importance of phosphotyrosyl phosphatases in antagonizing tyrosine kinase action. It is suggested that autophosphorylation may constitute a molecular switch by which some protein-tyrosine kinases are activated.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkins B., Hunter T., Beemon K. Expression of the PRC II avian sarcoma virus genome. J Virol. 1982 Mar;41(3):767–780. doi: 10.1128/jvi.41.3.767-780.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adkins B., Hunter T. Two structurally and functionally different forms of the transforming protein of PRC II avian sarcoma virus. Mol Cell Biol. 1982 Aug;2(8):890–896. doi: 10.1128/mcb.2.8.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. J., Gordon J. A. The stimulation of pp60v-src kinase activity by vanadate in intact cells accompanies a new phosphorylation state of the enzyme. J Biol Chem. 1984 Aug 10;259(15):9580–9586. [PubMed] [Google Scholar]
- Carmier J. F., Samarut J. Chicken myeloid stem cells infected by retroviruses carrying the v-fps oncogene do not require exogenous growth factors to differentiate in vitro. Cell. 1986 Jan 17;44(1):159–165. doi: 10.1016/0092-8674(86)90494-0. [DOI] [PubMed] [Google Scholar]
- Cartwright C. A., Kaplan P. L., Cooper J. A., Hunter T., Eckhart W. Altered sites of tyrosine phosphorylation in pp60c-src associated with polyomavirus middle tumor antigen. Mol Cell Biol. 1986 May;6(5):1562–1570. doi: 10.1128/mcb.6.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett M. S., Belzer S. K., Purchio A. F. Structurally and functionally modified forms of pp60v-src in Rous sarcoma virus-transformed cell lysates. Mol Cell Biol. 1984 Jul;4(7):1213–1220. doi: 10.1128/mcb.4.7.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Esch F. S., Taylor S. S., Hunter T. Phosphorylation sites in enolase and lactate dehydrogenase utilized by tyrosine protein kinases in vivo and in vitro. J Biol Chem. 1984 Jun 25;259(12):7835–7841. [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Sefton B. M., Hunter T. Detection and quantification of phosphotyrosine in proteins. Methods Enzymol. 1983;99:387–402. doi: 10.1016/0076-6879(83)99075-4. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coussens P. M., Cooper J. A., Hunter T., Shalloway D. Restriction of the in vitro and in vivo tyrosine protein kinase activities of pp60c-src relative to pp60v-src. Mol Cell Biol. 1985 Oct;5(10):2753–2763. doi: 10.1128/mcb.5.10.2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Hanafusa H. Local mutagenesis of Rous sarcoma virus: the major sites of tyrosine and serine phosphorylation of pp60src are dispensable for transformation. Cell. 1983 Sep;34(2):597–607. doi: 10.1016/0092-8674(83)90392-6. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Gabrilove J. L., Tam J. P., Moore M. A., Hanafusa H. Specific expression of the human cellular fps/fes-encoded protein NCP92 in normal and leukemic myeloid cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2379–2383. doi: 10.1073/pnas.82.8.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman R. A., Wang E., Hanafusa H. Cytoplasmic localization of the transforming protein of Fujinami sarcoma virus: salt-sensitive association with subcellular components. J Virol. 1983 Feb;45(2):782–791. doi: 10.1128/jvi.45.2.782-791.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Cooper J. A., Buss J. E., Shalloway D., Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985 Oct;42(3):849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Huang C. C., Hammond C., Bishop J. M. Nucleotide sequence and topography of chicken c-fps. Genesis of a retroviral oncogene encoding a tyrosine-specific protein kinase. J Mol Biol. 1985 Jan 20;181(2):175–186. doi: 10.1016/0022-2836(85)90083-x. [DOI] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Hunter T., Ling N., Cooper J. A. Protein kinase C phosphorylation of the EGF receptor at a threonine residue close to the cytoplasmic face of the plasma membrane. Nature. 1984 Oct 4;311(5985):480–483. doi: 10.1038/311480a0. [DOI] [PubMed] [Google Scholar]
- Klarlund J. K. Transformation of cells by an inhibitor of phosphatases acting on phosphotyrosine in proteins. Cell. 1985 Jul;41(3):707–717. doi: 10.1016/s0092-8674(85)80051-9. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Bister K., Moscovici C., Duesberg P. H. Temperature-sensitive mutants of Fujinami sarcoma virus: tumorigenicity and reversible phosphorylation of the transforming p140 protein. J Virol. 1981 Jun;38(3):1064–1076. doi: 10.1128/jvi.38.3.1064-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J. E. A mechanism for memory storage insensitive to molecular turnover: a bistable autophosphorylating kinase. Proc Natl Acad Sci U S A. 1985 May;82(9):3055–3057. doi: 10.1073/pnas.82.9.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald I., Levy J., Pawson T. Expression of the mammalian c-fes protein in hematopoietic cells and identification of a distinct fes-related protein. Mol Cell Biol. 1985 Oct;5(10):2543–2551. doi: 10.1128/mcb.5.10.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Moss P., Radke K., Carter V. C., Young J., Gilmore T., Martin G. S. Cellular localization of the transforming protein of wild-type and temperature-sensitive Fujinami sarcoma virus. J Virol. 1984 Nov;52(2):557–565. doi: 10.1128/jvi.52.2.557-565.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. L., Branton P. E. Identification, purification, and characterization of phosphotyrosine-specific protein phosphatases from cultured chicken embryo fibroblasts. Mol Cell Biol. 1984 Jun;4(6):1003–1012. doi: 10.1128/mcb.4.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawson T., Guyden J., Kung T. H., Radke K., Gilmore T., Martin G. S. A strain of Fujinami sarcoma virus which is temperature-sensitive in protein phosphorylation and cellular transformation. Cell. 1980 Dec;22(3):767–775. doi: 10.1016/0092-8674(80)90553-x. [DOI] [PubMed] [Google Scholar]
- Resh M. D., Erikson R. L. Characterization of pp60src phosphorylation in vitro in Rous sarcoma virus-transformed cell membranes. Mol Cell Biol. 1985 May;5(5):916–922. doi: 10.1128/mcb.5.5.916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroek A. J., Schalken J. A., Verbeek J. S., Van den Ouweland A. M., Onnekink C., Bloemers H. P., Van de Ven W. J. The structure of the human c-fes/fps proto-oncogene. EMBO J. 1985 Nov;4(11):2897–2903. doi: 10.1002/j.1460-2075.1985.tb04020.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samarut J., Mathey-Prevot B., Hanafusa H. Preferential expression of the c-fps protein in chicken macrophages and granulocytic cells. Mol Cell Biol. 1985 May;5(5):1067–1072. doi: 10.1128/mcb.5.5.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder M. A., Bishop J. M., Colby W. W., Levinson A. D. Phosphorylation of tyrosine-416 is not required for the transforming properties and kinase activity of pp60v-src. Cell. 1983 Mar;32(3):891–901. doi: 10.1016/0092-8674(83)90074-0. [DOI] [PubMed] [Google Scholar]
- Swarup G., Cohen S., Garbers D. L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982 Aug;107(3):1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Tracey A. S., Gresser M. J. Interaction of vanadate with phenol and tyrosine: implications for the effects of vanadate on systems regulated by tyrosine phosphorylation. Proc Natl Acad Sci U S A. 1986 Feb;83(3):609–613. doi: 10.1073/pnas.83.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G., Hinze E., Pawson T. Mapping of multiple phosphorylation sites within the structural and catalytic domains of the Fujinami avian sarcoma virus transforming protein. J Virol. 1983 Apr;46(1):29–41. doi: 10.1128/jvi.46.1.29-41.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmaster G., Pawson T. Protein kinase activity of FSV (Fujinami sarcoma virus) P130gag-fps shows a strict specificity for tyrosine residues. J Biol Chem. 1986 Jan 5;261(1):328–333. [PubMed] [Google Scholar]
- Weinmaster G., Zoller M. J., Smith M., Hinze E., Pawson T. Mutagenesis of Fujinami sarcoma virus: evidence that tyrosine phosphorylation of P130gag-fps modulates its biological activity. Cell. 1984 Jun;37(2):559–568. doi: 10.1016/0092-8674(84)90386-6. [DOI] [PubMed] [Google Scholar]
- Young J. C., Martin G. S. Cellular localization of c-fps gene product NCP98. J Virol. 1984 Dec;52(3):913–918. doi: 10.1128/jvi.52.3.913-918.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]