Abstract
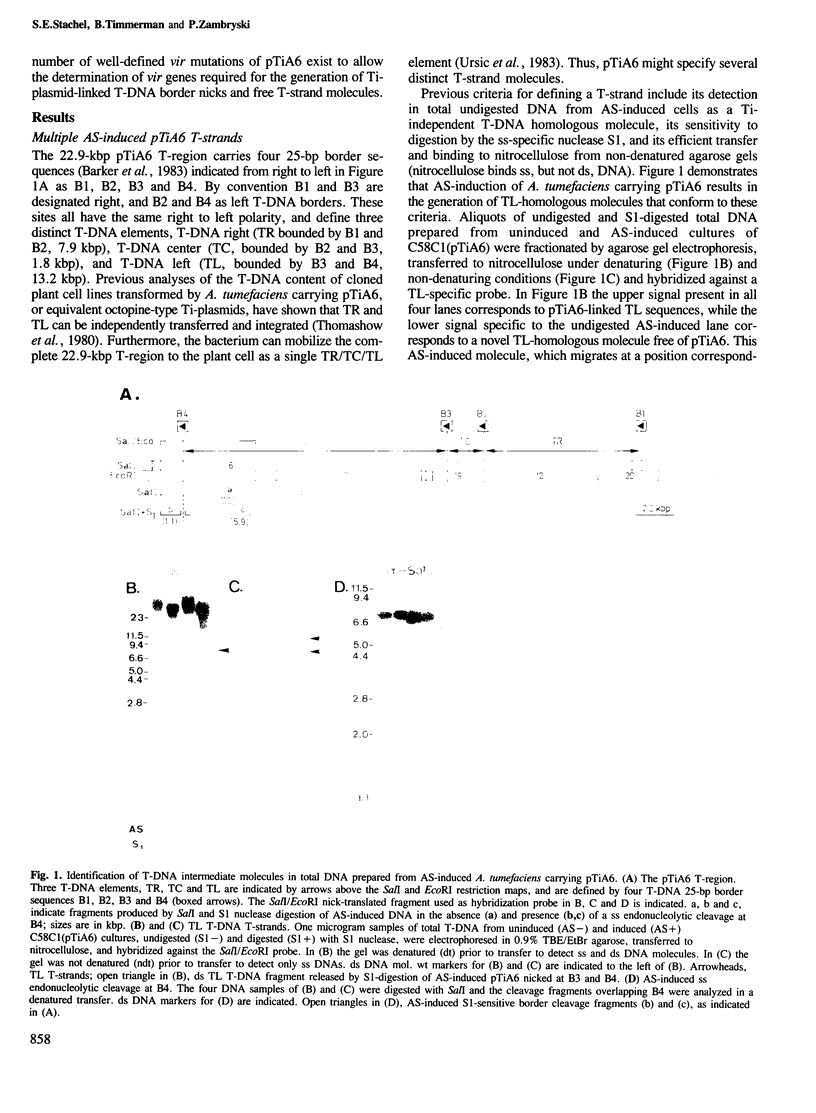
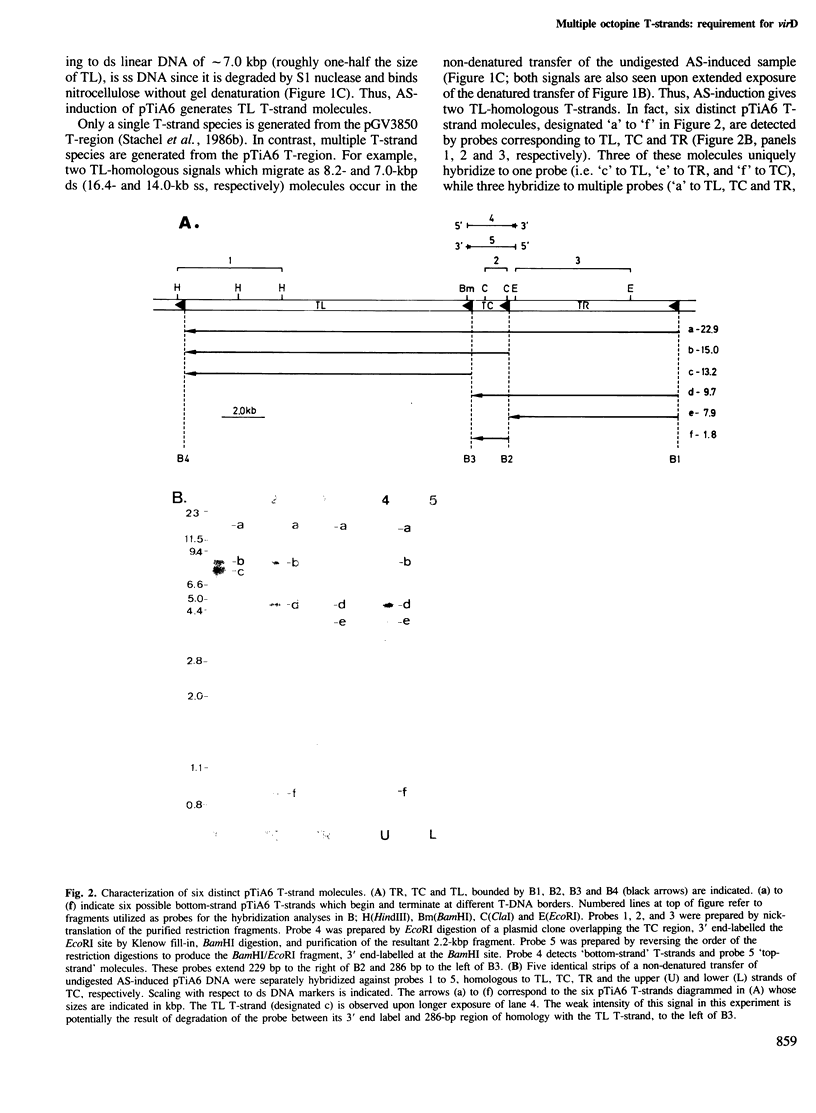
Agrobacterium tumefaciens transfers its Ti-plasmid T-DNA to plant cells. This process is initiated by plant-induced activation of the Ti-plasmid virulence loci, resulting in the generation of single stranded (ss) cleavages of the Ti-plasmid T-DNA border sequences (border nicks) and ss linear unipolar T-DNA molecules (T-strands). A single T-strand is produced from the two-border T-region of the pGV3850 nopaline plasmid. In this paper the induced molecular events for the complex T-region of the pTiA6 octopine plasmid are analyzed. This T-region carries four T-DNA borders delimiting three T-DNA elements (TR, TC and TL). Induction of pTiA6 generates cleavages independently at its border repeats, and six distinct T-strand species corresponding to TR, TR/TC, TR/TC/TL, TC, TC/TL and TL. These T-strand molecules are linear and correspond to the bottom strand of the pTiA6 T-region. Thus, borders can function for both initiation and termination of T-strand synthesis. We propose that the different pTiA6 T-strands are independently generated, and that the distribution of border nicks within the parental T-region determines which T-strand is produced. To identify genes involved in T-strand production, pTiA6 virulence (vir) and chromosomal virulence (chv) mutant strains were analyzed. VirA and VirG, the vir regulatory loci are required. Furthermore, the two 5' cistrons of virD are required for both border nicks and T-strands, suggesting that these genes encode the border endonuclease, and that T-strand production is dependent on border nicks. That no mutants are defective for T-strands alone suggests that functions encoded outside of vir and chv might mediate some of the later reactions of T-strand synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alt-Moerbe J., Rak B., Schröder J. A 3.6-kbp segment from the vir region of Ti plasmids contains genes responsible for border-sequence-directed production of T region circles in E. coli. EMBO J. 1986 Jun;5(6):1129–1135. doi: 10.1002/j.1460-2075.1986.tb04337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. J., Staneloni R. J., Rubin R. A., Nester E. W. Identification and genetic analysis of an Agrobacterium tumefaciens chromosomal virulence region. J Bacteriol. 1985 Mar;161(3):850–860. doi: 10.1128/jb.161.3.850-860.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everett R., Willetts N. Characterisation of an in vivo system for nicking at the origin of conjugal DNA transfer of the sex factor F. J Mol Biol. 1980 Jan 15;136(2):129–150. doi: 10.1016/0022-2836(80)90309-5. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joos H., Timmerman B., Montagu M. V., Schell J. Genetic analysis of transfer and stabilization of Agrobacterium DNA in plant cells. EMBO J. 1983;2(12):2151–2160. doi: 10.1002/j.1460-2075.1983.tb01716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Hellmiss R., Ream W. Overdrive, a T-DNA transmission enhancer on the A. tumefaciens tumour-inducing plasmid. EMBO J. 1986 Jun;5(6):1137–1142. doi: 10.1002/j.1460-2075.1986.tb04338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta E. G., Ream L. W. T-DNA border sequences required for crown gall tumorigenesis. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5112–5116. doi: 10.1073/pnas.82.15.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slightom J. L., Jouanin L., Leach F., Drong R. F., Tepfer D. Isolation and identification of TL-DNA/plant junctions in Convolvulus arvensis transformed by Agrobacterium rhizogenes strain A4. EMBO J. 1985 Dec 1;4(12):3069–3077. doi: 10.1002/j.1460-2075.1985.tb04047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W. The genetic and transcriptional organization of the vir region of the A6 Ti plasmid of Agrobacterium tumefaciens. EMBO J. 1986 Jul;5(7):1445–1454. doi: 10.1002/j.1460-2075.1986.tb04381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Nester E. W., Zambryski P. C. A plant cell factor induces Agrobacterium tumefaciens vir gene expression. Proc Natl Acad Sci U S A. 1986 Jan;83(2):379–383. doi: 10.1073/pnas.83.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. Agrobacterium tumefaciens and the susceptible plant cell: a novel adaptation of extracellular recognition and DNA conjugation. Cell. 1986 Oct 24;47(2):155–157. doi: 10.1016/0092-8674(86)90437-x. [DOI] [PubMed] [Google Scholar]
- Stachel S. E., Zambryski P. C. virA and virG control the plant-induced activation of the T-DNA transfer process of A. tumefaciens. Cell. 1986 Aug 1;46(3):325–333. doi: 10.1016/0092-8674(86)90653-7. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Wang K., Herrera-Estrella L., Van Montagu M., Zambryski P. Right 25 bp terminus sequence of the nopaline T-DNA is essential for and determines direction of DNA transfer from agrobacterium to the plant genome. Cell. 1984 Sep;38(2):455–462. doi: 10.1016/0092-8674(84)90500-2. [DOI] [PubMed] [Google Scholar]
- Wang K., Stachel S. E., Timmerman B., VAN Montagu M., Zambryski P. C. Site-Specific Nick in the T-DNA Border Sequence as a Result of Agrobacterium vir Gene Expression. Science. 1987 Jan 30;235(4788):587–591. doi: 10.1126/science.235.4788.587. [DOI] [PubMed] [Google Scholar]
- Yanofsky M. F., Porter S. G., Young C., Albright L. M., Gordon M. P., Nester E. W. The virD operon of Agrobacterium tumefaciens encodes a site-specific endonuclease. Cell. 1986 Nov 7;47(3):471–477. doi: 10.1016/0092-8674(86)90604-5. [DOI] [PubMed] [Google Scholar]





