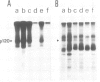
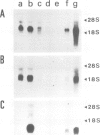
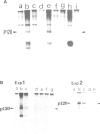
Abstract
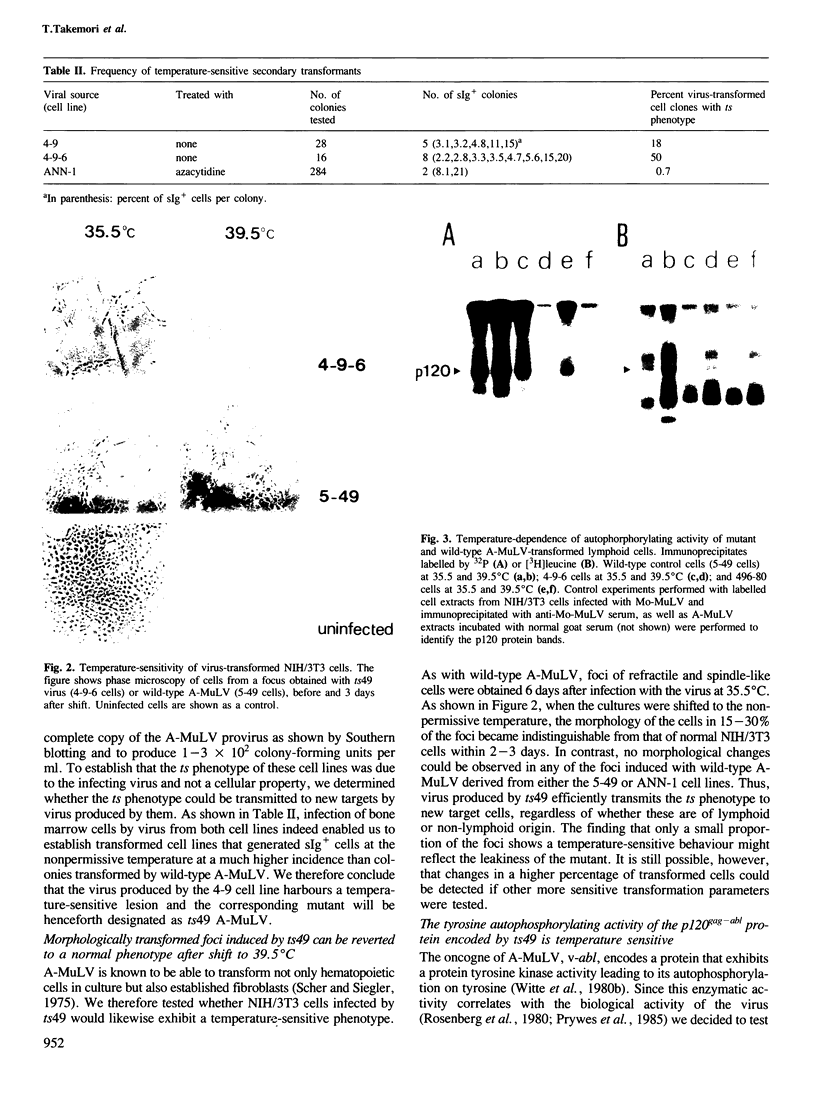
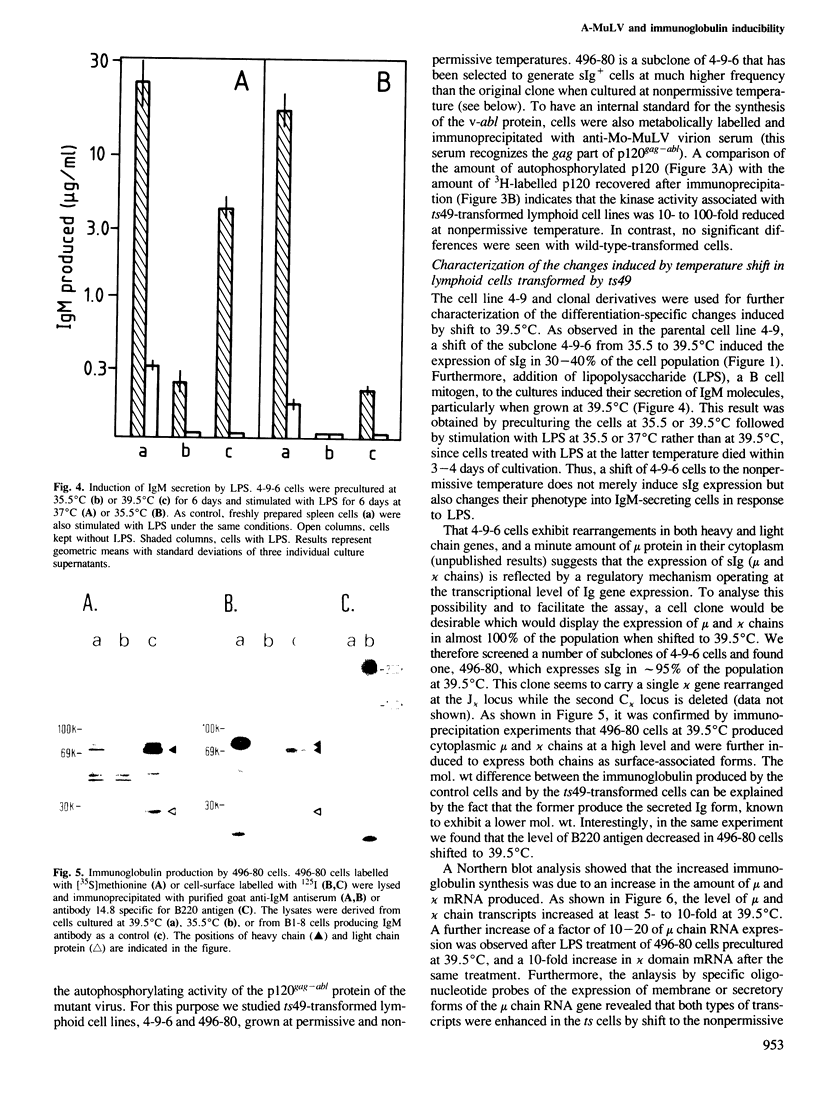
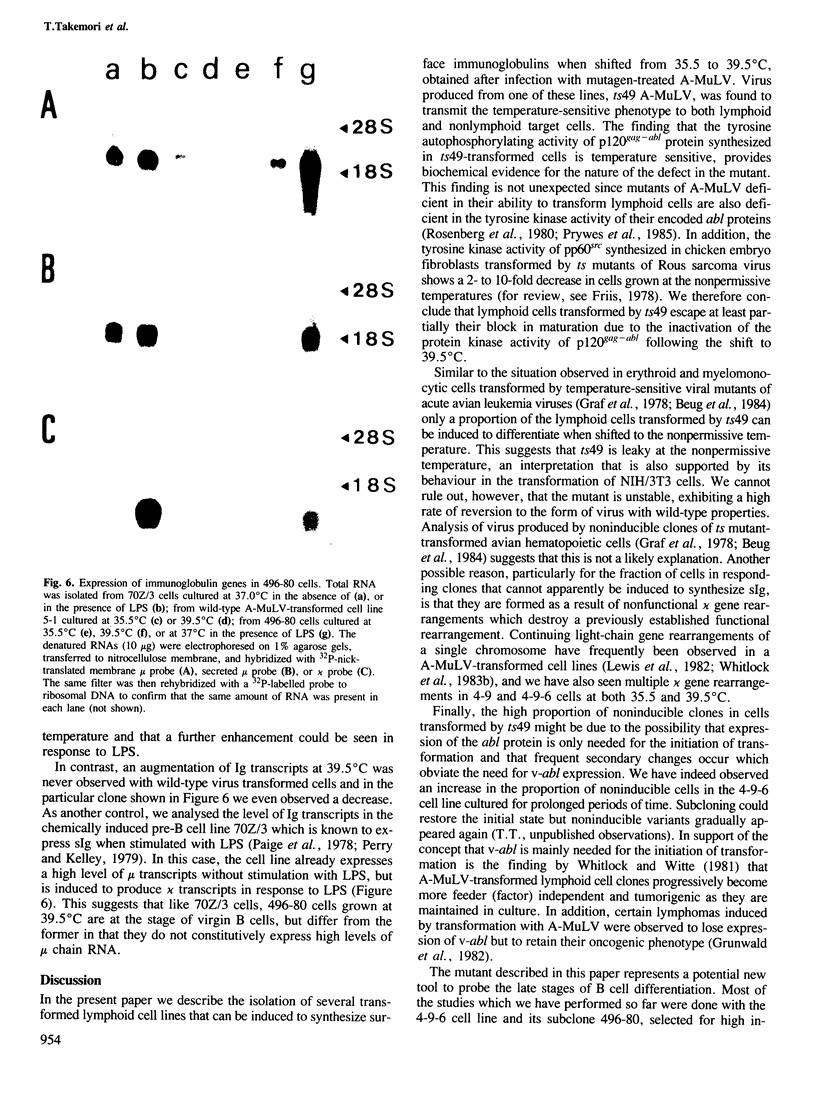
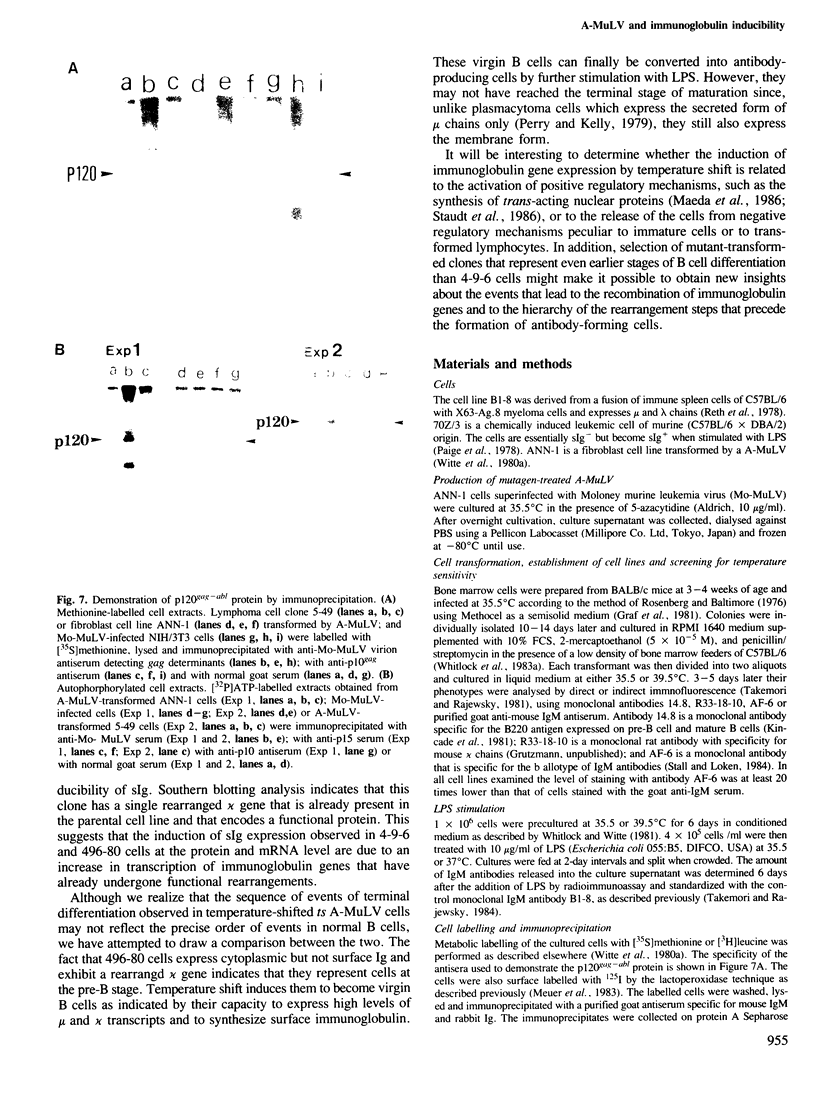
Lymphoid cell lines were isolated that were inducible for the expression of surface immunoglobulin by shift from 35.5 to 39.5 degrees C after infection of mouse bone marrow cells with a mutagen-treated Abelson murine leukemia virus. Virus produced by one of the cell lines (ts49) transmitted the temperature-sensitive phenotype to new lymphoid transformants as well as to NIH/3T3 cells. In addition, the tyrosine autophosphorylating activity of the p120gag-abl protein synthesized in ts49-transformed cells was found to be temperature-sensitive. Shift experiments using ts49-transformed lymphoid cells showed that at 39.5 degrees C they synthesize increased amounts of mu and kappa chain RNA and protein, and that they can be further induced to secrete IgM when treated with lipopolysaccharide.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Alt F. W., Rosenberg N., Enea V., Siden E., Baltimore D. Multiple immunoglobulin heavy-chain gene transcripts in Abelson murine leukemia virus-transformed lymphoid cell lines. Mol Cell Biol. 1982 Apr;2(4):386–400. doi: 10.1128/mcb.2.4.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beug H., Leutz A., Kahn P., Graf T. Ts mutants of E26 leukemia virus allow transformed myeloblasts, but not erythroblasts or fibroblasts, to differentiate at the nonpermissive temperature. Cell. 1984 Dec;39(3 Pt 2):579–588. doi: 10.1016/0092-8674(84)90465-3. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Graf T., von Kirchbach A., Beug H. Characterization of the hematopoietic target cells of AEV, MC29 and AMV avian leukemia viruses. Exp Cell Res. 1981 Feb;131(2):331–343. doi: 10.1016/0014-4827(81)90236-6. [DOI] [PubMed] [Google Scholar]
- Grunwald D. J., Dale B., Dudley J., Lamph W., Sugden B., Ozanne B., Risser R. Loss of viral gene expression and retention of tumorigenicity by Abelson lymphoma cells. J Virol. 1982 Jul;43(1):92–103. doi: 10.1128/jvi.43.1.92-103.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade P. W., Lee G., Watanabe T., Sun L., Scheid M. P. Antigens displayed on murine B lymphocyte precursors. J Immunol. 1981 Dec;127(6):2262–2268. [PubMed] [Google Scholar]
- Konopka J. B., Davis R. L., Watanabe S. M., Ponticelli A. S., Schiff-Maker L., Rosenberg N., Witte O. N. Only site-directed antibodies reactive with the highly conserved src-homologous region of the v-abl protein neutralize kinase activity. J Virol. 1984 Jul;51(1):223–232. doi: 10.1128/jvi.51.1.223-232.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis S., Rosenberg N., Alt F., Baltimore D. Continuing kappa-gene rearrangement in a cell line transformed by Abelson murine leukemia virus. Cell. 1982 Oct;30(3):807–816. doi: 10.1016/0092-8674(82)90285-9. [DOI] [PubMed] [Google Scholar]
- Maeda H., Kitamura D., Kudo A., Araki K., Watanabe T. Trans-acting nuclear protein responsible for induction of rearranged human immunoglobulin heavy chain gene. Cell. 1986 Apr 11;45(1):25–33. doi: 10.1016/0092-8674(86)90534-9. [DOI] [PubMed] [Google Scholar]
- Meuer S. C., Fitzgerald K. A., Hussey R. E., Hodgdon J. C., Schlossman S. F., Reinherz E. L. Clonotypic structures involved in antigen-specific human T cell function. Relationship to the T3 molecular complex. J Exp Med. 1983 Feb 1;157(2):705–719. doi: 10.1084/jem.157.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuberger M. S. Expression and regulation of immunoglobulin heavy chain gene transfected into lymphoid cells. EMBO J. 1983;2(8):1373–1378. doi: 10.1002/j.1460-2075.1983.tb01594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige C. J., Kincade P. W., Ralph P. Murine B cell leukemia line with inducible surface immunoglobulin expression. J Immunol. 1978 Aug;121(2):641–647. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Immunoglobulin messenger RNAs in murine cell lines that have characteristics of immature B lymphocytes. Cell. 1979 Dec;18(4):1333–1339. doi: 10.1016/0092-8674(79)90243-5. [DOI] [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Baltimore D. The minimum transforming region of v-abl is the segment encoding protein-tyrosine kinase. J Virol. 1985 Apr;54(1):114–122. doi: 10.1128/jvi.54.1.114-122.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reth M. G., Alt F. W. Novel immunoglobulin heavy chains are produced from DJH gene segment rearrangements in lymphoid cells. 1984 Nov 29-Dec 5Nature. 312(5993):418–423. doi: 10.1038/312418a0. [DOI] [PubMed] [Google Scholar]
- Reth M. G., Ammirati P., Jackson S., Alt F. W. Regulated progression of a cultured pre-B-cell line to the B-cell stage. 1985 Sep 26-Oct 2Nature. 317(6035):353–355. doi: 10.1038/317353a0. [DOI] [PubMed] [Google Scholar]
- Reth M., Hämmerling G. J., Rajewsky K. Analysis of the repertoire of anti-NP antibodies in C57BL/6 mice by cell fusion. I. Characterization of antibody families in the primary and hyperimmune response. Eur J Immunol. 1978 Jun;8(6):393–400. doi: 10.1002/eji.1830080605. [DOI] [PubMed] [Google Scholar]
- Rosenberg N. E., Clark D. R., Witte O. N. Abelson murine leukemia virus mutants deficient in kinase activity and lymphoid cell transformation. J Virol. 1980 Dec;36(3):766–774. doi: 10.1128/jvi.36.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakano H., Hüppi K., Heinrich G., Tonegawa S. Sequences at the somatic recombination sites of immunoglobulin light-chain genes. Nature. 1979 Jul 26;280(5720):288–294. doi: 10.1038/280288a0. [DOI] [PubMed] [Google Scholar]
- Scher C. D. Effect of pseudotype on Abelson virus and Kirsten sarcoma virus-induced leukemia. J Exp Med. 1978 Apr 1;147(4):1044–1053. doi: 10.1084/jem.147.4.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Schibler U., Marcu K. B., Perry R. P. The synthesis and processing of the messenger RNAs specifying heavy and light chain immunoglobulins in MPC-11 cells. Cell. 1978 Dec;15(4):1495–1509. doi: 10.1016/0092-8674(78)90072-7. [DOI] [PubMed] [Google Scholar]
- Stall A. M., Loken M. R. Allotypic specificities of murine IgD and IgM recognized by monoclonal antibodies. J Immunol. 1984 Feb;132(2):787–795. [PubMed] [Google Scholar]
- Staudt L. M., Singh H., Sen R., Wirth T., Sharp P. A., Baltimore D. A lymphoid-specific protein binding to the octamer motif of immunoglobulin genes. Nature. 1986 Oct 16;323(6089):640–643. doi: 10.1038/323640a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Akira S., Kikutani H., Kishimoto S., Yamamura Y., Kishimoto T. Functional V region formation during in vitro culture of a murine immature B precursor cell line. Nature. 1983 Jun 30;303(5920):812–815. doi: 10.1038/303812a0. [DOI] [PubMed] [Google Scholar]
- Takemori T., Rajewsky K. Lambda chain expression at different stages of ontogeny in C57BL/6, BALB/c and SJL mice. Eur J Immunol. 1981 Aug;11(8):618–625. doi: 10.1002/eji.1830110806. [DOI] [PubMed] [Google Scholar]
- Takemori T., Rajewsky K. Specificity, duration and mechanism of idiotype suppression induced by neonatal injection of monoclonal anti-idiotope antibodies into mice. Eur J Immunol. 1984 Jul;14(7):656–667. doi: 10.1002/eji.1830140714. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Robertson D., Witte O. N. Murine B cell lymphopoiesis in long term culture. J Immunol Methods. 1984 Mar 16;67(2):353–369. doi: 10.1016/0022-1759(84)90475-7. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Abelson virus-infected cells can exhibit restricted in vitro growth and low oncogenic potential. J Virol. 1981 Nov;40(2):577–584. doi: 10.1128/jvi.40.2.577-584.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock C. A., Ziegler S. F., Treiman L. J., Stafford J. I., Witte O. N. Differentiation of cloned populations of immature B cells after transformation with Abelson murine leukemia virus. Cell. 1983 Mar;32(3):903–911. doi: 10.1016/0092-8674(83)90075-2. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Treiman L. J., Witte O. N. kappa gene diversity among the clonal progeny of pre-B lymphocytes. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1529–1533. doi: 10.1073/pnas.81.5.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]