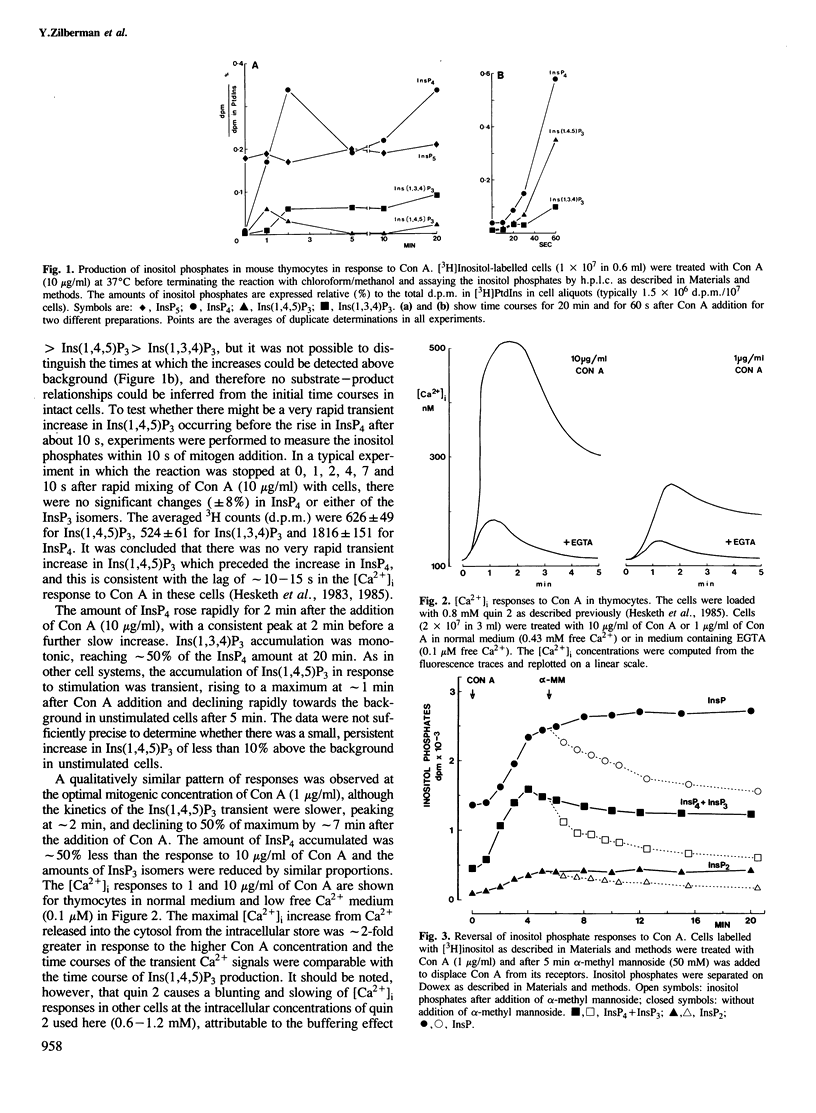
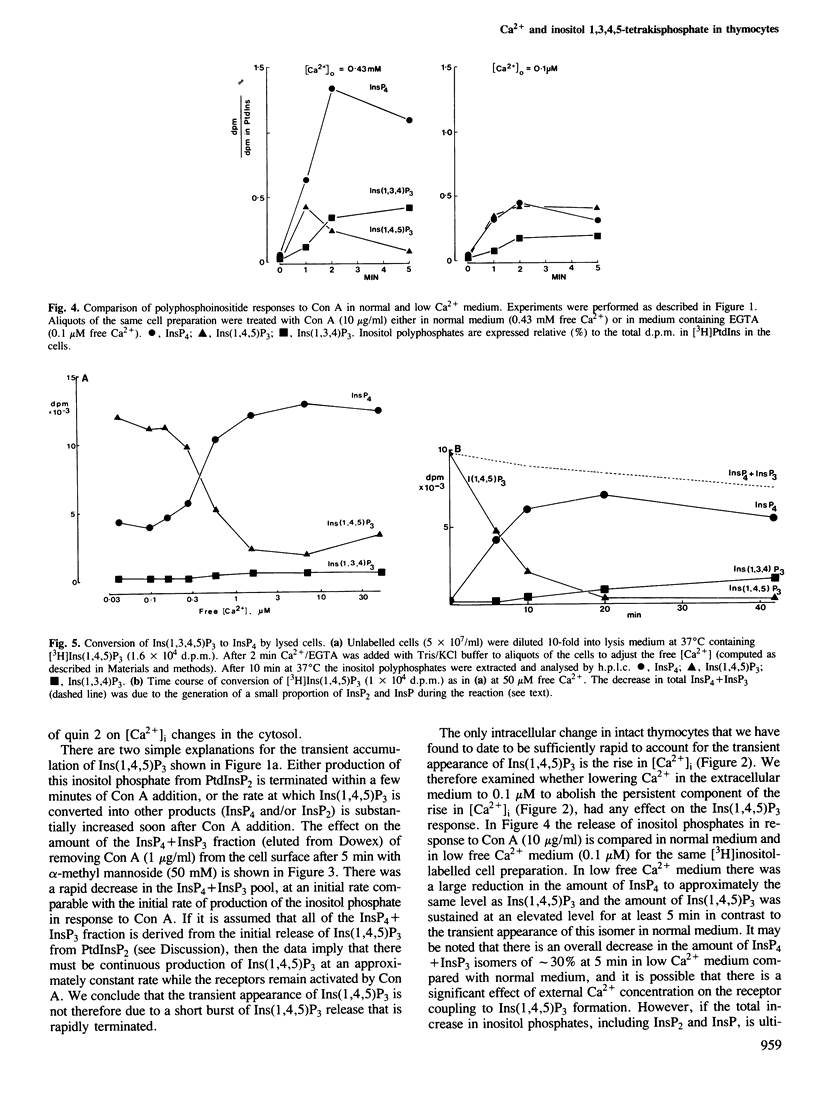
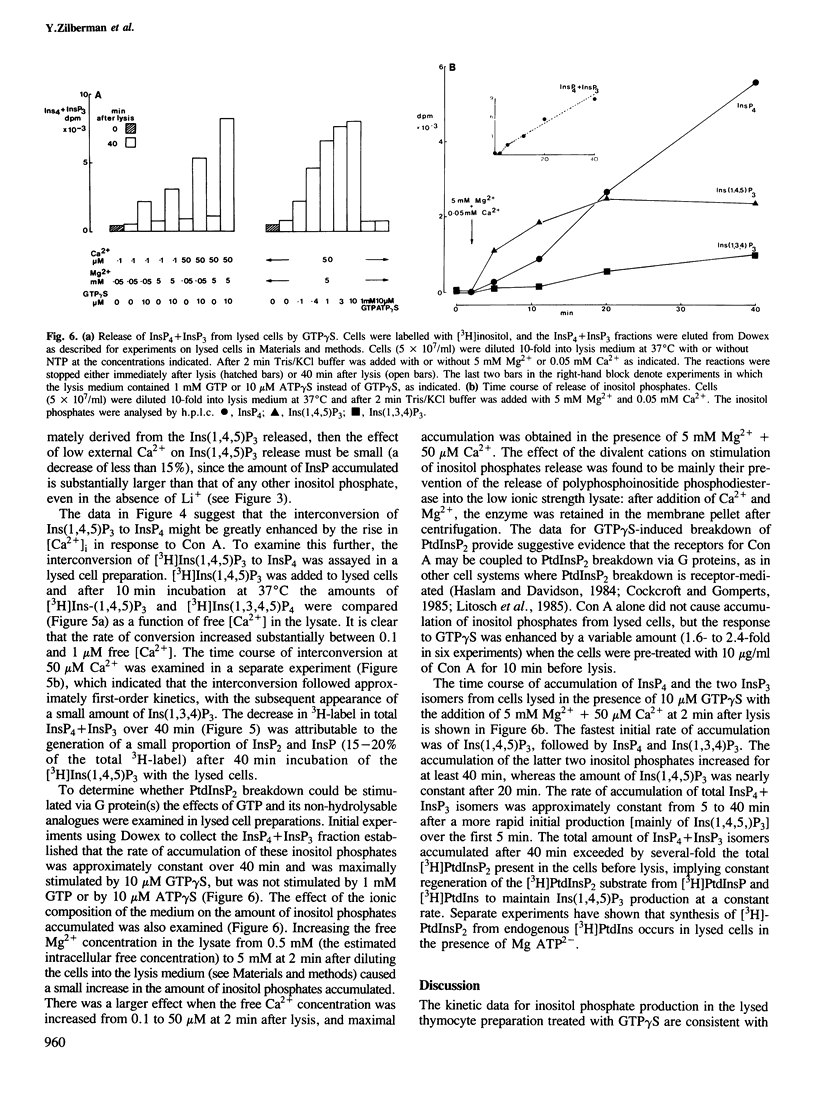
Abstract
Lysed mouse thymocytes release [3H]inositol 1,4,5 trisphosphate from [3H]inositol-labelled phosphatidyl inositol 4,5-bisphosphate in response to GTP gamma S, and rapidly phosphorylate [3H]inositol 1,4,5-trisphosphate to [3H]inositol 1,3,4,5-tetrakisphosphate. The rate of phosphorylation is increased approximately 7-fold when the free [Ca2+] in the lysate is increased from 0.1 to 1 microM, the range in which the cytosolic free [Ca2+] increases in intact thymocytes in response to the mitogen concanavalin A. Stimulation of the intact cells with concanavalin A also results in a rapid and sustained increase in the amount of inositol 1,3,4,5-tetrakisphosphate, and a much smaller transient increase in 1,4,5-trisphosphate. Lowering [Ca2+] in the medium from 0.4 mM to 0.1 microM before addition of concanavalin A reduces accumulation of inositol 1,3,4,5-tetrakisphosphate by at least 3-fold whereas the increase in inositol 1,4,5-trisphosphate is sustained rather than transient. The data imply that in normal medium the activity of the inositol 1,4,5-trisphosphate kinase increases substantially in response to the rise in cytosolic free [Ca2+] generated by concanavalin A, accounting for both the transient accumulation of inositol 1,4,5-trisphosphate and the sustained high levels of inositol 1,3,4,5-tetrakisphosphate. Inositol 1,3,4,5-tetrakisphosphate is a strong candidate for the second messenger for Ca2+ entry across the plasma membrane. This would imply that the inositol polyphosphates regulate both Ca2+ entry and intracellular Ca2+ release, with feedback control of the inositol polyphosphate levels by Ca2+.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Batty I. R., Nahorski S. R., Irvine R. F. Rapid formation of inositol 1,3,4,5-tetrakisphosphate following muscarinic receptor stimulation of rat cerebral cortical slices. Biochem J. 1985 Nov 15;232(1):211–215. doi: 10.1042/bj2320211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven M. A., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7137–7142. [PubMed] [Google Scholar]
- Berridge M. J., Dawson R. M., Downes C. P., Heslop J. P., Irvine R. F. Changes in the levels of inositol phosphates after agonist-dependent hydrolysis of membrane phosphoinositides. Biochem J. 1983 May 15;212(2):473–482. doi: 10.1042/bj2120473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biden T. J., Wollheim C. B. Ca2+ regulates the inositol tris/tetrakisphosphate pathway in intact and broken preparations of insulin-secreting RINm5F cells. J Biol Chem. 1986 Sep 15;261(26):11931–11934. [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Haslam R. J., Davidson M. M. Receptor-induced diacylglycerol formation in permeabilized platelets; possible role for a GTP-binding protein. J Recept Res. 1984;4(1-6):605–629. doi: 10.3109/10799898409042576. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Moore J. P., Morris J. D., Taylor M. V., Rogers J., Smith G. A., Metcalfe J. C. A common sequence of calcium and pH signals in the mitogenic stimulation of eukaryotic cells. Nature. 1985 Feb 7;313(6002):481–484. doi: 10.1038/313481a0. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Heslop J. P., Irvine R. F., Tashjian A. H., Jr, Berridge M. J. Inositol tetrakis- and pentakisphosphates in GH4 cells. J Exp Biol. 1985 Nov;119:395–401. doi: 10.1242/jeb.119.1.395. [DOI] [PubMed] [Google Scholar]
- Irvine R. F., Anggård E. E., Letcher A. J., Downes C. P. Metabolism of inositol 1,4,5-trisphosphate and inositol 1,3,4-trisphosphate in rat parotid glands. Biochem J. 1985 Jul 15;229(2):505–511. doi: 10.1042/bj2290505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine R. F., Letcher A. J., Heslop J. P., Berridge M. J. The inositol tris/tetrakisphosphate pathway--demonstration of Ins(1,4,5)P3 3-kinase activity in animal tissues. Nature. 1986 Apr 17;320(6063):631–634. doi: 10.1038/320631a0. [DOI] [PubMed] [Google Scholar]
- Kuno M., Goronzy J., Weyand C. M., Gardner P. Single-channel and whole-cell recordings of mitogen-regulated inward currents in human cloned helper T lymphocytes. Nature. 1986 Sep 18;323(6085):269–273. doi: 10.1038/323269a0. [DOI] [PubMed] [Google Scholar]
- Litosch I., Wallis C., Fain J. N. 5-Hydroxytryptamine stimulates inositol phosphate production in a cell-free system from blowfly salivary glands. Evidence for a role of GTP in coupling receptor activation to phosphoinositide breakdown. J Biol Chem. 1985 May 10;260(9):5464–5471. [PubMed] [Google Scholar]
- Moore J. P., Johannsson A., Hesketh T. R., Smith G. A., Metcalfe J. C. Calcium signals and phospholipid methylation in eukaryotic cells. Biochem J. 1984 Aug 1;221(3):675–684. doi: 10.1042/bj2210675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. The bivalent-cation dependence of phosphatidylinositol synthesis in a cell-free system from lymphocytes. Biochem J. 1983 Jun 15;212(3):691–697. doi: 10.1042/bj2120691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. V., Metcalfe J. C., Hesketh T. R., Smith G. A., Moore J. P. Mitogens increase phosphorylation of phosphoinositides in thymocytes. 1984 Nov 29-Dec 5Nature. 312(5993):462–465. doi: 10.1038/312462a0. [DOI] [PubMed] [Google Scholar]
- Wakelam M. J., Davies S. A., Houslay M. D., McKay I., Marshall C. J., Hall A. Normal p21N-ras couples bombesin and other growth factor receptors to inositol phosphate production. Nature. 1986 Sep 11;323(6084):173–176. doi: 10.1038/323173a0. [DOI] [PubMed] [Google Scholar]


