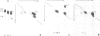Abstract
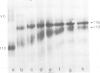
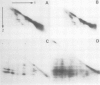
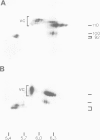
The Drosophila position-specific (PS) antigens are a family of cell surface glycoprotein complexes thought to be involved in morphogenesis. Their overall structures and biochemical properties are similar to those of a group of vertebrate receptors, including those for fibronectin, fibrinogen and vitronectin, and also the leukocyte antigens Mac-1, LFA-1 and p150,95 and the VLA family of cell surface antigens. The N-terminal sequences of the alpha subunits of some of these molecules are homologous to the N-terminus of a PS antigen component. The Drosophila PS antigens thus appear to be homologous to these vertebrate receptors.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Teplow D. B., Hood L. E., Kent S. B. Electroblotting onto activated glass. High efficiency preparation of proteins from analytical sodium dodecyl sulfate-polyacrylamide gels for direct sequence analysis. J Biol Chem. 1986 Mar 25;261(9):4229–4238. [PubMed] [Google Scholar]
- Akiyama S. K., Yamada S. S., Yamada K. M. Characterization of a 140-kD avian cell surface antigen as a fibronectin-binding molecule. J Cell Biol. 1986 Feb;102(2):442–448. doi: 10.1083/jcb.102.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barclay A. N., Letarte-Muirhead M., Williams A. F., Faulkes R. A. Chemical characterisation of the Thy-1 glycoproteins from the membranes of rat thymocytes and brain. Nature. 1976 Oct 14;263(5578):563–567. doi: 10.1038/263563a0. [DOI] [PubMed] [Google Scholar]
- Bordier C., Crettol-Järvinen A. Peptide mapping of heterogeneous protein samples. J Biol Chem. 1979 Apr 25;254(8):2565–2567. [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Boucaut J. C., Darribère T., Poole T. J., Aoyama H., Yamada K. M., Thiery J. P. Biologically active synthetic peptides as probes of embryonic development: a competitive peptide inhibitor of fibronectin function inhibits gastrulation in amphibian embryos and neural crest cell migration in avian embryos. J Cell Biol. 1984 Nov;99(5):1822–1830. doi: 10.1083/jcb.99.5.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower D. L., Lawrence P. A., Wilcox M. Clonal analysis of the undifferentiated wing disk of Drosophila. Dev Biol. 1981 Sep;86(2):448–455. doi: 10.1016/0012-1606(81)90203-7. [DOI] [PubMed] [Google Scholar]
- Brower D. L., Piovant M., Reger L. A. Developmental analysis of Drosophila position-specific antigens. Dev Biol. 1985 Mar;108(1):120–130. doi: 10.1016/0012-1606(85)90014-4. [DOI] [PubMed] [Google Scholar]
- Brower D. L. Posterior-to-anterior transformation in engrailed wing imaginal disks of Drosophila. Nature. 1984 Aug 9;310(5977):496–497. doi: 10.1038/310496a0. [DOI] [PubMed] [Google Scholar]
- Brower D. L., Wilcox M., Piovant M., Smith R. J., Reger L. A. Related cell-surface antigens expressed with positional specificity in Drosophila imaginal discs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7485–7489. doi: 10.1073/pnas.81.23.7485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P. J., Juliano R. L. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985 Jun 21;228(4706):1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Chan L. N., Gehring W. Determination of blastoderm cells in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2217–2221. doi: 10.1073/pnas.68.9.2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen S. E., Kindle C. S., Shreffler D. C., Cowing C. Differential glycosylation of murine B cell and spleen adherent cell Ia antigens. J Immunol. 1981 Oct;127(4):1478–1484. [PubMed] [Google Scholar]
- Elder J. H., Alexander S. endo-beta-N-acetylglucosaminidase F: endoglycosidase from Flavobacterium meningosepticum that cleaves both high-mannose and complex glycoproteins. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4540–4544. doi: 10.1073/pnas.79.15.4540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Bellido A., Ripoll P., Morata G. Developmental compartmentalization in the dorsal mesothoracic disc of Drosophila. Dev Biol. 1976 Jan;48(1):132–147. doi: 10.1016/0012-1606(76)90052-x. [DOI] [PubMed] [Google Scholar]
- Gardner J. M., Hynes R. O. Interaction of fibronectin with its receptor on platelets. Cell. 1985 Sep;42(2):439–448. doi: 10.1016/0092-8674(85)90101-1. [DOI] [PubMed] [Google Scholar]
- Geisow M. J. Cell-surface receptors: puzzles and paradigms. Bioessays. 1986 Apr;4(4):149–151. doi: 10.1002/bies.950040403. [DOI] [PubMed] [Google Scholar]
- Giancotti F. G., Tarone G., Knudsen K., Damsky C., Comoglio P. M. Cleavage of a 135 kD cell surface glycoprotein correlates with loss of fibroblast adhesion to fibronectin. Exp Cell Res. 1985 Jan;156(1):182–190. doi: 10.1016/0014-4827(85)90272-1. [DOI] [PubMed] [Google Scholar]
- Hasegawa T., Hasegawa E., Chen W. T., Yamada K. M. Characterization of a membrane-associated glycoprotein complex implicated in cell adhesion to fibronectin. J Cell Biochem. 1985;28(4):307–318. doi: 10.1002/jcb.240280409. [DOI] [PubMed] [Google Scholar]
- Hawkes R. Identification of concanavalin A-binding proteins after sodium dodecyl sulfate--gel electrophoresis and protein blotting. Anal Biochem. 1982 Jun;123(1):143–146. doi: 10.1016/0003-2697(82)90634-0. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hunkapiller M. W., Hood L. E. Analysis of phenylthiohydantoins by ultrasensitive gradient high-performance liquid chromatography. Methods Enzymol. 1983;91:486–493. doi: 10.1016/s0076-6879(83)91045-5. [DOI] [PubMed] [Google Scholar]
- Hunkapiller T., Hood L. The growing immunoglobulin gene superfamily. Nature. 1986 Sep 4;323(6083):15–16. doi: 10.1038/323015a0. [DOI] [PubMed] [Google Scholar]
- Keizer G. D., Borst J., Figdor C. G., Spits H., Miedema F., Terhorst C., De Vries J. E. Biochemical and functional characteristics of the human leukocyte membrane antigen family LFA-1, Mo-1 and p150,95. Eur J Immunol. 1985 Nov;15(11):1142–1148. doi: 10.1002/eji.1830151114. [DOI] [PubMed] [Google Scholar]
- Knudsen K. A., Horwitz A. F., Buck C. A. A monoclonal antibody identifies a glycoprotein complex involved in cell-substratum adhesion. Exp Cell Res. 1985 Mar;157(1):218–226. doi: 10.1016/0014-4827(85)90164-8. [DOI] [PubMed] [Google Scholar]
- Le Douarin N. M. Cell migrations in embryos. Cell. 1984 Sep;38(2):353–360. doi: 10.1016/0092-8674(84)90490-2. [DOI] [PubMed] [Google Scholar]
- McGregor J. L., Clemetson K. J., James E., Capitanio A., Greenland T., Lüscher E. F., Dechavanne M. Glycoproteins of platelet membranes from Glanzmann's thrombasthenia. A comparison with normal using carbohydrate-specific or protein-specific labelling techniques and high-resolution two-dimensional gel electrophoresis. Eur J Biochem. 1981 May 15;116(2):379–388. doi: 10.1111/j.1432-1033.1981.tb05346.x. [DOI] [PubMed] [Google Scholar]
- Morata G., Lawrence P. A. Control of compartment development by the engrailed gene in Drosophila. Nature. 1975 Jun 19;255(5510):614–617. doi: 10.1038/255614a0. [DOI] [PubMed] [Google Scholar]
- Naidet C., Sémériva M., Yamada K. M., Thiery J. P. Peptides containing the cell-attachment recognition signal Arg-Gly-Asp prevent gastrulation in Drosophila embryos. Nature. 1987 Jan 22;325(6102):348–350. doi: 10.1038/325348a0. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Patel V. P., Lodish H. F. The fibronectin receptor on mammalian erythroid precursor cells: characterization and developmental regulation. J Cell Biol. 1986 Feb;102(2):449–456. doi: 10.1083/jcb.102.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plow E. F., Pierschbacher M. D., Ruoslahti E., Marguerie G. A., Ginsberg M. H. The effect of Arg-Gly-Asp-containing peptides on fibrinogen and von Willebrand factor binding to platelets. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8057–8061. doi: 10.1073/pnas.82.23.8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. A 125/115-kDa cell surface receptor specific for vitronectin interacts with the arginine-glycine-aspartic acid adhesion sequence derived from fibronectin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5766–5770. doi: 10.1073/pnas.82.17.5766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothbard J. B., Brackenbury R., Cunningham B. A., Edelman G. M. Differences in the carbohydrate structures of neural cell-adhesion molecules from adult and embryonic chicken brains. J Biol Chem. 1982 Sep 25;257(18):11064–11069. [PubMed] [Google Scholar]
- Ruoslahti E., Pierschbacher M. D. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986 Feb 28;44(4):517–518. doi: 10.1016/0092-8674(86)90259-x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spits H., Yssel H., Takebe Y., Arai N., Yokota T., Lee F., Arai K., Banchereau J., de Vries J. E. Recombinant interleukin 4 promotes the growth of human T cells. J Immunol. 1987 Aug 15;139(4):1142–1147. [PubMed] [Google Scholar]
- Springer T. A., Teplow D. B., Dreyer W. J. Sequence homology of the LFA-1 and Mac-1 leukocyte adhesion glycoproteins and unexpected relation to leukocyte interferon. Nature. 1985 Apr 11;314(6011):540–542. doi: 10.1038/314540a0. [DOI] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Pytela R., Arai H., Krusius T., Pierschbacher M. D., Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamkun J. W., DeSimone D. W., Fonda D., Patel R. S., Buck C., Horwitz A. F., Hynes R. O. Structure of integrin, a glycoprotein involved in the transmembrane linkage between fibronectin and actin. Cell. 1986 Jul 18;46(2):271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Tarone G., Galetto G., Prat M., Comoglio P. M. Cell surface molecules and fibronectin-mediated cell adhesion: effect of proteolytic digestion of membrane proteins. J Cell Biol. 1982 Jul;94(1):179–186. doi: 10.1083/jcb.94.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox M., Brower D. L., Smith R. J. A position-specific cell surface antigen in the drosophila wing imaginal disc. Cell. 1981 Jul;25(1):159–164. doi: 10.1016/0092-8674(81)90240-3. [DOI] [PubMed] [Google Scholar]
- Wilcox M., Brown N., Piovant M., Smith R. J., White R. A. The Drosophila position-specific antigens are a family of cell surface glycoprotein complexes. EMBO J. 1984 Oct;3(10):2307–2313. doi: 10.1002/j.1460-2075.1984.tb02131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]