Abstract
Background and Purpose
Sex and race reportedly influence outcome after recombinant tissue-type plasminogen activator (rtPA). It is, however, unclear whether baseline imbalances (eg, stroke severity) or lack of response to thrombolysis is responsible. We applied balancing methods to test the hypothesis that race and sex influence outcome after rtPA independent of baseline conditions.
Methods
We mapped group outcomes from the National Institute of Neurological Disorders and Stroke (NINDS) dataset based on race and sex onto a surrogate-control function to assess differences from expected outcomes at their respective National Institutes of Health Stroke Scale and age. Outcomes were also compared for subjects matched individually on key baseline factors using NINDS and 2 recent datasets from southeastern United States.
Results
At similar National Institutes of Health Stroke Scale and age, 90-day good outcomes (modified Rankin Score, 0–2) in NINDS were similarly improved after rtPA for white men and women. There was a strong trend for improvement in black men. Conversely, black women treated with rtPA showed response rates no different from the controls. After baseline matching, there were nonsignificant trends in outcomes except for significantly fewer good outcomes in black versus matched white women (37% versus 63%; P=0.027). Pooling the 3 datasets showed a similar trend for poorer short-term outcome for black women (P=0.054; modified Rankin Score, 0–1).
Conclusions
Matching for key baseline factors indicated that race and sex influence outcome most strikingly in black women who demonstrated poorest outcomes after rtPA. This finding supports the hypothesis that poor response to rtPA, rather than differences in baseline conditions, contributes to the worse outcome. This finding requires prospective confirmation.
Keywords: alteplase, race, recombinant tissue-type plasminogen activator, sex, stroke, thrombolysis
Recombinant tissue-type plasminogen activator (rtPA) remains the only Food and Drug Administration (FDA)–approved therapy to improve outcome after acute ischemic stroke.1 The effectiveness in various populations remains incompletely explored and is of considerable importance, given the interest in expanding the ischemic stroke populations eligible for rtPA.2–4 As an example, effectiveness of rtPA in women compared with men remains inconclusive, with studies complicated by differences in initial stroke severity and underlying stroke pathophysiology.5 Although blacks bear a disproportionate burden of stroke incidence and stroke-related morbidity and mortality,6–11 clarification of the role of race in influencing rtPA outcomes has been hampered by small numbers of non-white patients in randomized controlled trials. Qureshi et al12 have shown long-term mortality for black women was highest among a cohort of blacks and whites. However, the differences in baseline factors, such as stroke severity or differences in subtype, could have explained the higher longer term mortality within this study. It is important to explore the relative safety and effectiveness of rtPA in populations with similar baseline characteristics to better define factors that influence any differences that are found. Available datasets include very few blacks treated with rtPA. For example, from the National Hospital Discharge Survey database of 22 842 patients hospitalized with ischemic stroke in the years 2001 to 2006, only 29 blacks received rtPA.13
In the National Institute of Neurological Disorders and Stroke (NINDS) rtPA trial parts A and B, a total of 80 blacks received active drug. A post hoc multivariable analysis of the NINDS dataset was published in 1997 to evaluate multiple factors, including race, with regard to influence on rtPA treatment outcomes.14 Neither race nor other baseline factors were found to influence or independently predict stroke outcomes after rtPA administration, after controlling for the strongly predictive variable of stroke severity at baseline. However, the small subgroup sample sizes and limitations of available analytic techniques suggested that further study was needed to ascertain differences in subgroup response to rtPA.15
We have argued that conventional analytic methodologies used for stroke treatment trials present challenges, particularly for subgroup analyses where sample sizes tend to be small and thus have a greater likelihood of imbalances of baseline factors that influence outcome.16 In this study, we use 2 distinct, complementary techniques to address issues of heterogeneity and small sample numbers to analyze 3 datasets with respect to sex and race. pPREDICTS is based on the generation of a surrogate-control function from the placebo arm of published randomized clinical trials, which relates selected group baseline characteristics to selected efficacy and safety outcomes (eg, modified Rankin Score [mRS], mortality).17 This enables comparison of treatment arm outcomes to an outcome model with similar baseline characteristics.
The second technique pPAIRS matches subjects on an individual basis. Matching is based on dichotomous (eg, race or sex) and continuous (eg, National Institutes of Health Stroke Scale [NIHSS], age, and glucose) variables in Euclidean multidimensional space.18 Unlike other matching methods, such as the Propensity Score, this method requires few assumptions regarding the relationship (eg, linear versus nonlinear) among these factors. Propensity Score accomplishes a similar goal of balancing populations based on selected factors, but has been validated only for large populations.19 pPAIRS can be applied to smaller populations, as long as there is sufficient overlap of subject characteristics for matching.18,20,21 Despite being completed nearly 2 decades ago, the NINDS rtPA dataset remains one of the best characterized datasets, and the only randomized controlled clinical trial of rtPA with a reasonably proportionate enrollment of blacks. Therefore, we used this dataset to investigate potential race, sex, and race-plus-sex outcome differences after rigorous consideration of baseline variables. We then combined the NINDS results with more recently acquired data from 2 southeastern US university stroke programs. A disadvantage of using nonrandomized control populations to compare outcomes after rtPA is there may have been contraindications to rtPA that could worsen outcome in the non–tPA-treated controls. To minimize this potential bias, we include an analysis of baseline-matched populations all of whom received rtPA and compare outcomes. We used these methods to test the hypothesis that race and sex influence outcome after rtPA independent of baseline conditions.
Methods
pPREDICTS Group Comparison
pPREDICTS is a predictive model developed from proportions of subjects in the control arms of 28 randomized controlled trials comprising a total of 7136 subjects.17 Although it was not the primary end point in the NINDS trial, we used an mRS of 0 to 2 as the functional outcome end point because it is the most commonly used end point in published stroke trials. The custom Matlab program generates a surface in 3-dimensional (3D) space that represents an mRS of 0 to 2 proportions expected for a particular baseline NIHSS and age. This surface is bounded on either side by a ±95% prediction interval surface.17 Our model accounted for 90% of the mRS of 0 to 2 and 83% of the mortality variance with respect to NIHSS and age.17
Outcome Assessment
The complete NINDS database was accessed.22 The median NIHSS and mean ages of the NINDS rtPA subgroups of white men and women and black men and women were superimposed on the pPRE-DICTS mRS of 0 to 2 and mortality models.
pPAIRS Euclidean Matching Method
A custom Matlab program was written to match subjects on baseline characteristics, with elimination of outliers as defined a priori.18,20,21 A matching method based on weighted Euclidean distances was adapted to obtain a pair of subjects considered to be the nearest neighbors in an 3D space of NIHSS, age, and glucose.18,23,24 Because there were fewer blacks relative to whites available to match, we were able to find a suitable white subject for each black subject. However, because even among these matches, there may be outliers, to eliminate matched pairs with extreme values of interpair distances, a threshold on the basis of 25th and 75th percentile was chosen.18,25
Study Populations and Outcome Measures
For pPAIRS analysis of the NINDS dataset, we first matched baseline NIHSS, age, glucose, and stroke subtype by race and sex in subjects all of whom had received rtPA to assess the role of race and sex on outcomes after thrombolysis independent of baseline severity. We next assessed the relative effectiveness of rtPA by matching baseline factors for subjects in the rtPA arm to subjects in the placebo arm. Specifically in the matched sample of white and black women, we performed exploratory analyses of multiple demographic and historical factors between the 2 groups.
pPAIRS was then applied to 2 more recently acquired datasets from stroke programs at the University of Alabama at Birmingham and Tulane University. These datasets included patients from 2009 to 2011, (n=90) white and (n=43) black women (University of Alabama at Birmingham) and 2008 to 2010, (n=23) white and (n=46) black women (Tulane). Factors collected included baseline demographic factors and NIHSS, but did not universally include stroke subtype, and for the most part, discharge assessment time point was limited to hospital discharge. Therefore, we pooled all the black and white women treated with rtPA and matched based on factors other than stroke subtype. We used the 7- to 10-day NINDS end point for this analysis. Our interest in this analysis was to confirm the outcomes after rtPA comparing white and black women, and hence we included only those patients that received rtPA.
Statistical Methods
We compared pre- and postmatch baseline factors using Student t test of means (NIHSS, age, and glucose) and Wilcoxon rank sum test of medians (NIHSS). We compared prematch 90-day outcomes (mRS) using Fisher test of proportions. Postmatch continuous demographic variables were compared using t test, and dichotomous variables were compared using McNemar test. We assessed the range of outcomes included in the rtPA NINDS dataset without regard to a predetermined primary outcome measure, as the intent of this experiment is hypothesis generation.
Results
National Institute of Neurological Disorders and Stroke Database
Race and Sex Considered Separately
We examined outcomes based on either sex or race after baseline matching for NIHSS, age, glucose, and stroke subtype using pPAIRS. The full results can be seen in Tables I and II in the online-only Data Supplement. Men and women show similar outcomes after receiving rtPA, with a trend toward less favorable response to rtPA in women compared with men when compared against sex-matched placebo outcomes. For example, among baseline-matched samples, 43.1% of men and 44.0% of women achieved an mRS of 0 to 1 after receiving rtPA, (P=1.00; Table IA in the online-only Data Supplement). Relative to placebo, 50% more men and 21.8% more women achieved an mRS of 0 to 1 after rtPA (Table IB in the online-only Data Supplement).
Comparing outcomes based on race, and not considering sex, we found a nonsignificant trend toward poorer outcomes after rtPA in blacks compared with whites, and lower response to rtPA compared with placebo (Table IIA in the online-only Data Supplement). For example, 46.5% of whites achieved an mRS of 0 to 1 after rtPA, compared with 36.6% of blacks (P=0.190). Relative to placebo, 47.6% more whites achieved an mRS of 0 to 2 compared with 10.8% for blacks (Table IIB in the online-only Data Supplement).
Simultaneous Consideration of Race and Sex
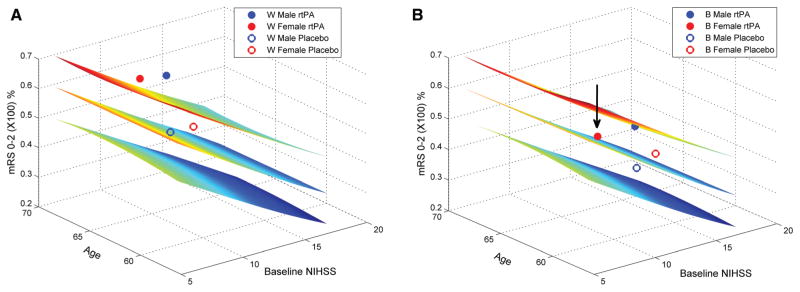
Using the pPREDICTS model, the dataset as divided by race plus sex is shown in the Figure. This analysis relates baseline NIHSS and age to a 90-day mRS of 0 to 2 (z axis). The middle surface is the fitted function. The surfaces above and below this function represent the P=0.05 prediction interval around this surface. Hence, a point above or below this surface indicates outcomes that are greater or less than those of the control arm function. The result for each subgroup is shown, superimposed on the surface at the median NIHSS and mean age of each subgroup.
Figure 1.
Good clinical outcome at 90 days for National Institute of Neurological Disorders and Stroke (NINDS) race-by-sex subgroups relative to the pPREDICTS natural history model of acute ischemic stroke. The pPREDICTS outcome model shows percentage of subjects achieving modified Rankin Score (mRS) ≤2 based on baseline NIHSS and age. The NINDS subgroup results are superimposed on the model at the corresponding baseline NIHSS and age. Placebo outcomes were close to the predicted model for all subgroups (proximity of the control results to the middle outcome surface). Significant improvement with recombinant tissue-type plasminogen activator (rtPA) was seen for both white sexes (A; both rtPA outcomes are above the P=0.05 interval). Black men showed improvement with rtPA that was only slightly below the P=0.05 interval. However, there was no improvement for black women (arrow; B). Although the percentage that achieved an mRS of 0–2 was higher in the rtPA-treated black women group, this increase can be accounted for by baseline imbalance that favored better outcomes. NIHSS indicates National Institutes of Health Stroke Scale.
In Figure (A), the results for white men and women are shown. The groups differ on baseline characteristics (the open circles are not superimposable for the 2 sexes; eg, the women group has a higher baseline NIHSS). However, both control arms are near the pooled surface, indicating that they are comparable with the expected outcomes for the larger pooled sample. This finding suggests that overall outcomes for the placebo arm of randomized controlled trials published since the NINDS trial have not appreciably changed. rtPA treatment outcomes are above the upper surface, for both white men and women, suggesting that rtPA treatment is beneficial for both white sexes (P<0.05).
In Figure (B), the results for black men and women cohort are shown. Both control arms are near to the middle surface, although baseline characteristics differ between the 2 sexes. However, although the treatment arm for black men is near the P=0.05 surface, the treatment arm for the black women is on the control surface, indicating little or no response to thrombolytic treatment (arrow) compared with expected outcomes. This result indicates that outcomes for black women treated with rtPA were not different than for those who received placebo at comparable NIHSS and age.
To pursue these findings, simultaneous matching for sex, race, baseline NIHSS, age, glucose, and stroke subtype was performed using pPAIRS (Tables 1 and 2). The number of subjects available for this analysis after outlier elimination was 42 white men, 30 white women, 42 black men, and 30 black women.
Table 1.
Post-Euclidean Matching Results
| Men
|
Women
|
|||||
|---|---|---|---|---|---|---|
| White rtPA | Black rtPA | P Value | White rtPA | Black rtPA | P Value | |
| Median NIHSS | 16.0 | 15.0 | 0.861 | 15.0 | 15.0 | 0.604 |
| Mean NIHSS | 15.3±6.63 | 15.1±6.79 | 0.897 | 14.4±7.70 | 15.5±7.5 | 0.589 |
| Age, y | 66.5±12.7 | 65.2±11.3 | 0.629 | 70.6±9.1 | 67.4±10.2 | 0.204 |
| Glucose | 130±47.52 | 130±52.00 | 0.970 | 146±61.20 | 159±80.69 | 0.489 |
| SV/LV/CE, n | 5/19/18 | 5/19/18 | … | 7/7/16 | 7/7/16 | … |
| SV/LV/CE, % | 12/45/43 | 12/45/43 | … | 23/23/53 | 23/23/53 | … |
| mRS 0–1 | 0.452 | 0.381 | 0.579 | 0.567 | 0.367 | 0.077 |
| mRS 0–2 | 0.571 | 0.452 | 0.302 | 0.633 | 0.367 | 0.027 |
| BI 95–100 | 0.548 | 0.476 | 0.450 | 0.667 | 0.400 | 0.077 |
| GOS 0–1 | 0.500 | 0.429 | 0.606 | 0.600 | 0.367 | 0.046 |
| NIHSS 0–1 | 0.429 | 0.238 | 0.043 | 0.300 | 0.333 | 1.000 |
| Mortality | 0.238 | 0.143 | 0.343 | 0.067 | 0.233 | 0.131 |
| Sym Hem | 0.024 | 0.024 | 0.480 | 0.067 | 0.000 | 0.480 |
| Asym Hem | 0.024 | 0.000 | 1.000 | 0.033 | 0.067 | 1.000 |
All treated with rtPA. Postmatching sample of rtPA-treated whites and blacks matched for NIHSS, age, glucose, and stroke subtype. SV/LV/CE indicates the number (n) and percentage (%) of subjects with the subtype; postmatching is identical between the 2 groups.
Asym Hem indicates Asymptomatic Hemorrhage; BI, Barthel Index; CE, cardioembolic; GOS, Glasgow Outcome Scale; LV, large vessel; mRS, modified Rankin Score; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue-type plasminogen activator; Sym Hem, Symptomatic hemorrhage; and SV, small vessel.
Table 2.
Post-Euclidean Matching for Black Women Treated With Placebo or Recombinant Tissue-Type Plasminogen Activator
| Black rtPA Post match | Black Placebo Post match | P Value | |
|---|---|---|---|
| Med NIHSS | 16.0 | 16.0 | 0.876 |
| Mean NIHSS | 16.0±7.61 | 16.0±7.14 | 0.869 |
| Age, y | 65.1±11.9 | 66.2±13.9 | 0.254 |
| Glucose | 145±63.15 | 141±65.53 | 0.819 |
| SV/LV/CE, n | 4/7/16 | 4/7/16 | … |
| SV/LV/CE, % | 15/26/59 | 15/26/59 | … |
| mRS 0–1 | 0.333 | 0.333 | 0.683 |
| mRS 0–2 | 0.333 | 0.333 | 0.683 |
| BI 95–100 | 0.370 | 0.296 | 0.221 |
| GOS 0–1 | 0.333 | 0.333 | 0.683 |
| Mortality | 0.259 | 0.222 | 1.000 |
| Sym Hem | 0.000 | 0.037 | 1.000 |
| Asym Hem | 0.074 | 0.037 | 1.000 |
Postmatching sample of black women matched for NIHSS, age, glucose, and stroke subtype, comparing treatment response to rtPA compared with placebo. SV/LV/CE indicates the number (n) and percentage (%) of subjects; postmatching is identical between the 2 groups.
CE indicates cardioembolic; LV, large vessel; mRS, modified Rankin Score; NIHSS, National Institutes of Health Stroke Scale; rtPA, recombinant tissue-type plasminogen activator; and SV, small vessel.
There was a trend toward a lower percentage of black men compared with white men who achieved an mRS of 0 to 2 after receiving rtPA, and fewer black men versus white men achieved NIHSS 0 to 1 (23.8% versus 42.9%, respectively; P=0.043). Among black women who received rtPA, the proportion with good poststroke outcome (mRS, 0–2) was lower when compared with matched white women: 37% for black women versus 63% for white women (P=0.027). Compared with white women, 42% fewer black women had a good poststroke functional outcome after rtPA. Mortality was nonsignificantly higher in black women (23.3% versus 6.7%; P=0.131; Table 1). We further examined this trend of higher mortality by superimposing the original women group data onto our pPREDICTS mortality function (Figure I in the online-only Data Supplement). The highest mortality can be seen in black women who received rtPA, approaching the P=0.05 upper boundary.
We hypothesized that lack of response to rtPA compared with placebo is 1 possible explanation for the poorer outcome of black women treated with rtPA compared with white women. Among black women, after balancing on baseline NIHSS, age, glucose, and stroke subtype, the percentage that achieved either an mRS of 0 to 1 or 0 to 2 was 33% in both rtPA and placebo groups (Table 2), confirming this hypothesis.
Exploratory results of the post hoc test of demographics and historical factors between baseline-matched white and black women showed that history of diabetes mellitus, weight, and blood urea nitrogen differed between black and white women (Table III in the online-only Data Supplement). Notably, time to treatment was nonsignificantly higher in black women with a difference of 15 minutes (P=0.15).
Outcomes of Post-rtPA women patients from the combined NINDS, Tulane, and University of Alabama at Birmingham Datasets
From the combined datasets of NINDS, Tulane, and University of Alabama at Birmingham, 120 black women and 201 white subjects were available for analysis. Euclidean matching identified 120 white women as nearest neighbors to the 120 black women. Among these pairs, 8 outlier black–white pairs (7%) were subsequently eliminated because of prespecified outlier definition. Comparison of baseline variables, efficacy, and safety outcomes prematch, postmatch, and for pairs eliminated postmatch are shown in Table IV in the online-only Data Supplement for women (results for men are shown in Table V in the online-only Data Supplement). In general, eliminated subjects had a lower NIHSS and in the case of blacks, much higher glucose.
Baseline characteristics for the resulting final matched sample were similar in both white and black women. We found a strong trend for white women treated with rtPA to have better short-term outcomes in terms of mRS of 0 to 1 (P=0.054). In contrast to the 90-day NINDS outcomes, mortality at this time point was identical between the 2 races and the mRS of 0 to 2 did not differ. Results for this cohort are shown in Table 3. No significant differences were found in the combined database comparing white and black men treated with rtPA (Table 4).
Table 3.
Postmatch Results for Women, by Race for the Combined National Institute of Neurological Disorders and Stroke, Tulane, and University of Alabama at Birmingham Cohort
| White rtPA | Black rtPA | P Value | |
|---|---|---|---|
| Median NIHSS | 12.5 | 13.0 | 0.922 |
| Mean NIHSS | 12.8±7.04 | 12.9±7.07 | 0.902 |
| Age, y | 68.5±15.5 | 67.6±17.2 | 0.690 |
| Glucose | 138±62.45 | 138±62.37 | 0.951 |
| Number | 112 | 112 | … |
| mRS 0–1 | 0.304 | 0.205 | 0.054 |
| mRS 0–2 | 0.357 | 0.339 | 0.864 |
| Mortality | 0.071 | 0.071 | 0.803 |
| Sym Hem | 0.045 | 0.036 | 1.000 |
All treated with rtPA. Tulane and UAB patients were evaluated at discharge and NINDS patients were assessed 7 to 10 days after treatment.
mRS indicates modified Rankin Score; NIHSS, National Institutes of Health Stroke Scale; NINDS, National Institute of Neurological Disorders and Stroke; rtPA, recombinant tissue-type plasminogen activator; and UAB, University of Alabama at Birmingham.
Table 4.
Postmatch Results for Men, by Race for the Combined National Institute of Neurological Disorders and Stroke, Tulane, and University of Alabama at Birmingham Cohort
| White rtPA | Black rtPA | P Value | |
|---|---|---|---|
| Median NIHSS | 11.0 | 10.5 | 0.967 |
| Mean NIHSS | 11.6±7.18 | 11.6±7.21 | 0.993 |
| Age, y | 62.7±13.4 | 62.6±13.9 | 0.946 |
| Glucose | 125±48.58 | 126±48.15 | 0.841 |
| Number | 136 | 136 | |
| mRS 0–1 | 0.331 | 0.287 | 0.417 |
| mRS 0–2 | 0.434 | 0.404 | 0.658 |
| Mortality | 0.088 | 0.066 | 0.628 |
| Sym Hem | 0.015 | 0.059 | 0.114 |
All treated with rtPA. Tulane and UAB patients were evaluated at discharge, and NINDS patients were assessed 7 to 10 days after treatment.
mRS indicates modified Rankin Score; NIHSS, National Institutes of Health Stroke Scale; NINDS, National Institute of Neurological Disorders and Stroke; rtPA, recombinant tissue-type plasminogen activator; and UAB, University of Alabama at Birmingham.
Discussion
Application of methods to accommodate baseline imbalances in the NINDS dataset showed differences in response to rtPA after consideration of both race and sex simultaneously. After rtPA, outcomes among white women were similar to those of white men after baseline factor matching, although there was a trend toward lower response relative to placebo. There was a nonsignificant trend toward less benefit from rtPA in black men compared with white men. However, the most salient finding was a significantly lower proportion of black women treated with rtPA had a good or excellent functional outcome at 90 days compared with baseline-matched white women. This finding supports our hypothesis that poor response to rtPA, rather than more severe baseline conditions, explains previous findings of worse outcomes in black women.
Consistent with longer emergency department wait times in blacks,26 time to treatment (TTT) was nonsignificantly higher in black women and may have had some influence on the poorer outcome after rtPA in black women, but the difference was small. Uchino et al27 and others have shown that, within usual time windows, NIHSS is a much greater predictor of outcome than TTT. Estimates based on the analysis of Lees et al’s28 outcome versus the TTT model suggest that a small difference in odds ratios would have been expected between the 1.78 seen for white women at a TTT of 117 minutes compared with 1.72 for black women at a TTT of 132 minutes.
Ninety-day follow-up of the rtPA-treated white and black women from the NINDS dataset indicated a poorer functional outcome after rtPA in black women and a trend toward higher mortality. These results were partly confirmed by expanding the dataset with more recently acquired patients and looking at short-term results, demonstrating the same trend toward poorer functional outcome, although higher mortality did not manifest for this shorter time. This finding, plus Qureshi et al’s12 report of higher long-term mortality (without baseline matching, however), suggests that longer term factors may be more important than short-term recovery to survival for black women after stroke. The consequences of a much higher incidence of diabetes mellitus could be one such factor.
It is biologically plausible that there are race-based differences that bear on benefit and risk of rtPA administration. In a study of 95 healthy black and 95 healthy European Americans, a prothrombotic genotype of plasma inhibitor-1 was present in 55% of the blacks compared with 16% of European Americans (PAI-1-668delG G/G; P<0.001).29 Plasminogen activator inhibitor acts to inhibit rtPA’s enzymatic conversion of the inactive proenzyme plasminogen to an active trypsin-like protease, plasmin, critical for fibrin clot lyses. The effectiveness of rtPA is dependent on the amount of delivered drug that becomes enzymatically active. An interaction between rtPA and its main plasma inhibitor PAI-1 could differentially influence efficacy and safety of rtPA among blacks versus European Americans and in black women, in particular.30 Pandolfi et al31 found increased PAI-1 in arterial walls of diabetics versus controls, raising the possibility of an interaction among diabetes mellitus, race, and sex in conferring relative resistance to benefits of rtPA among black women.
It is unclear whether body mass index, weight, appropriateness of rtPA dose, type of stroke or distribution of atherosclerosis (eg, extra versus intracranial), and interaction of these factors play a role in poorer outcome of black women. Interestingly weight of black women was lower than white women. We are unable to calculate body mass index of the patients from NINDS database because of unavailability of height parameter. We explicitly matched for type of stroke in the NINDS database but broad conclusions on the influence of stroke subtype on outcome cannot be made because of small sample sizes. The interaction of race, sex, stroke risk factors, metabolic indices, and biological/imaging markers requires further study.
There are several alternative approaches to our matching methodology to balance baseline factors that may influence outcome. Simple case control matching based on a few characteristics can be accomplished manually. However, simultaneous manual matching on multiple variables is more difficult, and the algorithm used here guarantees nearest neighbor matching ≤12 variables in an efficient manner.24 Covariate adjustment of baseline factors is a widely used alternative to matching to assess the contribution of confounders (eg, imbalances).32 However, covariate adjustment of baseline factors also makes outcomes assumptions that are not typically tested or met in stroke trials. Unsubstantiated assumptions include that treatment effect is consistent across different levels of baseline variables, and that there is considerable overlap in the distributions of baseline variables in treatment and control arms of a study.33 Also, the relationship between functional outcome and NIHSS does not follow a linear relationship across the entire range.16,34
Our study is limited by the fact that our findings are mainly based on the nearly 2 decade old NINDS dataset. However, it is reassuring that the control arms from NINDS are still consistent with current outcomes generated from a contemporary pooled sample using pPREDICTS analysis. The same trend regarding poorer functional outcomes with fewer black women achieving an mRS of 0 to 1 was found when pooled with the more recently acquired data from 2008 to 2011. Because there may be many factors that contribute to the poorer outcomes found here, including factors not measured,35 a prospective evaluation of comparative outcomes in blacks versus whites with ischemic stroke that receive rtPA using rigorous baseline assessments and carefully matched on key baseline factors is needed to confirm these results and identify factors that could influence longer term outcomes.
Supplementary Material
Footnotes
Disclosures
Drs Mandava and Kent have a copyright but no financial interest in pPREDICTS and pPAIRS. The Stroke Outcomes Laboratory was established through a grant from the pilot grant program of the Institute for Clinical and Translational Research at the Baylor College of Medicine. No relevant disclosures for Drs Murthy, Munoz, Simon, Alexandrov, Albright, Boehme, Martin-Schild, and Martini. Dr McGuire serves on the speaker’s bureau of AAN, Ethics Research Colloquium (<$10k), serves on the advisory board of Morehouse School of Medicine Health Disparities in Neurological Diseases (<$10k), and has received honoraria from University of Iowa School of Medicine Brain Aging Panel (<$10k).
The online-only Data Supplement is available with this article at http://stroke.ahajournals.org/lookup/suppl/doi:10.1161/STROKEAHA.113.001116/-/DC1.
References
- 1.The National Institute of Neurological Disorders and Stroke rtPA Study Group. Tissue plasminogen activator for acute ischemic stroke. N Eng J Med. 1995;333:1581–1588. [Google Scholar]
- 2.Hacke W, Kaste M, Bluhmki E, Brozman M, Davalos A, Guidetti D, et al. Thrombolysis with alteplase in 3 to 4.5 hours after ischemic stroke. N Eng J Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 3.The IST-3 Collaborative Group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke: a randomized controlled trial. Lancet. 2012;379:2352–2363. doi: 10.1016/S0140-6736(12)60768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bushnell CD. Stroke and the female brain. Nat Clin Pract Neurol. 2008;4:22–33. doi: 10.1038/ncpneuro0686. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, et al. Heart disease and stroke statistics--2010 update: A report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 7.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and postacute outcomes. Stroke. 2005;36:374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 8.Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, et al. Stroke incidence and survival among middle-aged adults: 9-year follow-up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- 9.Howard G, Howard VJ REasons for Geographic And Racial Differences in Stroke (REGARDS) Investigators. Ethnic disparities in stroke: the scope of the problem. Ethn Dis. 2001;11:761–768. [PubMed] [Google Scholar]
- 10.Casper ML, Nwaise IA, Croft JB, Nilasena DS. Atlas of Stroke Hospitalizations Among Medicare Beneficiaries. Atlanta: Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 11.Schwamm LH, Reeves MJ, Pan W, Smith EE, Frankel MR, Olson D, et al. Race/ethnicity, quality of care, and outcomes in ischemic stroke. Circulation. 2010;121:1492–1501. doi: 10.1161/CIRCULATIONAHA.109.881490. [DOI] [PubMed] [Google Scholar]
- 12.Qureshi AI, Suri MF, Zhou J, Divani AA. African American women have poor long-term survival following ischemic stroke. Neurology. 2006;67:1623–1629. doi: 10.1212/01.wnl.0000242756.00084.f9. [DOI] [PubMed] [Google Scholar]
- 13.Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med. 2010;5:406–409. doi: 10.1002/jhm.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Generalized efficacy of t-PA for acute stroke. Subgroup analysis of the NINDS t-PA Stroke Trial. The NINDS tPA Study Group. Stroke. 1997;28:2119–2125. doi: 10.1161/01.str.28.11.2119. [DOI] [PubMed] [Google Scholar]
- 15.Ingall TJ, O’Fallon WM, Asplund K, Goldfrank LR, Hertzberg VS, Louis TA, et al. Findings from the reanalysis of the NINDS tissue plasminogen activator for acute ischemic stroke treatment trial. Stroke. 2004;35:2418–2424. doi: 10.1161/01.STR.0000140891.70547.56. [DOI] [PubMed] [Google Scholar]
- 16.Mandava P, Krumpelman CS, Murthy SB, Kent TA. A critical review of stroke trial analytical methodology: outcome measures, study design and correction for imbalances. In: Lapchak PA, Zhang JH, editors. Translational Stroke Research: From Target Selection to Clinical Trials. New York, NY: Springer; 2012. pp. 833–862. [Google Scholar]
- 17.Mandava P, Kent TA. A method to determine stroke trial success using multidimensional pooled control functions. Stroke. 2009;40:1803–1810. doi: 10.1161/STROKEAHA.108.532820. [DOI] [PubMed] [Google Scholar]
- 18.Mandava P, Kalkonde YV, Rochat RH, Kent TA. A matching algorithm to address imbalances in study populations: application to the National Institute of Neurological Diseases and Stroke Recombinant Tissue Plasminogen Activator acute stroke trial. Stroke. 2010;41:765–770. doi: 10.1161/STROKEAHA.109.574103. [DOI] [PubMed] [Google Scholar]
- 19.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 20.Kunte H, Busch MA, Trostdorf K, Vollnberg B, Harms L, Mehta RI, et al. Hemorrhagic transformation of ischemic stroke in diabetics on sulfonylureas. Ann Neurol. 2012;72:799–806. doi: 10.1002/ana.23680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mandava P, Dalmeida W, Anderson JA, Thiagarajan P, Fabian RH, Weir RU, et al. A pilot trial of low-dose intravenous abciximab and unfractionated heparin for acute ischemic stroke: Translating GP IIb/IIIa receptor inhibition to clinical practice. Transl Stroke Res. 2010;1:170–177. doi: 10.1007/s12975-010-0026-4. [DOI] [PubMed] [Google Scholar]
- 22.Dachs RJ, Burton JH, Joslin J. A user’s guide to the NINDS rt-PA stroke trial database. PLoS Med. 2008;5:e113. doi: 10.1371/journal.pmed.0050113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bergstralh EJ, Kosanke JL. Computerized matching of cases to controls. [Accessed on March 9, 2013];Technical report 56. Available at: http://mayoresearch.mayo.edu/mayo/research/biostat/upload/56.pdf.
- 24.Friedman JH, Bentley JL, Finkel RA. An algorithm for finding best matches in logarithmic time. ACM Trans Math Softw. 1977;3:209–226. [Google Scholar]
- 25.McGill R, Tukey JW, Larsen WA. Variations of box plots. Am Stat. 1978;32:12–16. [Google Scholar]
- 26.Karve SJ, Balkrishnan R, Mohammad YM, Levine DA. Racial/ethnic disparities in emergency department waiting time for stroke patients in the United States. J Stroke Cerebrovasc Dis. 2011;20:30–40. doi: 10.1016/j.jstrokecerebrovasdis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 27.Uchino K, Billheimer D, Cramer SC. Entry criteria and baseline characteristics predict outcome in acute stroke trials. Stroke. 2001;32:909–916. doi: 10.1161/01.str.32.4.909. [DOI] [PubMed] [Google Scholar]
- 28.Lees KR, Bluhmki E, von Kummer R, Brott TG, Toni D, Grotta JC, et al. ECASS, ATLANTIS, NINDS and EPITHET rt-PA Study Group. Time to treatment with intravenous alteplase and outcome in stroke: an updated pooled analysis of ECASS, ATLANTIS, NINDS, and EPITHET trials. Lancet. 2010;375:1695–1703. doi: 10.1016/S0140-6736(10)60491-6. [DOI] [PubMed] [Google Scholar]
- 29.Lanfear DE, Marsh S, Cresci S, Shannon WD, Spertus JA, McLeod HL. Genotypes associated with myocardial infarction risk are more common in African Americans than in European Americans. J Am Coll Cardiol. 2004;44:165–167. doi: 10.1016/j.jacc.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 30.Perry A, Wang X, Goldberg R, Ross R, Jackson L. The relationship between cardiometabolic and hemostatic variables: influence of race. Metabolism. 2008;57:200–206. doi: 10.1016/j.metabol.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 31.Pandolfi A, Cetrullo D, Polishuck R, Alberta MM, Calafiore A, Pellegrini G, et al. Plasminogen activator inhibitor type 1 is increased in the arterial wall of type II diabetic subjects. Arterioscler Thromb Vasc Biol. 2001;21:1378–1382. doi: 10.1161/hq0801.093667. [DOI] [PubMed] [Google Scholar]
- 32.O’Fallon WM, Asplund K, Goldfrank LR, Hertzberg VS, Ingall TJ, Louis TA. Report of the t-PA Review Committee. National Institute of Neurologic Diseases and Stroke; 2004. [Accessed on February 11, 2012]. Available at: http://www.ninds.nih.gov/funding/review_committees/t-pa_review_committee/t-pa_committee_report.pdf. [Google Scholar]
- 33.Committee for Proprietary Medicinal Products. Points to consider on adjustment of baseline covariates. Stat Med. 2004;23:701–709. doi: 10.1002/sim.1647. [DOI] [PubMed] [Google Scholar]
- 34.Adams HP, Jr, Davis PH, Leira EC, Chang KC, Bendixen BH, Clarke WR, et al. Baseline NIH Stroke Scale score strongly predicts outcome after stroke: A report of the Trial of Org 10172 in Acute Stroke Treatment (TOAST) Neurology. 1999;53:126–131. doi: 10.1212/wnl.53.1.126. [DOI] [PubMed] [Google Scholar]
- 35.Cruz-Flores S, Rabinstein A, Biller J, Elkind MS, Griffith P, Gorelick PB, et al. Racialethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.