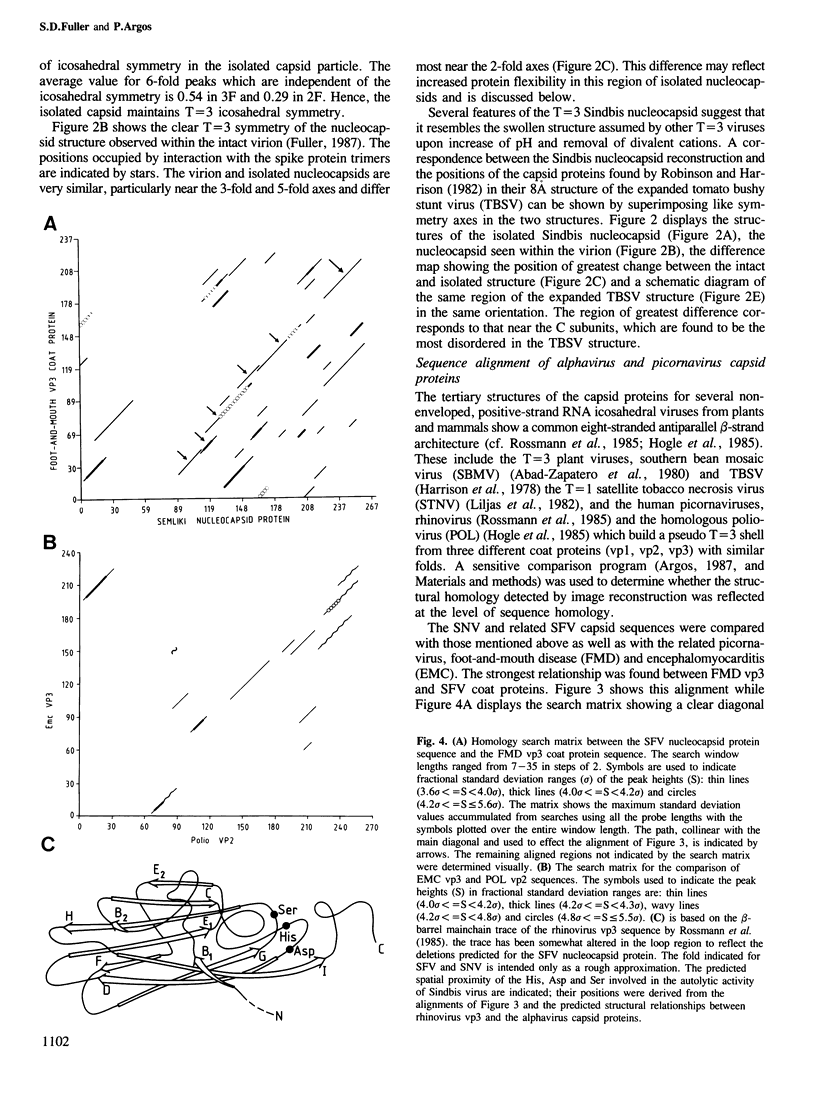
Abstract
A three-dimensional image reconstruction was performed from cryo-electron micrographs of isolated Sindbis (SNV) nucleocapsids. The isolated capsid is a smooth but fenestrated T = 3 structure. Comparison with the nucleocapsid seen within the whole virion indicated that the structure resembles the swollen forms which some non-enveloped viruses adopt after removal of divalent cations. A sensitive comparison method was used to align SNV capsid protein sequences with those of picornavirus vp3 capsid proteins whose high resolution structures display an eight-stranded beta-barrel fold found in many icosahedral viruses. The alignment predicted a similar folding for the Sindbis protein which juxtaposes several sets of residues known to be essential for its serine proteolytic activity. These results suggest that the capsid proteins of the enveloped alphaviruses and the non-enveloped picornaviruses may have arisen through divergent evolution from a simple, vp3-like ancestor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argos P. A sensitive procedure to compare amino acid sequences. J Mol Biol. 1987 Jan 20;193(2):385–396. doi: 10.1016/0022-2836(87)90226-9. [DOI] [PubMed] [Google Scholar]
- Argos P. Evidence for a repeating domain in type I restriction enzymes. EMBO J. 1985 May;4(5):1351–1355. doi: 10.1002/j.1460-2075.1985.tb03784.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argos P., Garavito R. M., Eventoff W., Rossmann M. G., Brändén C. I. Similarities in active center geometries of zinc-containing enzymes, proteases and dehydrogenases. J Mol Biol. 1978 Dec 5;126(2):141–158. doi: 10.1016/0022-2836(78)90356-x. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Brown D. T., Gliedman J. B. Morphological variants of Sindbis virus obtained from infected mosquito tissue culture cells. J Virol. 1973 Dec;12(6):1534–1539. doi: 10.1128/jvi.12.6.1534-1539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Enzmann P. J., Weiland F. Studies on the morphology of alphaviruses. Virology. 1979 Jun;95(2):501–510. doi: 10.1016/0042-6822(79)90504-x. [DOI] [PubMed] [Google Scholar]
- Garavito R. M., Rossmann M. G., Argos P., Eventoff W. Convergence of active center geometries. Biochemistry. 1977 Nov 15;16(23):5065–5071. doi: 10.1021/bi00642a019. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. Nucleotide sequence of cdna coding for Semliki Forest virus membrane glycoproteins. Nature. 1980 Nov 20;288(5788):236–241. doi: 10.1038/288236a0. [DOI] [PubMed] [Google Scholar]
- Garoff H., Frischauf A. M., Simons K., Lehrach H., Delius H. The capsid protein of Semliki Forest virus has clusters of basic amino acids and prolines in its amino-terminal region. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6376–6380. doi: 10.1073/pnas.77.11.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn C. S., Strauss E. G., Strauss J. H. Sequence analysis of three Sindbis virus mutants temperature-sensitive in the capsid protein autoprotease. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4648–4652. doi: 10.1073/pnas.82.14.4648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogle J. M., Chow M., Filman D. J. Three-dimensional structure of poliovirus at 2.9 A resolution. Science. 1985 Sep 27;229(4720):1358–1365. doi: 10.1126/science.2994218. [DOI] [PubMed] [Google Scholar]
- Jones D. D. Amino acid properties and side-chain orientation in proteins: a cross correlation appraoch. J Theor Biol. 1975 Mar;50(1):167–183. doi: 10.1016/0022-5193(75)90031-4. [DOI] [PubMed] [Google Scholar]
- Kamer G., Argos P. Primary structural comparison of RNA-dependent polymerases from plant, animal and bacterial viruses. Nucleic Acids Res. 1984 Sep 25;12(18):7269–7282. doi: 10.1093/nar/12.18.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- Kraut J., Robertus J. D., Birktoft J. J., Alden R. A., Wilcox P. E., Powers J. C. The aromatic substrate binding site in subtilisin BPN' and its resemblance to chymotrypsin. Cold Spring Harb Symp Quant Biol. 1972;36:117–123. doi: 10.1101/sqb.1972.036.01.017. [DOI] [PubMed] [Google Scholar]
- Liljas L., Unge T., Jones T. A., Fridborg K., Lövgren S., Skoglund U., Strandberg B. Structure of satellite tobacco necrosis virus at 3.0 A resolution. J Mol Biol. 1982 Jul 25;159(1):93–108. doi: 10.1016/0022-2836(82)90033-x. [DOI] [PubMed] [Google Scholar]
- Manavalan P., Ponnuswamy P. K. Hydrophobic character of amino acid residues in globular proteins. Nature. 1978 Oct 19;275(5681):673–674. doi: 10.1038/275673a0. [DOI] [PubMed] [Google Scholar]
- Matthews B. W. Solvent content of protein crystals. J Mol Biol. 1968 Apr 28;33(2):491–497. doi: 10.1016/0022-2836(68)90205-2. [DOI] [PubMed] [Google Scholar]
- Palau J., Argos P., Puigdomenech P. Protein secondary structure. Studies on the limits of prediction accuracy. Int J Pept Protein Res. 1982 Apr;19(4):394–401. [PubMed] [Google Scholar]
- Palmenberg A. C., Kirby E. M., Janda M. R., Drake N. L., Duke G. M., Potratz K. F., Collett M. S. The nucleotide and deduced amino acid sequences of the encephalomyocarditis viral polyprotein coding region. Nucleic Acids Res. 1984 Mar 26;12(6):2969–2985. doi: 10.1093/nar/12.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Kirby E. M., Janda M. R., Drake N. L., Duke G. M., Potratz K. F., Collett M. S. The nucleotide and deduced amino acid sequences of the encephalomyocarditis viral polyprotein coding region. Nucleic Acids Res. 1984 Mar 26;12(6):2969–2985. doi: 10.1093/nar/12.6.2969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann M. G., Abad-Zapatero C., Murthy M. R., Liljas L., Jones T. A., Strandberg B. Structural comparisons of some small spherical plant viruses. J Mol Biol. 1983 Apr 25;165(4):711–736. doi: 10.1016/s0022-2836(83)80276-9. [DOI] [PubMed] [Google Scholar]
- Rossmann M. G., Argos P. A comparison of the heme binding pocket in globins and cytochrome b5. J Biol Chem. 1975 Sep 25;250(18):7525–7532. [PubMed] [Google Scholar]
- Rossmann M. G., Arnold E., Erickson J. W., Frankenberger E. A., Griffith J. P., Hecht H. J., Johnson J. E., Kamer G., Luo M., Mosser A. G. Structure of a human common cold virus and functional relationship to other picornaviruses. Nature. 1985 Sep 12;317(6033):145–153. doi: 10.1038/317145a0. [DOI] [PubMed] [Google Scholar]
- Simons K., Warren G. Semliki Forest virus: a probe for membrane traffic in the animal cell. Adv Protein Chem. 1984;36:79–132. doi: 10.1016/S0065-3233(08)60296-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. G., Rice C. M., Strauss J. H. Complete nucleotide sequence of the genomic RNA of Sindbis virus. Virology. 1984 Feb;133(1):92–110. doi: 10.1016/0042-6822(84)90428-8. [DOI] [PubMed] [Google Scholar]
- Söderlund H., Käriäinen L., von Bonsdorff C. H. Properties of Semliki Forest virus nucleocapsid. Med Biol. 1975 Oct;53(5):412–417. [PubMed] [Google Scholar]
- Söderlund H., Käriänen L., Von Bonsdorff C. H., Weckström P. Properties of Semliki forest virus nucleocapsid. II. An irreversible contraction by acid pH. Virology. 1972 Mar;47(3):753–760. doi: 10.1016/0042-6822(72)90565-x. [DOI] [PubMed] [Google Scholar]
- Söderlund H., von Bonsdorff C. H., Ulmanen I. Comparison of the structural properties of Sindbis and Semliki forest virus nucleocapsids. J Gen Virol. 1979 Oct;45(1):15–26. doi: 10.1099/0022-1317-45-1-15. [DOI] [PubMed] [Google Scholar]
- Vogel R. H., Provencher S. W., von Bonsdorff C. H., Adrian M., Dubochet J. Envelope structure of Semliki Forest virus reconstructed from cryo-electron micrographs. Nature. 1986 Apr 10;320(6062):533–535. doi: 10.1038/320533a0. [DOI] [PubMed] [Google Scholar]