Abstract
Introduction
Excess adiposity constitute an important public health problem because of the associated increased risk of hypertension, coronary heart disease, type 2 diabetes and other disorders. Not only the contribution of general measures of adiposity but also central measures of adiposity have been observed.
Aim
To compare and correlate the central and general adiposity indices with ventricular electrocardiographic variables and vascular stiffness indices in normal, overweight and obese young adults.
Materials and Methods
This was a cross-sectional study. Nearly ninety subjects were enrolled and were divided into 30 normal weight, 30 overweight and 30 obese group based on the BMI classification of WHO for Asian population with their age ranging from 18-25 years. Waist and hip circumferences were measured using stretchable tape. Two novel indices-conicity index and abdominal volume index were calculated using standard formula.
ECG and pulse wave were recorded using the Physiopac – Medicaid systems. Statistical analysis was done using SPSS version 19.0 software. ANOVA test was done to compare the variables among three groups. Pearson’s correlation coefficients were applied to establish the correlations between variables.
Results
In our study Body Mass Index (BMI) and Conicity Index (CI) was significantly and strongly correlated with the ventricular electrocardiographic variables especially with QRS duration, QTc interval and with vascular stiffness indices. These indices can be used to assess the electrocardiographic abnormalities and vascular stiffness status.
Conclusion
Excess adipose tissue in young adults was closely linked to ventricular depolarization and repolarization abnormalities and also to increased vascular stiffness. Adiposity indices in particular (BMI, CI) provide a simple and non invasive approach to assess these abnormalities at the earliest in order to prevent future complications.
Keywords: Abdominal fat, Abdominal volume index, Conicity index, Reflection index, Stiffness index, Total fat
Introduction
Obesity is associated with numerous comorbidities such as coronary heart disease, type 2 diabetes, hypertension, certain cancers, sleep apnea and many other disorders [1]. Because of the lifestyle, socioeconomic status, advancement in entertainment and technology such as television, computer, and video games the prevalence of overweight and obesity has been increased [2].
Various adiposity indices have been used to assess the total and abdominal fat [3,4]. Certain adiposity indices have been useful in assessing cardiovascular risk [5], few others in prediction of diabetes mellitus [6]. The results of previous studies on adiposity and arterial stiffness have not been consistent. In many studies association of BMI with vascular stiffness, electrocardiographic variables have been observed [7-9]. Abdominal volume index and conicity index are two recent novel obesity indices which were included in this study [10]. Moreover, in majority of the studies, observations have been done based on the WHO classification of body weight. But we have considered the BMI classification for Asian population, according to which BMI >25 will be considered as obese [11]. A recent study observed abdominal obesity to be associated with arterial stiffness in middle-aged adults [12] but in the present study young adults were considered.
The aim of this study was to record and compare the central and general adiposity indices, electrocardiographic variables and vascular stiffness indices in normal, overweight and obese young adults. To correlate adiposity indices with QRS duration and amplitude, corrected QT interval and T peak-Tend interval in young adults a to determine the adiposity index which is more strongly correlated with change in electrocardiographic variables and to correlate adiposity indices with vascular stiffness and reflection indices in young adults and to determine the adiposity index which is more strongly correlated with change in vascular stiffness indices.
Materials and Methods
This was a cross-sectional study conducted in the research lab, Department of Physiology, SRM Medical College Hospital and Research Institute, Kattankulathur, Chennai, Tamilnadu, India, for a period of two months from March 30th to May 30th 2016. The subjects enrolled in this study had been divided into control, overweight and obese group based on the BMI classification of WHO for Asian population [11].
Group 1 was considered as control group (BMI: 18.5-22.9). It included 30 apparently healthy male volunteers, with their age ranging from 18-25 years.
Group 2 was considered as overweight group (BMI: 23-24.9). It included 30 apparently healthy male volunteers, with their age ranging from 18-25 years.
Group 3 was considered as obese group (BMI: >25). It included 30 apparently healthy male volunteers, with their age ranging from 18-25 years.
Institutional Ethical Clearance was obtained. Written informed consent was obtained from all the participants prior to the initiation of study. A complete record of medical and personal history was obtained from all the subjects.
Apparently healthy obese, overweight and normal male individuals within the age group of 18-25 years were involved. Those who were smokers and alcoholics and with history of cardiovascular diseases, kidney disease, thyroid disorders, diabetes, hypertension, syncope were excluded. Those on medications such as diuretics, antidepressants, antipsychotic and other drugs which might prolong QT interval were also excluded from the study.
Measurements
Blood pressure was measured in accordance with the British hypertension society guidelines [13] using a mercury sphygmomanometer and stethoscope. Standing height was recorded without shoes and with light clothes using stadiometer to the nearest of centimeters. Weight was recorded without shoes and with light clothes on a weighing machine.
BMI was calculated as body weight (kg) divided by body height (m2).
Waist and hip circumferences were obtained using a stretchable measuring tape in standing position. Waist circumference was measured at approximate midpoint between the lower margin of the last costal rib and the top of the iliac crest. Hip circumference was taken around the widest portion of the buttocks [14].
Waist-to-hip Ratio (WHR) was calculated from the above measurements by using the formula, WHR = waist circumference (cm) divided by hip circumference (cm).
Waist to height ratio was calculated from the above measurements by using the formula, waist circumference (cm) divided by height (cm).
The conicity index (C index) was derived using the following formula [15]:
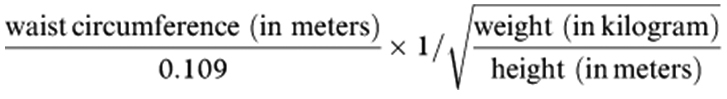 |
Abdominal volume index was obtained from the formula [5]:
 |
Recording of ECG
While the subjects were resting in supine position, electrocardiogram was performed with the paper speed of the 25 mm/sec and amplitude of 10 mm/mV. Heart rate, QRS duration and amplitude, QT interval, T peak-T end interval was measured. Corrected QT interval was calculated according to Bazett’s formula [16].
Recording of Pulse Wave
Pulse wave was recorded using the Physiopac – Medicaid systems. Stiffness index and reflection index were calculated.
Stiffness index: It is the measure of large artery stiffness. It was calculated by subject’s height in meters divided by time delay between direct and reflected waves.
Reflection index: It is the measure of vascular tone of small arteries. It was calculated by the formula RI = a/b *100, where ‘a’ and ‘b’ are the amplitudes of the pulse wave and reflection wave respectively.
Statistical Analysis
Statistical analysis was done using SPSS version 19.0 software. For comparison of variables among three groups, ANOVA test was done. Pearson’s correlation coefficients were applied to establish the correlations between variables. A p-value <0.05 was considered to be significant.
Results
The study included 90 male subjects who were divided into three equal groups of 30 each based on their BMI. [Table/Fig-1] shows anthropometric measurements of Group 1,2 and 3 individuals.
[Table/Fig-1]:
Anthropometric measurements of the subjects.
| Parameters | Group 1 (Mean±SD) | Group 2 Mean±SD) | Group 3 Mean±SD) |
|---|---|---|---|
| Height (cm) | 173.43±5.78 | 170.87±5.42 | 174.05±7.5 |
| Weight (kg) | 62.43±6.43 | 70.20±4.87 | 92.07±11.07 |
| WC (cm) | 76.8±7.8 | 82.3±6.76 | 93.67±9.9 |
| HC (cm) | 89.27±5.94 | 93.95±5.27 | 105.07±9.16 |
(WC: Waist Circumference, HC: Hip Circumference)
[Table/Fig-2] shows comparison of various adiposity indices between groups. There was significant difference in the body mass index, waist circumference, waist height ratio, conicity index and abdominal volume index between the groups.
[Table/Fig-2]:
Comparision of adiposity indices between groups.
| Parameters | Group 1 Mean±SD) | Group 2 Mean±SD) | Group 3 Mean±SD) | p-value |
|---|---|---|---|---|
| BMI | 20.7±1.67 | 24±0.52 | 30.4±3.5 | <0.001* |
| WC (cm) | 76.8±7.8 | 82.3±6.76 | 93.67±9.9 | <0.001* |
| WH | 0.86±0.09 | 0.88±0.05 | 0.89±0.06 | 0.228 |
| WHT | 0.44±0.05 | 0.48±0.03 | 0.54±0.06 | <0.001* |
| CI | 0.33±0.02 | 0.31±0.01 | 0.28±0.01 | <0.001* |
| AVI | 12.0±2.39 | 13.76±2.22 | 17.86±3.63 | <0.001* |
(BMI: Body Mass Index, WC: Waist Circumference, WH: Waist and Hip Circumference, WHT: Waist to Height Ratio, CI: Coincity Index, AVI: Abdominal Volume Index, SD: Standard Deviation, ANOVA was done to compare the variables between groups,
: p-value<0.001)
[Table/Fig-3] indicates that there was significant difference in heart rate, QRS duration, corrected QT interval and in systolic blood pressure between the groups.
[Table/Fig-3]:
Comparison of electrocardiographic variables and blood pressure between groups.
| Parameters | Group 1 (Mean±SD) | Group 2 (Mean±SD) | Group 3 (Mean±SD) | p-value |
|---|---|---|---|---|
| HR (beats/min) | 74.35±11.12 | 74.93±11.93 | 82.1±10.94 | 0.015* |
| QRSD (s) | 0.09±0.01 | 0.11±0.01 | 0.13±0.02 | <0.001* |
| QRSA (mv) | 1.58±0.27 | 1.46±0.34 | 1.4±0.37 | 0.087 |
| QTC (s) | 0.38±0.03 | 0.39±0.04 | 0.43±0.05 | <0.001* |
| TpTe (s) | 0.09±0.01 | 0.11±0.11 | 0.11±0.15 | 0.591 |
| SBP (mmHg) | 111.47±8.03 | 116.9±8.5 | 117.97±9.78 | 0.011* |
| DBP (mmHg) | 72.0±5.0 | 72.63±5.0 | 74.7±5.0 | 0.101 |
(HR: Heart Rate, QRSD: QRS Duration, QRSA: QRS Amplitude, QTC: Corrected QT Interval, TpTe: T-peak-T-end Interval, SBP: Systolic Blood Pressure, DBP: Diastolic Blood Pressure, ANOVA was done to compare the variables between groups
: p-value<0.05)
[Table/Fig-4] compares vascular stiffness indices between groups. There was significant difference between stiffness and reflection indices between normal, overweight and obese individuals.
[Table/Fig-4]:
Comparision of stiffness and reflection indices between groups.
| Parameters | Group 1 (Mean±SD) | Group 2 (Mean±SD) | Group 3 (Mean±SD) | p-value |
|---|---|---|---|---|
| SI | 4.13±1.02 | 5.0±0.21 | 6.6±1.0 | <0.001* |
| RI | 9.89±3.86 | 12.28±4.70 | 13.12±6.10 | 0.037* |
(SI: Stiffness Index, RI: Reflection Index, SD: Standard Deviation, ANOVA was done to compare the variables between groups
: p-value<0.05)
[Table/Fig-5] correlates various adiposity indices with electrocardiographic variables. Of all the adiposity indices, BMI was found to be strongly positively correlated and CI to be strongly negatively correlated with QRS duration.
[Table/Fig-5]:
Correlation between adiposity indices and electrocardiographic variables.
| Parameters | HR | QRSD | QRSA | QTC | TpTe | |
|---|---|---|---|---|---|---|
| BMI | r-value | 0.3 | 0.8 | -0.2 | 0.6 | 0.1 |
| p-value | 0.001* | <0.001* | 0.038* | <0.001* | 0.553 | |
| WC | r-value | 0.3 | 0.6 | -0.2 | 0.5 | 0.1 |
| p-value | 0.001* | <0.001* | 0.022* | <0.001* | 0.439 | |
| WH | r-value | 0.2 | 0.2 | -0.2 | 0.1 | -0.05 |
| p-value | 0.019* | 0.109 | 0.136 | 0.225 | 0.619 | |
| WHT | r-value | 0.4 | 0.6 | -0.2 | 0.5 | 0.1 |
| p-value | <0.001* | <0.001* | 0.058 | <0.001* | 0.445 | |
| CI | r-value | -0.3 | -0.8 | 0.2 | -0.6 | -0.1 |
| p-value | 0.004* | <0.001* | 0.034* | <0.001* | 0.492 | |
| AVI | r-value | 0.3 | 0.6 | -0.3 | 0.5 | 0.1 |
| p-value | 0.001* | <0.001* | 0.016* | <0.001* | 0.443 |
(BMI: Body Mass Index, WC: Waist Circumference, WH: Waist and Hip Circumference, WHT: Waist to Height Ratio, CI: Conicity Index, AVI: Abdominal Volume Index, HR: Heart Rate, QRSD: QRS Duration, QRSA: QRS Amplitude, QTC: QT Interval, TpTe: T-peak-T-end Interval, Pearson’s correlation analysis was done to correlate the variables
: p-value<0.05)
[Table/Fig-6] correlates various adiposity indices with vascular stiffness indices. BMI was significantly and positively correlated and CI was significantly negatively correlated to reflection and stiffness indices.
[Table/Fig-6]:
Correlation between adiposity indices and stiffness and reflection indices.
| Parameters | RI | si | |
|---|---|---|---|
| BMI | r-value | 0.5 | 0.9 |
| p-value | <0.001* | <0.001* | |
| WC | r-value | 0.3 | 0.7 |
| p-value | 0.007* | <0.001* | |
| WH | r-value | 0.1 | 0.2 |
| p-value | 0.270 | 0.087 | |
| WHT | r-value | 0.3 | 0.7 |
| p-value | 0.001* | <0.001* | |
| CI | r-value | -0.4 | -0.9 |
| p-value | <0.001* | <0.001* | |
| AVI | r-value | 0.3 | 0.7 |
| p-value | 0.006* | <0.001* |
(RI: Reflection Index, SI: Stiffness Index, Pearson’s correlation analysis was done to correlate the variables
: p-value<0.05)
Discussion
The relationship between obesity and electrocardiographic variables has been investigated in previous studies especially in middle aged or older adults [17-19]. Our study observed influence of adiposity on electrocardiographic variables especially in young adults. Both total fat and abdominal fat contributed to variations in ventricular electrocardiographic variables [20].
Resting heart rate was found to be significantly increased in obese and overweight young individuals when compared to normal weight individuals. In obese individuals, there is excess production of leptin by the adipose tissue [21]. Consequently, hyperleptinaemia lead to the activation of the sympathetic nervous system which results in increased resting heart rate [21]. Resting heart rate, which is a marker of sympathetic tone, was significantly correlated with both total fat and abdominal fat. It was found to be strongly correlated with waist height ratio which is an indicator of abdominal obesity. Our study suggests increased abdominal obesity is well associated with increased resting heart rate.
Obese groups demonstrated significantly wider QRS duration (greater than 0.12 seconds) when compared with overweight and normal weight groups. Studies have shown that QRS duration, which represents the period of myocardial depolarization, is consistently prolonged in obese humans [22]. Our study confirms that both the abdominal and general obesity correlates significantly with QRS duration. Of all the adiposity indices, BMI was found to be strongly positively correlated and CI to be strongly negatively correlated with QRS duration. Though QRS amplitude appeared to decrease with increased adiposity, there was no significant difference between the groups. Both increase in abdominal and total fat would decrease the QRS amplitude. It was found to be significantly strongly negatively correlated with abdominal obesity especially with abdominal volume index which indicates the abdominal fat.
There had been significant difference in corrected QT interval in obese and overweight individuals when compared to normal weight individuals. Our study shows significant correlation of QTc interval with adiposity indices which is in accordance with various other studies which have reported correlation between obesity and QTc interval prolongation [23]. Our finding contradicts with one study which showed no correlation between BMI and QTc interval in a healthy population aged 22–25 years [24]. Our finding establishes significant strong positive correlation of QTc interval with BMI and negative correlation of QTc interval with conicity index, an indicator of abdominal obesity. Both the total fat and abdominal fat influences the ventricular depolarization and repolarization activity.
T-peak-T-end interval was found to be prolonged in overweight and obese individuals but not to the significant level. T wave peak to end interval is an ECG index of repolarization of the left ventricle. Prolongation of this interval is potential indicator of ventricular arrhythmia risk [25,26]. Morbid obese individuals would have shown significant prolongation of this interval.
Obese and overweight individuals exhibited significantly increased vascular stiffness when compared to normal weight individuals. Our study indicates that both total fat and abdominal fat increases the arterial stiffness. Few studies have shown abdominal fat as a major risk factor for early arterial stiffening and few others have shown BMI which is an indicator of total fat to be a risk factor [27,28]. But in this study, it was found that BMI (indicator of total fat) and CI (indicator of abdominal fat), was significantly strongly correlated with the stiffness and reflection indices. BMI was positively correlated and CI was negatively correlated to vascular stiffness indices. These adiposity indices can be used to assess the vascular stiffness status in young obese individuals. Thus by simple and non invasive method of calculation of BMI and conicity index it could predict the stiffness status of the vessel. Even in young adults increased adiposity leads to early vascular aging leading to cardiovascular complications.
The probable mechanism linking adiposity to vascular stiffening would be due to elevated lipolytic activity of visceral adipocytes resulting in increased free fatty acids release contributing to insulin resistance. Insulin resistance may exert its vascular effects through hyperinsulinemia [29] which causes stimulation of vascular smooth muscle growth. Other mechanisms could be due to increased levels of leptin in obese individuals which leads to reduction in arterial distensibility [30]. Excess adiposity also contributes to arterial stiffness through low-grade inflammation [31].
Limitation
Females would have also been included and compared with that of males in the study. Future studies with larger sample size and including both males and females are required to provide any generalized results.
Conclusion
Thus adiposity indices especially BMI and CI can be used to assess the electrocardiographic abnormalities and vascular stiffness status. Arterial stiffness is a silent killer and electrocardiographic abnormalities are associated with an increased risk of adverse cardiovascular outcomes. Screening for arterial stiffness and electrocardiographic abnormalities using these adiposity indices will be useful to identify high risk young adults. Early interventions can be done in high risk groups in terms of strict diet (avoiding fatty and junk foods) and exercise as research has shown exercise can reverse arterial stiffness.
Acknowledgments
It’s an ICMR STS approved project. I would like to thank ICMR profusely for providing the fund. I thank my Guide and Head of the Department for constantly motivating me and providing full support till the end.
Financial or Other Competing Interests
None.
References
- [1].Indumathy J, Pal GK, Pal P, Ananthanarayanan PH, Parija SC, Balachander J, et al. Association of obesity indices and metabolic markers with sympathovagal imbalance and cardiovascular risks in obesity in Indian population. Obes Res Clin Pract. 2015;9:55–66. doi: 10.1016/j.orcp.2014.01.007. [DOI] [PubMed] [Google Scholar]
- [2].Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013:A systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;14:60460–68. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Valdez R. A simple model-based index of abdominal adiposity. J Clin Epidemiol. 1991;44:955–56. doi: 10.1016/0895-4356(91)90059-i. [DOI] [PubMed] [Google Scholar]
- [4].Valdez R, Seidell JC, Ahn YI, Weiss KM. A new index of abdominal adiposity as an indicator of risk for cardiovascular disease:a cross population study. Int J Obes Relat Metab Disord. 1993;17:77–82. [PubMed] [Google Scholar]
- [5].Motamed N, Perumal D, Zamani F, Ashrafi H, Haghjoo M, Saeedian FS. Conicity index and waist-to-hip ratio are superior obesity indices in predicting 10-year cardiovascular risk among men and women. Clin Cardiol. 2015;38(9):527–34. doi: 10.1002/clc.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Xiao X, Liu Y, Sun C, Gang X, Cheng J, Tian S, et al. Evaluation of different obesity indices as predictors of type 2 diabetes mellitus in a Chinese population. J Diabetes. 2015;7(3):386–92. doi: 10.1111/1753-0407.12201. [DOI] [PubMed] [Google Scholar]
- [7].Tarnoky AD, Tarnoki DL, Bogl LH, Medda E, Fagnani C, Nisticò L, et al. Association of body mass index with arterial stiffness and blood pressure components:A twin study. Atherosclerosis. 2013;229(2):388–95. doi: 10.1016/j.atherosclerosis.2013.05.001. [DOI] [PubMed] [Google Scholar]
- [8].Zebekakis PE, Nawrot T, Thijs L, Balkestein EJ, Van der Heijden-Spek J, Van Bortel LM, et al. Obesity is associated with increased arterial stiffness from adolescence until old age. Journal of Hypertension. 2005;23:1839–46. doi: 10.1097/01.hjh.0000179511.93889.e9. [DOI] [PubMed] [Google Scholar]
- [9].Arslan E. Effect of uncomplicated obesity on QT interval in young men. Pol Arch Med Wewn. 2010;120(6):209–13. [PubMed] [Google Scholar]
- [10].Motamed N, Perumal D, Zamani F, Ashrafi H, Haghjoo M, Saeedian FS, et al. Conicity Index and waist-to-hip ratio are superior obesity indices in predicting 10-year cardiovascular risk among men and women. Clin Cardiol. 2015;38(9):527–34. doi: 10.1002/clc.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Misra A, Chowbey P, Makkar BM, Vikram NK, Wasir JS, Chadha D. Consensus statement for diagnosis of obesity, abdominal obesity and the metabolic syndrome for asian Indians and recommendations for physical activity, medical and surgical management. J Assoc Physicians India. 2009;57:163–70. [PubMed] [Google Scholar]
- [12].Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, Tanaka H. Abdominal obesity is associated with arterial stiffness in middle-aged adults Nutrition. Metabolism & Cardiovascular Diseases. 2015;25:495–502. doi: 10.1016/j.numecd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [13].British hypertension society guidelines. [August 24 2011]. Available at: http://guidance.nice.org.uk/CG127/NICEGuidance/pdf/English .
- [14].Nishida C, Ko GT, Kumanyika S. Body fat distribution and noncommunicable diseases in populations:overview of the 2008 WHO expert consultation on waist circumference and waist-hip ratio. European Journal of Clinical Nutrition. 2010;64(1):2–5. doi: 10.1038/ejcn.2009.139. [DOI] [PubMed] [Google Scholar]
- [15].Sabbah I, Sabbah H, Sabbah S, Akoum H, Droubi N. Central obesity and comorbidity risk in hemodialysis patients:a cross sectional study in Lebanon. Open Journal of Nephrology. 2012;2(4):109–15. [Google Scholar]
- [16].Bazzet HC. An analysis of the time relations of electrocardiograms. Heart. 1920;7:353–70. [Google Scholar]
- [17].Kim HK, Kim CH, Ko KH, Park SW, Park JY, Lee KU. Variable association between components of the metabolic syndrome and electrocardiographic abnormalities in Korean adults. Korean J Intern Medicine. 2010;25(2):174–80. doi: 10.3904/kjim.2010.25.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rautaharju PM, Prineas R, Kadish A, Larson JC, Hsia J, Lund B. Normal standards for QT and QT subintervals derived from a large ethinically diverse population of women aged 50- 79 years. Am J Cardiol. 2006;97:730–37. doi: 10.1016/j.amjcard.2005.09.108. [DOI] [PubMed] [Google Scholar]
- [19].Rautaharju PM, Manolio TA, Psaty BM, Borhani NO, Furberg CD. Correlates of QT prolongation in older adults (the cardiovascular health study) Am J Cardiol. 1994;73:999–1002. doi: 10.1016/0002-9149(94)90156-2. [DOI] [PubMed] [Google Scholar]
- [20].Savage DD, Levy D, Dannenberg AL, Garrison RJ, Castelli WP. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study) Am J Cardiol. 1990;65:371–76. doi: 10.1016/0002-9149(90)90304-j. [DOI] [PubMed] [Google Scholar]
- [21].Fraley MA, Birchem JA, Senkottaiyan N, Alpert MA. Obesity and the electrocardiogram. Obes Rev. 2005;6:275–81. doi: 10.1111/j.1467-789X.2005.00199.x. [DOI] [PubMed] [Google Scholar]
- [22].Frank S, Colliver JA, Frank A. The electrocardiogram in obesity:statistical analysis of 1,029 patients. J Am Coll Cardiol. 1986;7:295–99. doi: 10.1016/s0735-1097(86)80494-6. [DOI] [PubMed] [Google Scholar]
- [23].Arslan E, Yiğiner O, Yavasoğlu I, Ozcelik F, Kardesoğlu E, Nalbant S. Effect of uncomplicated obesity on QT interval in young men. Pol Arch Med Wewn. 2010;120:209. [PubMed] [Google Scholar]
- [24].Leotta G, Maule S, Rabbia F, Del Colle S, Tredici M, Canadè A, et al. Relationship between QT interval and cardiovascular risk factors in healthy young subjects. J Hum Hypertens. 2005;19:623–27. doi: 10.1038/sj.jhh.1001874. [DOI] [PubMed] [Google Scholar]
- [25].Taggart P, Sutton PM, Opthof T, Coronel R, Trimlett R, Pugsley W, et al. Transmural repolarisation in the left ventricle in humans during normoxia and ischaemia. Cardiovasc Res. 2001;50:454–62. doi: 10.1016/s0008-6363(01)00223-1. [DOI] [PubMed] [Google Scholar]
- [26].Pthof T, Coronel R, Janse MJ. Is there a significant transmural gradient in repolarization time in the intact heart?Repolarization gradients in the intact heart. Circ Arrhythm Electrophysiol. 2009;2:89–96. doi: 10.1161/CIRCEP.108.825356. [DOI] [PubMed] [Google Scholar]
- [27].Strasser B, Arvandi M, Pasha EP, Haley AP, Stanforth P, Tanaka H. Abdominal obesity is associated with arterial stiffness in middle-aged adults. Nutr Metab Cardiovasc Dis. 2015;25:495–502. doi: 10.1016/j.numecd.2015.01.002. [DOI] [PubMed] [Google Scholar]
- [28].Wildman RP, Mackey RH, Bostom A, Thompson T, Sutton-Tyrrell K. Measures of obesity are associated with vascular stiffness in young and older adults. Hypertension. 2003;42:468–73. doi: 10.1161/01.HYP.0000090360.78539.CD. [DOI] [PubMed] [Google Scholar]
- [29].Montagnani M, Quon MJ. Insulin action in vascular endothelium:Potential mechanisms linking insulin resistance with hypertension. Diabetes Obes Metab. 2000;2:285–92. doi: 10.1046/j.1463-1326.2000.00092.x. [DOI] [PubMed] [Google Scholar]
- [30].Singhal A, Farooqi IS, Cole TJ, O’Rahilly S, Fewtrell M, Kattenhorn M, et al. Influence of leptin on arterial distensibility:A novel link between obesity and cardiovascular disease? Circulation. 2002;106:1919–24. doi: 10.1161/01.cir.0000033219.24717.52. [DOI] [PubMed] [Google Scholar]
- [31].Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25:932–43. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]


