Abstract
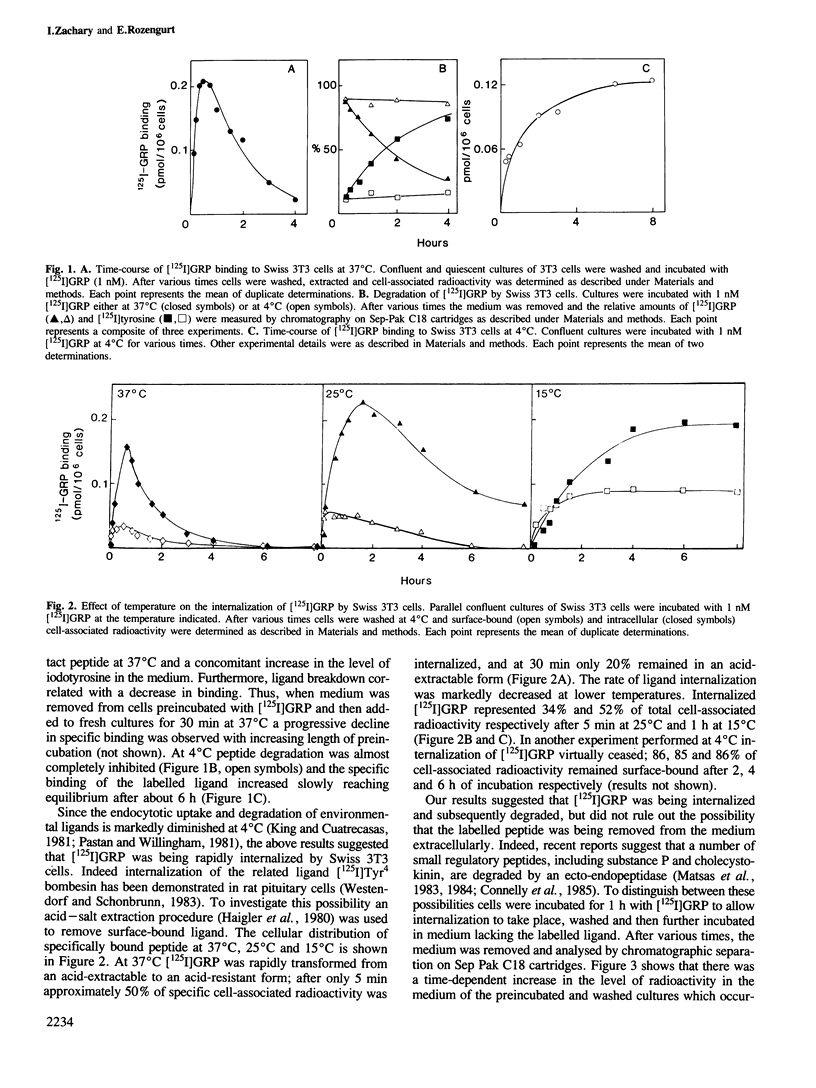
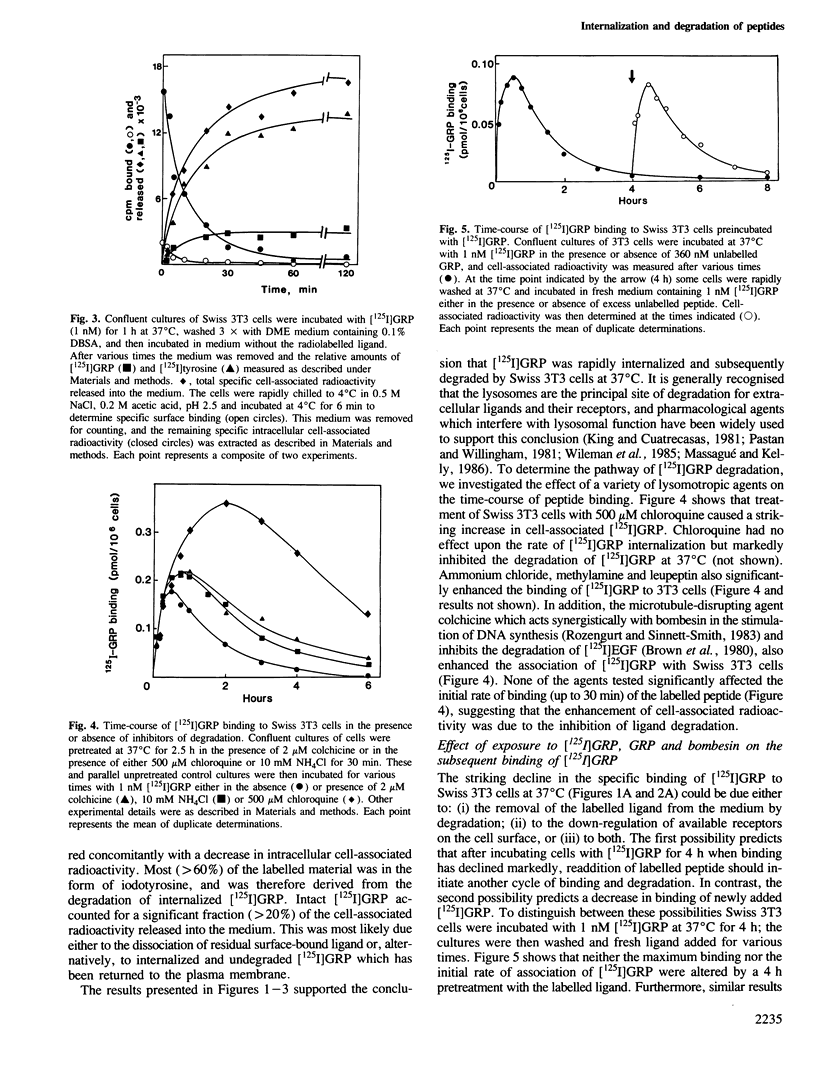
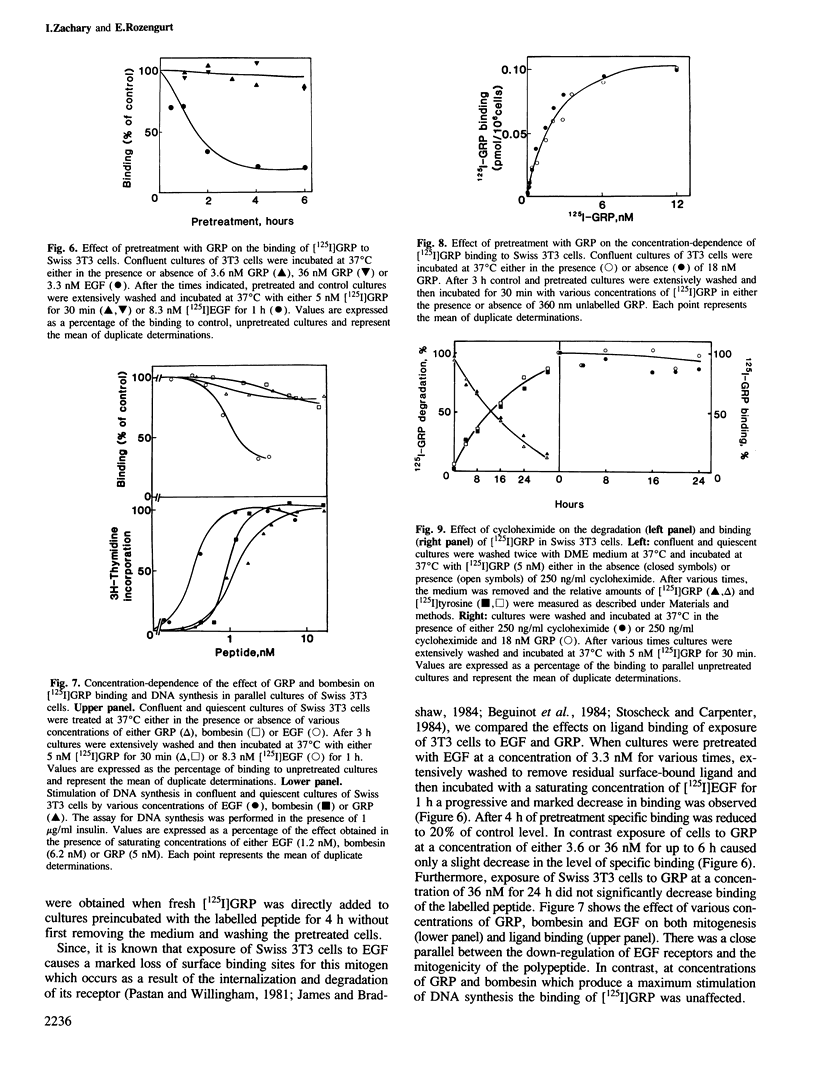
The binding of [125I]gastrin releasing peptide ([125I]GRP) to Swiss 3T3 cells at 37 degrees C increases rapidly, reaching a maximum after 30 min and decreasing afterwards. The decrease in cell-associated radioactivity at this temperature is accompanied by extensive degradation of the labelled peptide. At 4 degrees C equilibrium binding is achieved after 6 h and [125I]GRP degradation is markedly inhibited. Extraction of surface-bound ligand at low pH demonstrates that the iodinated peptide is internalized within minutes after addition to 3T3 cells at 37 degrees C. The rate of internalization is strikingly temperature-dependent and is virtually abolished at 4 degrees C. In addition, lysomotropic agents including chloroquine increase the cell-associated radioactivity in cells incubated with [125I]GRP. The binding of [125I]GRP to Swiss 3T3 cells was not affected by pretreatment for up to 24 h with either GRP or bombesin at mitogenic concentrations. Furthermore, pretreatment with GRP did not reduce the affinity labelling of a Mr 75,000-85,000 surface protein recently identified as a putative receptor for bombesin-like peptides. These results demonstrate that while peptides of the bombesin family are rapidly internalized and degraded by Swiss 3T3 cells, the cell surface receptors for these molecules are not down-regulated.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi A., Erspamer V., Bucci M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia. 1971 Feb 15;27(2):166–167. doi: 10.1007/BF02145873. [DOI] [PubMed] [Google Scholar]
- Beguinot L., Lyall R. M., Willingham M. C., Pastan I. Down-regulation of the epidermal growth factor receptor in KB cells is due to receptor internalization and subsequent degradation in lysosomes. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2384–2388. doi: 10.1073/pnas.81.8.2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergeron J. J., Cruz J., Khan M. N., Posner B. I. Uptake of insulin and other ligands into receptor-rich endocytic components of target cells: the endosomal apparatus. Annu Rev Physiol. 1985;47:383–403. doi: 10.1146/annurev.ph.47.030185.002123. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Ross R. Platelet-derived growth factor. II. Specific binding to cultured cells. J Biol Chem. 1982 May 10;257(9):5161–5171. [PubMed] [Google Scholar]
- Brown K. D., Blay J., Irvine R. F., Heslop J. P., Berridge M. J. Reduction of epidermal growth factor receptor affinity by heterologous ligands: evidence for a mechanism involving the breakdown of phosphoinositides and the activation of protein kinase C. Biochem Biophys Res Commun. 1984 Aug 30;123(1):377–384. doi: 10.1016/0006-291x(84)90424-8. [DOI] [PubMed] [Google Scholar]
- Brown K. D., Friedkin M., Rozengurt E. Colchicine inhibits epidermal growth factor degradation in 3T3 cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):480–484. doi: 10.1073/pnas.77.1.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown M. S., Anderson R. G., Goldstein J. L. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell. 1983 Mar;32(3):663–667. doi: 10.1016/0092-8674(83)90052-1. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. Epidermal growth factor. Annu Rev Biochem. 1979;48:193–216. doi: 10.1146/annurev.bi.48.070179.001205. [DOI] [PubMed] [Google Scholar]
- Connelly J. C., Skidgel R. A., Schulz W. W., Johnson A. R., Erdös E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuttitta F., Carney D. N., Mulshine J., Moody T. W., Fedorko J., Fischler A., Minna J. D. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. 1985 Aug 29-Sep 4Nature. 316(6031):823–826. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- Das M., Fox C. F. Molecular mechanism of mitogen action: processing of receptor induced by epidermal growth factor. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2644–2648. doi: 10.1073/pnas.75.6.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by tumour promoter and pure mitogenic factors. Nature. 1978 Dec 14;276(5689):723–726. doi: 10.1038/276723a0. [DOI] [PubMed] [Google Scholar]
- Erisman M. D., Linnoila R. I., Hernandez O., DiAugustine R. P., Lazarus L. H. Human lung small-cell carcinoma contains bombesin. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2379–2383. doi: 10.1073/pnas.79.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S., Anderson R. G., Russell D. W., Schneider W. J. Receptor-mediated endocytosis: concepts emerging from the LDL receptor system. Annu Rev Cell Biol. 1985;1:1–39. doi: 10.1146/annurev.cb.01.110185.000245. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., Maxfield F. R., Willingham M. C., Pastan I. Dansylcadaverine inhibits internalization of 125I-epidermal growth factor in BALB 3T3 cells. J Biol Chem. 1980 Feb 25;255(4):1239–1241. [PubMed] [Google Scholar]
- Heldin C. H., Wasteson A., Westermark B. Interaction of platelet-derived growth factor with its fibroblast receptor. Demonstration of ligand degradation and receptor modulation. J Biol Chem. 1982 Apr 25;257(8):4216–4221. [PubMed] [Google Scholar]
- Heslop J. P., Blakeley D. M., Brown K. D., Irvine R. F., Berridge M. J. Effects of bombesin and insulin on inositol (1,4,5)trisphosphate and inositol (1,3,4)trisphosphate formation in Swiss 3T3 cells. Cell. 1986 Dec 5;47(5):703–709. doi: 10.1016/0092-8674(86)90513-1. [DOI] [PubMed] [Google Scholar]
- Isacke C. M., Meisenhelder J., Brown K. D., Gould K. L., Gould S. J., Hunter T. Early phosphorylation events following the treatment of Swiss 3T3 cells with bombesin and the mammalian bombesin-related peptide, gastrin-releasing peptide. EMBO J. 1986 Nov;5(11):2889–2898. doi: 10.1002/j.1460-2075.1986.tb04584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James R., Bradshaw R. A. Polypeptide growth factors. Annu Rev Biochem. 1984;53:259–292. doi: 10.1146/annurev.bi.53.070184.001355. [DOI] [PubMed] [Google Scholar]
- Kaplan J., Keogh E. A. Analysis of the effect of amines on inhibition of receptor-mediated and fluid-phase pinocytosis in rabbit alveolar macrophages. Cell. 1981 Jun;24(3):925–932. doi: 10.1016/0092-8674(81)90118-5. [DOI] [PubMed] [Google Scholar]
- King A. C., Cuatrecasas P. Peptide hormone-induced receptor mobility, aggregation, and internalization. N Engl J Med. 1981 Jul 9;305(2):77–88. doi: 10.1056/NEJM198107093050206. [DOI] [PubMed] [Google Scholar]
- Krupp M. N., Connolly D. T., Lane M. D. Synthesis, turnover, and down-regulation of epidermal growth factor receptors in human A431 epidermoid carcinoma cells and skin fibroblasts. J Biol Chem. 1982 Oct 10;257(19):11489–11496. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Letterio J. J., Coughlin S. R., Williams L. T. Pertussis toxin-sensitive pathway in the stimulation of c-myc expression and DNA synthesis by bombesin. Science. 1986 Nov 28;234(4780):1117–1119. doi: 10.1126/science.3465038. [DOI] [PubMed] [Google Scholar]
- Lopez-Rivas A., Adelberg E. A., Rozengurt E. Intracellular K+ and the mitogenic response of 3T3 cells to peptide factors in serum-free medium. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6275–6279. doi: 10.1073/pnas.79.20.6275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Kelly B. Internalization of transforming growth factor-beta and its receptor in BALB/c 3T3 fibroblasts. J Cell Physiol. 1986 Aug;128(2):216–222. doi: 10.1002/jcp.1041280212. [DOI] [PubMed] [Google Scholar]
- Matsas R., Fulcher I. S., Kenny A. J., Turner A. J. Substance P and [Leu]enkephalin are hydrolyzed by an enzyme in pig caudate synaptic membranes that is identical with the endopeptidase of kidney microvilli. Proc Natl Acad Sci U S A. 1983 May;80(10):3111–3115. doi: 10.1073/pnas.80.10.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsas R., Turner A. J., Kenny A. J. Endopeptidase-24.11 and aminopeptidase activity in brain synaptic membranes are jointly responsible for the hydrolysis of cholecystokinin octapeptide (CCK-8). FEBS Lett. 1984 Sep 17;175(1):124–128. doi: 10.1016/0014-5793(84)80583-9. [DOI] [PubMed] [Google Scholar]
- McDonald T. J., Jörnvall H., Nilsson G., Vagne M., Ghatei M., Bloom S. R., Mutt V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem Biophys Res Commun. 1979 Sep 12;90(1):227–233. doi: 10.1016/0006-291x(79)91614-0. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Schneider J. A., Lopez-Rivas A., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. II. Changes in Na+ and Ca2+ fluxes, Na+/K+ pump activity, and intracellular pH. J Cell Biol. 1986 Jun;102(6):2223–2233. doi: 10.1083/jcb.102.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. Neuromedin B: a novel bombesin-like peptide identified in porcine spinal cord. Biochem Biophys Res Commun. 1983 Jul 29;114(2):541–548. doi: 10.1016/0006-291x(83)90814-8. [DOI] [PubMed] [Google Scholar]
- Minamino N., Kangawa K., Matsuo H. Neuromedin C: a bombesin-like peptide identified in porcine spinal cord. Biochem Biophys Res Commun. 1984 Feb 29;119(1):14–20. doi: 10.1016/0006-291x(84)91611-5. [DOI] [PubMed] [Google Scholar]
- Minamino N., Sudoh T., Kangawa K., Matsuo H. Neuromedin B-32 and B-30: two "big" neuromedin B identified in porcine brain and spinal cord. Biochem Biophys Res Commun. 1985 Jul 31;130(2):685–691. doi: 10.1016/0006-291x(85)90471-1. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B. Bombesin-like peptides in rat brain: quantitation and biochemical characterization. Biochem Biophys Res Commun. 1979 Sep 12;90(1):7–14. doi: 10.1016/0006-291x(79)91582-1. [DOI] [PubMed] [Google Scholar]
- Moody T. W., Pert C. B., Gazdar A. F., Carney D. N., Minna J. D. High levels of intracellular bombesin characterize human small-cell lung carcinoma. Science. 1981 Dec 11;214(4526):1246–1248. doi: 10.1126/science.6272398. [DOI] [PubMed] [Google Scholar]
- Nilsson J., von Euler A. M., Dalsgaard C. J. Stimulation of connective tissue cell growth by substance P and substance K. Nature. 1985 May 2;315(6014):61–63. doi: 10.1038/315061a0. [DOI] [PubMed] [Google Scholar]
- Oka Y., Czech M. P. The type II insulin-like growth factor receptor is internalized and recycles in the absence of ligand. J Biol Chem. 1986 Jul 15;261(20):9090–9093. [PubMed] [Google Scholar]
- Palumbo A. P., Rossino P., Comoglio P. M. Bombesin stimulation of c-fos and c-myc gene expression in cultures of Swiss 3T3 cells. Exp Cell Res. 1986 Nov;167(1):276–280. doi: 10.1016/0014-4827(86)90226-0. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Jensen R. T., Gardner J. D. Mechanism of [Tyr4]bombesin-induced desensitization in dispersed acini from guinea pig pancreas. J Biol Chem. 1982 Oct 25;257(20):12024–12029. [PubMed] [Google Scholar]
- Pastan I. H., Willingham M. C. Receptor-mediated endocytosis of hormones in cultured cells. Annu Rev Physiol. 1981;43:239–250. doi: 10.1146/annurev.ph.43.030181.001323. [DOI] [PubMed] [Google Scholar]
- Payan D. G. Receptor-mediated mitogenic effects of substance P on cultured smooth muscle cells. Biochem Biophys Res Commun. 1985 Jul 16;130(1):104–109. doi: 10.1016/0006-291x(85)90388-2. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Bowen-Pope D. F., Ross R., Krebs E. G. Characterization of platelet-derived growth factor-stimulated phosphorylation in cell membranes. J Biol Chem. 1983 Aug 10;258(15):9383–9390. [PubMed] [Google Scholar]
- Roth K. A., Evans C. J., Weber E., Barchas J. D., Bostwick D. G., Bensch K. G. Gastrin-releasing peptide-related peptides in a human malignant lung carcinoid tumor. Cancer Res. 1983 Nov;43(11):5411–5415. [PubMed] [Google Scholar]
- Rozengurt E., Brown K. D., Pettican P. Vasopressin inhibition of epidermal growth factor binding to cultured mouse cells. J Biol Chem. 1981 Jan 25;256(2):716–722. [PubMed] [Google Scholar]
- Rozengurt E., Collins M., Brown K. D., Pettican P. Inhibition of epidermal growth factor binding to mouse cultured cells by fibroblast-derived growth factor. Evidence for an indirect mechanism. J Biol Chem. 1982 Apr 10;257(7):3680–3686. [PubMed] [Google Scholar]
- Rozengurt E. Early signals in the mitogenic response. Science. 1986 Oct 10;234(4773):161–166. doi: 10.1126/science.3018928. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Mendoza S. A. Synergistic signals in mitogenesis: role of ion fluxes, cyclic nucleotides and protein kinase C in Swiss 3T3 cells. J Cell Sci Suppl. 1985;3:229–242. doi: 10.1242/jcs.1985.supplement_3.20. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A. L., Bolognesi A., Fridovich S. E. Recycling of the asialoglycoprotein receptor and the effect of lysosomotropic amines in hepatoma cells. J Cell Biol. 1984 Feb;98(2):732–738. doi: 10.1083/jcb.98.2.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Walker J. P., Townsend C. M., Jr, Thompson J. C. Role of gastrin and gastrin receptors on the growth of a transplantable mouse colon carcinoma (MC-26) in BALB/c mice. Cancer Res. 1986 Apr;46(4 Pt 1):1612–1616. [PubMed] [Google Scholar]
- Stahl P., Schwartz A. L. Receptor-mediated endocytosis. J Clin Invest. 1986 Mar;77(3):657–662. doi: 10.1172/JCI112359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoscheck C. M., Carpenter G. Down regulation of epidermal growth factor receptors: direct demonstration of receptor degradation in human fibroblasts. J Cell Biol. 1984 Mar;98(3):1048–1053. doi: 10.1083/jcb.98.3.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takuwa N., Takuwa Y., Bollag W. E., Rasmussen H. The effects of bombesin on polyphosphoinositide and calcium metabolism in Swiss 3T3 cells. J Biol Chem. 1987 Jan 5;262(1):182–188. [PubMed] [Google Scholar]
- Wakshull E. M., Wharton W. Stabilized complexes of epidermal growth factor and its receptor on the cell surface stimulate RNA synthesis but not mitogenesis. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8513–8517. doi: 10.1073/pnas.82.24.8513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf J. M., Schonbrunn A. Characterization of bombesin receptors in a rat pituitary cell line. J Biol Chem. 1983 Jun 25;258(12):7527–7535. [PubMed] [Google Scholar]
- Wharton J., Polak J. M., Bloom S. R., Ghatei M. A., Solcia E., Brown M. R., Pearse A. G. Bombesin-like immunoreactivity in the lung. Nature. 1978 Jun 29;273(5665):769–770. doi: 10.1038/273769a0. [DOI] [PubMed] [Google Scholar]
- Wileman T., Harding C., Stahl P. Receptor-mediated endocytosis. Biochem J. 1985 Nov 15;232(1):1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood S. M., Wood J. R., Ghatei M. A., Lee Y. C., O'Shaughnessy D., Bloom S. R. Bombesin, somatostatin and neurotensin-like immunoreactivity in bronchial carcinoma. J Clin Endocrinol Metab. 1981 Dec;53(6):1310–1312. doi: 10.1210/jcem-53-6-1310. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. High-affinity receptors for peptides of the bombesin family in Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7616–7620. doi: 10.1073/pnas.82.22.7616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Identification of a receptor for peptides of the bombesin family in Swiss 3T3 cells by affinity cross-linking. J Biol Chem. 1987 Mar 25;262(9):3947–3950. [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Modulation of the epidermal growth factor receptor by mitogenic ligands: effects of bombesin and role of protein kinase C. Cancer Surv. 1985;4(4):729–765. [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J. W., Rozengurt E. Early events elicited by bombesin and structurally related peptides in quiescent Swiss 3T3 cells. I. Activation of protein kinase C and inhibition of epidermal growth factor binding. J Cell Biol. 1986 Jun;102(6):2211–2222. doi: 10.1083/jcb.102.6.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]