Abstract
Magnetic nanoparticles are promising new tools for therapeutic applications, such as magnetic nanoparticle hyperthermia therapy and targeted drug delivery. Recent in vitro studies have demonstrated that a force application with magnetic tweezers can also affect cell fate, suggesting a therapeutic potential for magnetically modulated mechanical stimulation. The magnetic properties of nanoparticles that induce physical responses and the subtle responses that result from mechanically induced membrane damage and/or intracellular signaling are evaluated. Magnetic particles with various physical, geometric, and magnetic properties and specific functionalization can now be used to apply mechanical force to specific regions of cells, which permit the modulation of cellular behavior through the use of spatially and time controlled magnetic fields. On one hand, mechanochemical stimulation has been used to direct the outgrowth on neuronal growth cones, indicating a therapeutic potential for neural repair. On the other hand, it has been used to kill cancer cells that preferentially express specific receptors. Advances made in the synthesis and characterization of magnetic nanomaterials and a better understanding of cellular mechanotransduction mechanisms may support the translation of mechanochemical stimulation into the clinic as an emerging therapeutic approach.
1. Introduction
Recent advances in nanotechnology have resulted in the synthesis of an array of magnetic particles varying in size, shape, composition, and surface functionalization.[1] Magnetic nanoparticles (MNPs) have been used for enhancing magnetic resonance imaging (MRI) contrast and, more recently, considered for controlled drug delivery and for magnetic nanoparticle hyperthermia (MNH) therapy.[2] Drug delivery using magnetic nanoparticles can occur through two distinct mechanisms: Particles loaded with drugs can be directed from the blood circulation to a target tissue using a magnetizable implant.[3] Alternatively, drug-carrying particles can be designed such that they release their content upon heating. For example, MNPs have been functionalized with β-cyclodextrin to encapsulate tamoxifen and folic acid to target folate receptors, which are overexpressed in breast cancer tumors.[4] When exposed to high frequency alternating magnetic fields (AMF), these particles generated sufficient heat to release the drug, demonstrating a potential for targeted delivery and controlled release. If sustained, the heat generated by magnetic nanoparticles can also be used to induce apoptosis via activating tumor necrosis factor (TNF)-α,[5] or to directly ablate tumors.[6] MNH has recently been demonstrated for the treatment of human glioblastoma multiforme by directly injecting nanoparticles into the tumor to achieve high MNP concentration.[7] However, while the indirect, affinity-based targeting of tumors for MNH has been demonstrated in mice, the secondary uptake by other organs remains a problem.[8]
Recent work has suggested that, in addition to providing image contrast, drug delivery, and hyperthermia, MNPs can be used for the mechanical stimulation of cells to induce a therapeutic effect.[9] Mechanics plays important roles in numerous physiological and pathological processes and is particularly important for cancer, since metastatic cancer accounts for over 90% of cancer-related deaths and forces acting on cells can modulate the metastatic process.[10] Metastasizing cells detach from the primary tumor, migrate through the extracellular matrix, enter the vasculature by squeezing through the endothelial cells, adhere to the endothelial cells, and exit the vasculature once again by penetrating the endothelium to eventually form a secondary tumor.[11] Throughout this process, cells not only undergo morphological change, but also actively interact with their physical environment through motility and adhesionrelated pathways.[12] The most straightforward approach to translate this into the clinic would appear to be to interfere with these critical mechanical processes to block metastasis.[13] An alternative approach would be to use mechanical stimulation to selectively kill tumors and circulating tumor cells. This could be achieved either through damaging the target cell’s membrane or its intracellular structures,[9c] or through activating select mechanotransduction pathways to induce cell death.[9b]
Another area where mechanical force may induce a therapeutic effect is chronic spinal cord injury, where the glial scar tissue that forms after the injury not only poses a physical barrier for regenerating neurons, but also secretes repulsive factors that inhibit axon elongation.[14] A number of recent reviews detail the role of mechanics in neuronal growth and guidance.[15] Importantly, external mechanical stretch has been shown to enhance axon elongation under control conditions[16] and against concentration gradients of known axon repellents.[9a] Thus, stretch growth of regenerating axons through regions of low permissivity may complement existing therapeutic approaches after spinal cord injury, such as engineered biomaterial scaffolds, cell transplantation, and local delivery of neurotrophic factors.[17] To this end, delivering sufficient force to axonal growth cones in vivo appears to be a challenge to overcome. Ideally, this would be achieved noninvasively since a very long therapy period may be required depending on injury severity.
Among various techniques available for the direct mechanical stimulation of cells in vitro, magnetic tweezers (MTW), the non-invasive manipulation of magnetic particles using an externally applied magnetic field, has claimed particular attention.[18] Following the recent demonstration of in vivo MTW force application to study mechanotransduction in developing Xenopus[19] and Drosophila[20] embryos, the potential of using MNPs for mechanically modulated future cell therapies became evident. New classes of MNPs, such as those developed for clinical applications, e.g., MRI contrast or MNH, may be further optimized for delivering significant forces in deep tissues upon exposure to external magnetic fields. Here, we review the recent literature that describes the use of MNPs in targeted, mechanically modulated cell therapy. We describe the physical (magnetic) properties of nanoparticles and how they can be controlled to achieve higher MRI contrast, magnetic heating, and MTW force. We then discuss the potential of mechanical cell actuation in the context of neural repair and targeted cancer therapy.
2. Physical Properties of Magnetic Nanoparticles
To fully realize the potential of applications using MNPs, it is critical to understand both how the application makes use of the magnetic response of the nanomaterial as well as the magnetic behavior of the nanomaterial, especially in the operating regime of the application (Figure 1). For example, magnetic particle imaging utilizes the inherent non-linear response of a magnetic nanomaterial to an applied magnetic field,[21] which is controlled, in part, by the magnetic size, the magnetic anisotropy, and the switching field, as well as their distributions.[22] MRI uses the magnetic field generated by the nanomaterial to change the relaxation rate of the surrounding water protons (1H), which is controlled, in part, by the magnetization and nanoparticle stability.[23] MNH utilizes the heat generated from hysteresis losses, which is controlled, in part, by the saturation magnetization, dynamic coercivity, magnetic domain size, magnetic anisotropy, and interactions between the MNPs, as well as by the alternating current (AC) magnetic field amplitude and frequency.[24] Magnetic targeting/stimulation utilize the fact that a magnetic field gradient will translationally move a magnetic nanomaterial, which is controlled, in part, by the magnitude of the magnetic field gradient, the magnetization, the magnetic anisotropy, and the mass/shape of the nanomaterial.[25]
Figure 1.
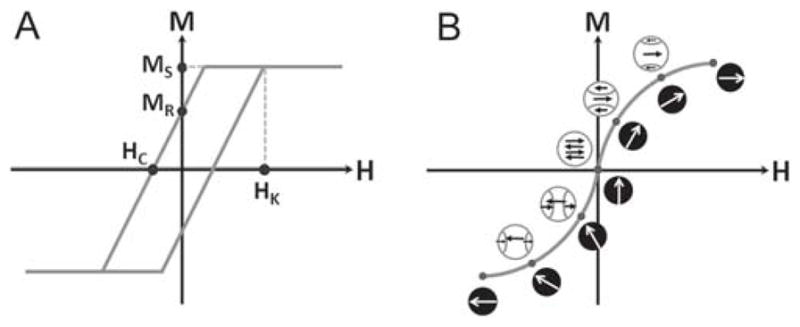
Magnetization behavior of nanomaterials. A) Schematic of a simple magnetic hysteresis loop for a magnetic material (M vs H) showing important parameters saturation magnetization (MS), remanence magnetization (MR), coercivity (HC), and anisotropy field (HK) which is proportional to the magnetic anisotropy (K). B) Ideal case of rapid magnetization reversal through coherent rotation of the magnetization of the magnetic nanoparticle (white arrows on black) and magnetization reversal through domain nucleation and growth (black arrows on white).
Understanding which property or properties are most critical to the application, as well as how the nanomaterial behaves magnetically, will lead to optimization of the magnetic and physical properties for the given application, without having to proceed using a costly “Edisonian” approach. This requires a thorough magnetic and physical characterization of the nanomaterial, as the physical properties (size, shape, crystallinity, grain size, defects, composition, etc.) are intimately entwined with the magnetic behavior (saturation magnetization, susceptibility, coercivity, magnetic anisotropy, type of magnetism, magnetic domain size, magnetic transition temperatures, etc.). Small changes in the former can result in significant changes in the latter.[26]
Basic magnetic characterization is well understood for nanomaterials, under static/direct current (DC) measurement conditions.[27] This includes saturation magnetization, saturating field, and coercivity. However, while the saturation magnetization is an intrinsic property of the material, the coercivity and saturating field are not.[28] The latter depends critically on the size, shape, crystallinity, and defect structure of the material. This leads to recognition of a fundamental issue for magnetic nanomaterials: no two nanoparticles/nanorods are alike. Instead, there is variability in the physical and, therefore, magnetic properties within a single synthesis batch, as well as variability between different batches for the same synthesis, and finally variability between different syntheses.[29] As a direct result, it is critical to characterize the distributions in magnetic properties of a given batch and then use this for quality control to minimize batch-to-batch variability for a specific synthesis for commercial applications. First-order reversal curves (FORCs) are being explored as a method for quantifying the magnetic property distributions in nanomaterials.[30] Magnetic property distributions are critical because it may be that one extreme or the other of a specific property (i.e., saturation magnetization, magnetic anisotropy) is controlling the effectiveness of the nanomaterial for its application;[31] alternatively, it may be that the extremes are detrimental to the effectiveness of the nanomaterial.[22]
Other more advanced types of magnetic characterization are significantly less well-understood for nanomaterials, as two (or more) parameters can be convoluted. For example, while magnetocrystalline anisotropy is well understood at the thin film and bulk level,[32] it is convoluted with magnetic surface anisotropy[33] in nanoparticles/nanorods, which is not understood, as well as interactions between the nanoparticles/nanorods,[34] which are also dependent upon the applied magnetic field. As a result, while there exist several methods for determining the magnetic anisotropy (hysteresis loops, torque magnetometry, longitudinal AC susceptibility, and transverse AC susceptibility), the data can be difficult to interpret.[27] Furthermore, there are anomalous behaviors in magnetic nanomaterials (i.e., anomalously large Keff) that originate from this convolution of several parameters (i.e., interactions) (C.L.D., unpublished data).
Finally, many biomedical applications use AC magnetic fields, with frequencies ranging from a few Hz (such as cell stimulation)[35] to kHz (such as 25 kHz for magnetic particle imaging[36] and 100’s of kHz for MNH[24] and drug delivery)[37] to MHz (such as in MRI).[38] In contrast, much of the magnetic characterization occurs under static (DC) conditions or at low frequencies/amplitudes (<1 kHz and < 1 mT for AC susceptibility). This results in significant errors when attempting to correlate DC behavior with the actual behavior under operating conditions with large AC magnetic fields. The most common example of this is measuring the magnetic hysteresis loop (with the virgin curve)[27,39] of a nanomaterial and determining that there are negligible interactions and zero coercivity, and concluding that the nanomaterial is “superparamagnetic”. Direct translation of this to the operating conditions under AC fields would presume be that the material is still superparamagnetic in the application. However, superparamagnetism is a time-dependent effect,[40] and may be “blocked” under AC field conditions.[41] Furthermore, many applications using AC magnetic fields do not achieve magnetic field magnitudes that saturate the sample. As a result, the nanomaterial operates under “minor” loop (nonsaturating loop) conditions, so the interpretation of the results becomes very complex.[26] Unfortunately, the experimental and theoretical characterization of magnetic nanomaterials under non-saturating conditions has lagged behind the characterization under saturating conditions. However, even if the reversal mechanisms/timescales are not well-understood, a measurement under the actual operating conditions yields critical information for the application, such as: Is the field large enough to reverse the magnetization? Is the loop open or closed? What is the maximum magnetization achievable? If the magnetic field is not large enough to overcome the magnetic anisotropy and reverse the magnetization, then no mechanical motion (back and forth) will occur at all. If the loop is open, there will be energy lost to hysteresis heating rather than mechanical motion, with the amount lost dependent upon the dynamic coercivity. If the non-saturating field does not align enough of the moments, the mechanical motion will be reduced. All of these help explain the behavior of the magnetic nanomaterials in a specific application.
2.1. Force Acting on a Magnetic Particle
When placed in an external magnetic field H, a MNP with a magnetic moment m results in magnetic induction B = μ0(H + m/V), where μ0 is the vacuum permeability and V is the volume of the nanoparticle. The particle is subjected to a torque τ = m × B that tends to align the particle’s magnetic moment with the magnetic field. If the magnetic field has a gradient, ∇H, the particle is subjected to a force F = (m∇) B directed towards regions with higher field density. Due to their high saturation magnetization, ferromagnetic nanoparticles are preferred in applications where the external magnetic field is weak and particle size is limited. This would be the case for deep tissue MTW force application. However, it is important to note that the magnetic moment m for the force equations above is actually m(H), and is a function of the applied magnetic field. As the external magnetic field is unlikely to be a saturating field, the internal magnetic domain structure (and its competing energies: magnetic anisotropy energy, Zeeman energy, exchange energy, etc.) will be a critical factor to optimize the magnetic response of the MNPs for MTW.[26] Furthermore, the ease and rapidity of reversal, which will require a small switching field and narrow switching field distribution, will play a critical role when a low frequency AMF is applied to oscillate the MNP to generate the force.
Physically, the magnetic material of choice has typically been an iron oxide (magnetite or maghemite), due to their known biocompatibility. However, there is a wide range of magnetic materials available, including Fe, Ni, and Co, and alloys/oxides thereof. From a magnetics point of view, the non-iron oxides, especially the metals, are particularly attractive with their larger saturation magnetization. Unfortunately, the biocompatibility suffers (e.g., Co and Ni are known carcinogens and pure Fe has a highly exothermic reaction upon exposure to oxygen/water), so the material choice is constrained by two competing sets of requirements – magnetic and physiological. However, alloys are possible, or core/shell or layered structures that have another coating (such as Au or polymers) to prevent absorption by the body. There are also several options for design of the nanomaterials. Core/shell MNPs with core diameters in the range of 10–100 nm can be used for MTW, but the choice of shell for stabilization is more challenging and critical for preventing irreversible agglomeration.[34] Alternatively, spherical magnetic beads (where magnetic nanocrystals are dispersed within a polymer matrix) 0.1–100 μm in diameter can be used.[42] (Novel emulsion-templated synthesis has been shown to increase the nanocrystal density of the beads such that the saturation magnetization is 6 times higher than commercial beads).[43] In both cases, there exist large selections of chemically modified surfaces, which can be tailored for affinity targeting of specific cells. Furthermore, recent work identified shape as an important property for MTW applications. Cylindrical particles, termed nanowires or nanorods, have recently been employed in MTW.[9b,44] Templated synthesis using a nanoporous membrane enables the sequential deposition of different metals[44c] or alloys[45] into a single structure, which permits the localized functionalization of the rod.[9b,46] As such, the shape is not only used to control the magnetic anisotropy. This sequential deposition also enables elements with strong magnetic properties (e.g., saturation magnetization), such as pure Fe, Co, or Ni (and alloys thereof), to be packed into nanorods to achieve the desired crystal structure and domain size/structure. For example, a 2 μm long, 100 nm diameter iron nanorod (with a saturation magnetization of 1.6 MA m−1 (or 1600 emu cm−3)[44c] provides 1.3 times higher pulling force compared to a 1 μm diameter commercial spherical bead despite having 33.3 times smaller volume.[9b] Another potential advantage of using composite nanorods is their capacity to arbitrarily define the segment size and element size, thereby enabling the design of multiplexed bioassays. For example, a mixture of different types of nanorods, which have short Au tips, functionalized with the same ligand, but Fe segments that vary in length. These rods would deliver forces of different magnitude to the same target and would be easily identified from their lengths. Alternatively, the mixture may consist of nanorods that have short Au tips, functionalized with different ligands, and have FePt alloy segments that provide the same force, but vary in their alloy composition. These rods would deliver the same force to different targets and would also be easily identified from their lengths. Adding additional segments, similar to metallic barcodes,[47] may be considered for further multiplexing, including the functionalization of the single particle with multiple ligands. In summary, developing MTW probes optimized for in vivo force application will benefit not only from novel synthetic approaches leading to different size, shape, and composition, but also from proper characterization of magnetic properties, within physiological constraints. Ultimately, these two competing requirements will have to be balanced to achieve optimal performance.
3. Force Application to Cells using Magnetic Tweezers
Mechanotransduction, the conversion of mechanical stimuli into biochemical signaling, contributes to numerous developmental and physiological processes.[48] Tissue cells are mechanically integrated structures where dynamic processes link the cytoskeleton (CSK) to transmembrane receptors that connect cells with extracellular matrix (ECM) proteins or with other cells. Externally applied forces are transmitted to mechanosensitive molecules, which, in turn, alter their conformation and thereby their function.[49] However, these so-called mechanical switches are not sufficient to describe the complexity of the cellular response to external mechanical stimuli, which depends on the loading rate[50] and the force application frequency,[51] apart from the force magnitude. In fact, it has been speculated that the cellular response to physical stimuli may be as complex as its biochemical and genetic signaling pathways.[52] In support of this idea, a dynamic model of mechanotransduction has recently been suggested where the cell’s response is modulated through signaling networks that govern the spatiotemporal dynamics of the intracellular mechanical stress.[53] It is widely accepted that forces regulate the folding states of proteins and unbinding rates of protein complexes. Since the force-bearing elements of the cell, i.e., the plasma membrane, cell adhesion complexes, and the cytoskeleton, are highly dynamic, it is perceivable that the basis of mechanotransduction is the force-dependent disassembly of intermolecular bonds and the force-depended changes in the folding states of mechanosensitive proteins. Therefore, the timescales of the externally applied forces need to match the intrinsic timescales of the target intracellular signaling processes, for the intended mechanical control of biological phenomena to occur. Accordingly, in their dynamic model of mechanotransduction, Hoffman and colleagues proposed that “mechanical stresses may convey large amounts of information through precise time-dependent and force-dependent modulation”.[53]
Several families of cell adhesion molecules (CAMs), integrins and cadherins in particular, and some of the proteins that link them to the CSK have been identified. For example, integrin, the essential component of focal adhesions, is linked to the actin CSK through a number of mechanosensitive proteins.
Talin binds to β-integrin and to filamentous actin (F-actin) via its N-terminal and C-terminal domains, respectively. Talin’s C-terminal domain contains vinculin binding sites that are buried in the relaxed state which become exposed when talin is stretched.[50b] This leads to vinculin binding and subsequent adhesion strengthening, since vinculin also binds to F-actin.[54] Interestingly, when integrins are stretched, they signal to other, unoccupied integrins to alter their affinity states, such that more integrins can bind to ECM proteins, further strengthening the adhesion.[55] Filamin A, another linker protein that binds to integrin and F-actin, has been shown to mediate Rac GTPase activity as a function of applied force.[56] Stretching of integrin also activates RhoA GTPase, through two distinct guanine exchange factors.[57] Rac and RhoA belong to the Rho family of GTPases, small signaling G proteins that regulate the actin CSK. RhoA activation regulates myosin-2, the main motor protein that generates contractile forces in the actin CSK. RhoA activation also induces zyxin phosphorylation, which, in turn, mediates a range of force responses, such as stabilizing the CSK and inducing gene expression related to inflammatory response.[58] Similar discoveries have been made for linkers of other CAMs such as catenins for cadherin[59] and p21-activated kinase 1 for neural CAM (NCAM).[60] Interestingly, adherens junctions, which link CSKs of adjacent cells through cadherin complexes, undergo stretch-mediated strengthening similar to focal adhesions.[61]
A critical aspect in MTW-based mechanical stimulation is that, unlike stretching entire cells, a particular membranebound receptor can be targeted, such that the downstream signaling pathways are selectively activated. The specificity in the target receptor has recently been demonstrated in mesenchymal stem cells, where mechanical stimulation of TREK1 K+ channels led to osteogenic differentiation,[62] whereas mechanical stimulation of Frizzled receptors led to the activation of Wnt pathway via β-catenin signaling.[63] The discovery of novel mechanosensitive targets,[64] and the dissection of complex signaling mechanisms downstream of known targets,[58,65] define the landscape for a putative “cell mechanotherapy” using MTW. However, off-target effects, such as the activation of mechanosensitive ion channels, cannot be disregarded.
3.1. Mechanotransduction to Promote Neural Growth
Axon growth and guidance during development is a complex mechanochemical process, where growth cones, highly dynamic tips of elongating axons, are responsible for sensing and generating forces.[66] Growth cones continuously probe their environment for chemical guidance cues, whose signals modulate the F-actin and microtubule dynamics, which, in turn, determines the subsequent axon behavior, i.e., elongation, turning, or retraction.[67] The mechanical regulation of neural growth is crucial not only for the development of the nervous system,[15a] but also for its repair after a localized trauma, e.g., spinal cord injury (SCI). SCI causes the degeneration of distal axons and results in glial scarring at the site of the lesion, which prohibits the endogenous regeneration of adult central nervous system (CNS) axons by acting as a mechanical barrier as well as a source of axon repellents.[14] While research geared towards spinal cord repair is currently focused on strategies that combine engineered biomaterial scaffolds with cell transplantation and local delivery of neurotrophic factors,[17] using force to promote axon elongation through a region of low permissivity, i.e., glial scar, should also be considered as an alternative/supplementary therapeutic approach.
Accelerated axon elongation has been repeatedly demonstrated for various types of neurons.[16,68] Continuously stretching integrated neurons using a microstepper motor resulted in axon lengths up to 10 cm.[69] Moreover, stretchgrown axons experiencing ≤ 25% strain (corresponding to an elongation rate of ≤ 3 mm per day) for two weeks exhibited normal electrical activity.[70] However, the repair of the endogenous CNS requires a less invasive therapeutic approach, leading to the idea of pulling growth cones of regenerating neurons with MTW forces. We have recently shown the MTWbased towing of cortical axons against concentration gradients of known axon repellents in vitro, by combining low magnitude force (≤ 12 pN) acting on NCAM with inhibitors of select molecular motors.[9a] The challenge to translate this “mechanochemical stimulation” approach into preclinical stage (in vivo) is at least two-fold: First, the magnetic particle is required to be small enough to navigate through the complex extracellular matrix and magnetic enough to deliver a considerable force magnitude to provide the desired therapeutic effect. Second, the magnetic particle is required to be functionalized in such a way that the magnetic particle selectively binds to the desired CAM on the regenerating growth cone, but avoids non-specific interactions. We believe that the recent emergence of magnetic particles with a multitude of physical and other properties,[1] as well as the various approaches developed for the bio-functionalization of their surfaces[42] will help identify the optimum magnetic nanoparticle for in vivo MTW force application to promote neural repair.
Actomyosin stress fibers are known to exert traction forces on the extracellular matrix via focal adhesions.[71] Recent evidence demonstrated that mechanical tension in cells is nonuniformly distributed[72] and that tension directly regulates the activities of linker proteins vinculin[73] and zyxin.[74] This suggests that not the magnitude of the externally applied force per se, but the tension it exerts on the transmembrane molecule, may be critical in the mechanotransduction process. This notion was supported by our axon towing results, where axons towed by relatively small magnetic beads exhibited higher elongation rates per applied force (8.3 μm h−1 per 10 pN)[9a] compared to earlier work (1 μm h−1 per 10 pN).[16] In fact, mechanical tension has been suggested to affect the local intracellular environment near the pulled receptor, leading to an increased probability of docking of growing microtubule tips,[68b] which randomly explore the growth cone periphery.[75] Accordingly, tension exerted on apCAM, the invertebrate homolog of NCAM has been shown to result in the translocation and polymerization of microtubules towards the site of force application.[76]
In this context, it is important to highlight the difference between uniformly functionalized spherical MNPs and tipfunctionalized cylindrical magnetic nanorods in terms of tensile stress they can deliver to CAMs. Using basic mechanics relations and assuming Hertzian contact, the contact area of a spherical particle of radius R will be ∝ R8/3.[9a] As force scales with R3, the tensile stress exerted will be ∝R1/3. Despite the tiny force magnitude, the tensile stress exerted by MNPs (e.g., during in vivo MTW force application) will not decrease drastically. On the other hand, the contact area of a tip-functionalized nanorod (diameter d, length L) will be ∝ d2. As the force scales with d2L, the tensile stress exerted will be ∝ L. The tensile stress exerted by an aligned nanorod will thus be independent of its diameter and will scale with rod length. Moreover, since the cross-sectional areas of these particles are equal, they are expected to navigate similarly in the body, provided that the aspect ratio is small enough to avoid triggering an immune response. To summarize, we propose that MNPs, tip-functionalized magnetic nanorods in particular, may be small enough to be considered safe for clinical applications and still able to produce sufficient tensile stress to promote axon growth in vivo.
3.2. Mechanotransduction to Induce Cell Death
Among the new classes of MNPs, those with asymmetric geometric structure and/or rapid/easy magnetic reversal (e.g., through magnetic anisotropy) are of particular interest, since they may physically oscillate to a greater extent than symmetrical particles when exposed to AMF.[9c] MNPs can be targeted to specific cell surface receptors or be internalized by specifically targeted cells, providing two distinct modes for force application. The first mode does not require internalized MNPs, and therefore, may utilize magnetic microparticles too large to be taken up by the cells, but small enough to navigate in the circulatory system. Periodic pulling of integrins with 3 pN per 4.5 μm-diameter magnetic particle, for example, has been shown to activate the mechanosensitive potassium channels in mesenchymal stem cells, which led to the reversible hyperpolarization of these cells.[77] Application of constant forces at much lower magnitudes (≈10−5 pN per 30 nm-diameter particle) has been shown to be sufficient for clustering individual Immunoglobulin E receptors in Mast cells, which, in turn, activated calcium influx.[78] The extent of the calcium influx depended on the force magnitude and the duration of rest period between consecutive actuations, demonstrating the level of control in inducing a physiological response with a remote physical input. However, mechanically modulating ion channel activity to induce cell death is yet to be demonstrated.
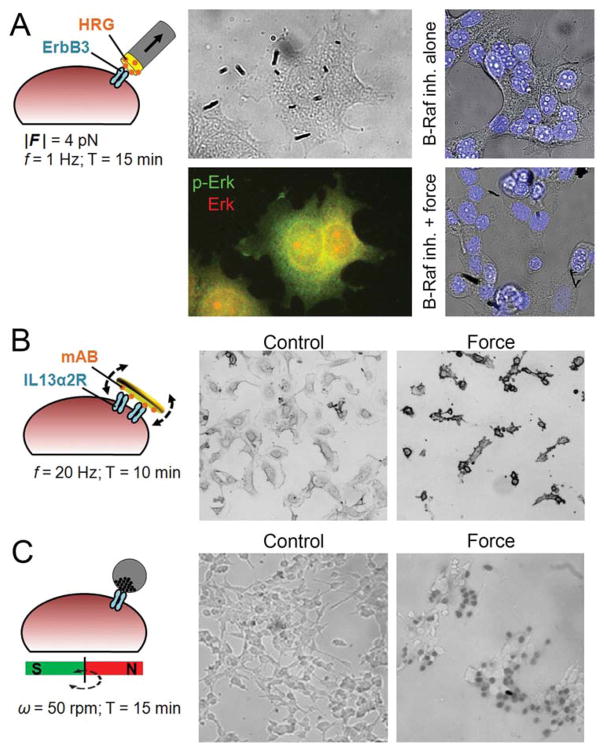
Mechanical activation of select signaling pathways may be combined with other (systemic) stimuli to induce cell death. We have recently demonstrated the activation of extracellular signal-regulated kinase (ERK) pathway through periodically stretching specifically targeted ErbB3 receptors in MCF7 cells with 4 pN using micrometer-long iron rods with functionalized gold tips.[9b] Interestingly, ERK phosphorylation (and receptor clustering) was observed in multiple foci that did not necessarily co-localize with nanorod binding locations. A very recent study has shown that ERK phosphorylation foci localized to actomyosin stress fibers in fibroblasts, and correlated with the level of tension acting on the stress fiber.[79] This suggests that ERK phosphorylation at remote foci may have been caused by the distribution of tension inside the cell upon localized force application. To benefit from the activated ERK signaling, we combined mechanical stimulation with low dose B-Raf inhibition, which also activates ERK, to achieve significant levels of cell death through hyperactivating this signaling pathway (Figure 2A).[9b]
Figure 2.
Examples of mechanically induced cancer cell death in vitro. A) MCF7 breast cancer cells targeted with FeAu composite nanorods that are tip-functionalized with Heregulin (HRG). Periodic pulling of ErbB3 receptor caused 2.4-fold increase in Erk phosphorylation, which, when combined with low dose B-Raf inhibition results in 15-fold increase in cell death. Reproduced with permission.[9b] Copyright 2014, John Wiley and Sons. B) Neuroblastoma cells targeted with micrometer-sized ferromagnetic disks, possessing a vortex spin state, functionalized with a monoclonal antibody (mAB) against interleukin-13 receptor α2. A brief exposure to alternating magnetic field resulted in membrane damage and apoptosis. Reproduced with permission.[9d] Copyright 2010, Macmillan Publishers Ltd: Nature Materials. C) LNCaP prostate cancer cells non-specifically targeted with asymmetric magnetic/fluorescent nanocomposites. Rotating a permanent magnet resulted in cell death as shown by trypan blue. Reproduced with permission.[9e] Copyright 2010, American Chemical Society.
The alternative mode, i.e., using internalized MNPs to deliver force from within the cell, benefits from some form of asymmetry in the particle shape or easy/rapid reversal of the MNP magnetization. Upon exposure to AMF, MNPs which reversed rapidly at small applied magnetic fields and could therefore be controlled, damaged the cellular structures they are in contact with, eventually killing the cell.[9c] The idea of oscillatory physical damage has been mostly demonstrated with non-internalized particles: Neuroblastoma cells that have been targeted with micrometer-sized ferromagnetic disks, which possess a magnetic-vortex spin state, underwent apoptosis when briefly exposed to AMF (Figure 2B).[9d] These disks exhibit zero in-plane magnetic moment in remanence, but magnetize in the direction of an externally applied field, thus rotate and align with the field. Asymmetric magnetic/fluorescent composites (Ø = 200 nm)[9e] and carbon nanotubes with iron impurity (Ø = 100 nm)[80] have also been used to kill cells upon exposure to a rotating magnetic field (Figure 2C). Despite the lack of specific cell targeting and the use of very high particle densities in these studies, these particles may be promising tools for mechanical cell therapy. The aspect ratio is considered to be a key factor in the cellular uptake of rod-shaped particles, where higher aspect ratios are less favored for membrane wrapping and subsequent internalization.[81] Cylindrical nanoparticles favoring internalization or membrane-binding may thus be designed depending on the mode of force application.
4. Conclusion
Beyond imaging, MNPs are promising tools for magnetically modulated new therapeutic approaches, such as MNH or heating-induced drug delivery. In this article, we highlighted recent in vitro studies where MNPs were used to mechanically stimulate cells to induce a biological response to ultimately control cell behavior. These studies show that MTW is not only a biophysical technique for characterizing the fundamental mechanisms of mechanotransduction; rather, force may be used induce cell growth and death, suggesting a therapeutic potential in spinal cord injury and cancer. In this context, creating the optimum particle for the application at hand, in terms of magnetic and other properties, becomes a crucial task. While much is already known about the correlations between magnetic and physical properties of nanomaterials, much remains to be studied. From the applications point of view, better understanding of the structure-property relationships is critical to balance the competing realities of physiological limitations (size, aspect ratio, toxicity, etc.) with maximizing the magnetic properties for optimization of the nanomaterial for the application. From the characterization point of view, more development work is needed, in theory and instrumentation, for deconvoluting different parameters (e.g., magnetocrystalline anisotropy from magnetic interactions) as well as characterizing nanomaterials under AC magnetic field conditions. For example, high frequency properties will dominate the design of a hyperthermia agent; whereas, DC or low frequency properties, such as saturation magnetization, will dominate the design of a force probe.
The hypothesis that mechanically induced axon growth or cell death can be induced in vivo using MNPs that are targeted to specific cell surface receptors, such as those over-expressed in cancer or in regenerating growth cones, remains to be tested. We argue that the sparse literature on magneto-mechanical stimulation to control cell growth and death is due to the limited accessibility of new classes of MNPs that would permit different modes of force application to achieve the desired therapeutic effect. Functionalizing particles to target and mechanically destroy tumors and subclinical metastases from within – analogous to Trojan horses – may be an emerging therapeutic strategy, as is towing regenerating axons through the glial scar tissue. Designing novel bio-nano-magnetic materials geared towards these clinical applications is a challenging endeavor, which will benefit from a thorough understanding of magnetism at the nanoscale.
Acknowledgments
This material is based upon works supported by Science Foundation Ireland (SFI) under Grant No. 08/RP1/81376 and 08/IN1/82072 (G.U.L.), the Nanoremedies Programme funded under the Programme for Research in Third-Level Institutions and co-funded under the European Regional Development Fund (G.U.L.), and a Marie Curie Intra-European Fellowship (D.K.).
References
- 1.Kilinc D, Lee GU. Integr Biol (Camb) 2014;6:27. doi: 10.1039/c3ib40185e. [DOI] [PubMed] [Google Scholar]
- 2.Ho VH, Barcza A, Chen R, Muller KH, Darton NJ, Slater NK. Biomaterials. 2009;30:6548. doi: 10.1016/j.biomaterials.2009.08.023. [DOI] [PubMed] [Google Scholar]
- 3.a) Polyak B, Fishbein I, Chorny M, Alferiev I, Williams D, Yellen B, Friedman G, Levy RJ. Proc Natl Acad Sci USA. 2008;105:698. doi: 10.1073/pnas.0708338105. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tietze R, Zaloga J, Unterweger H, Lyer S, Friedrich RP, Janko C, Pottler M, Durr S, Alexiou C. Biochem Biophys Res Commun. 2015 doi: 10.1016/j.bbrc.2015.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Hayashi K, Ono K, Suzuki H, Sawada M, Moriya M, Sakamoto W, Yogo T. ACS Appl Mater Interfaces. 2010;2:1903. doi: 10.1021/am100237p. [DOI] [PubMed] [Google Scholar]
- 5.Ito A, Shinkai M, Honda H, Kobayashi T. Cancer Gene Ther. 2001;8:649. doi: 10.1038/sj.cgt.7700357. [DOI] [PubMed] [Google Scholar]
- 6.Jordan A, Scholz R, Maier-Hauff K, van Landeghem FK, Waldoefner N, Teichgraeber U, Pinkernelle J, Bruhn H, Neumann F, Thiesen B, von Deimling A, Felix R. J Neurooncol. 2006;78:7. doi: 10.1007/s11060-005-9059-z. [DOI] [PubMed] [Google Scholar]
- 7.Maier-Hauff K, Rothe R, Scholz R, Gneveckow U, Wust P, Thiesen B, Feussner A, von Deimling A, Waldoefner N, Felix R, Jordan A. J Neurooncol. 2007;81:53. doi: 10.1007/s11060-006-9195-0. [DOI] [PubMed] [Google Scholar]
- 8.a) DeNardo SJ, DeNardo GL, Natarajan A, Miers LA, Foreman AR, Gruettner C, Adamson GN, Ivkov R. J Nucl Med. 2007;48:437. [PubMed] [Google Scholar]; b) DeNardo SJ, DeNardo GL, Miers LA, Natarajan A, Foreman AR, Gruettner C, Adamson GN, Ivkov R. Clin Cancer Res. 2005;11:7087s. doi: 10.1158/1078-0432.CCR-1004-0022. [DOI] [PubMed] [Google Scholar]
- 9.a) Kilinc D, Blasiak A, O’Mahony JJ, Lee GU. Sci Rep. 2014;4:7128. doi: 10.1038/srep07128. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Kilinc D, Lesniak A, Rashdan SA, Gandhi D, Blasiak A, Fannin PC, von Kriegsheim A, Kolch W, Lee GU. Adv Healthcare Mater. 2015;4:395. doi: 10.1002/adhm.201400391. [DOI] [PubMed] [Google Scholar]; c) Cheng D, Li X, Zhang G, Shi H. Nanoscale Res Lett. 2014;9:195. doi: 10.1186/1556-276X-9-195. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kim DH, Rozhkova EA, Ulasov IV, Bader SD, Rajh T, Lesniak MS, Novosad V. Nat Mater. 2009;9:165. doi: 10.1038/nmat2591. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Hu SH, Gao X. J Am Chem Soc. 2010;132:7234. doi: 10.1021/ja102489q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weigelt B, Peterse JL, van’t Veer LJ. Nat Rev Cancer. 2005;5:591. doi: 10.1038/nrc1670. [DOI] [PubMed] [Google Scholar]
- 11.Wirtz D, Konstantopoulos K, Searson PC. Nat Rev Cancer. 2011;11:512. doi: 10.1038/nrc3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bordeleau F, Alcoser TA, Reinhart-King CA. Am J Physiol Cell Physiol. 2014;306:C110. doi: 10.1152/ajpcell.00283.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nguyen HN, Yang JM, Afkari Y, Park BH, Sesaki H, Devreotes PN, Iijima M. Proc Natl Acad Sci USA. 2014;111:E2684. doi: 10.1073/pnas.1409433111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoichet MS, Tate CC, Baumann MD, LaPlaca MC. In: Indwelling Neural Implants: Strategies for Contending with the In Vivo Environment. Reichert WM, editor. CRC Press; Boca Raton, FL: 2007. pp. 221–244. [PubMed] [Google Scholar]
- 15.a) Franze K. Development. 2013;140:3069. doi: 10.1242/dev.079145. [DOI] [PubMed] [Google Scholar]; b) Suter DM, Miller KE. Prog Neurobiol. 2011;94:91. doi: 10.1016/j.pneurobio.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chada S, Lamoureux P, Buxbaum RE, Heidemann SR. J Cell Sci. 1997;110:1179. doi: 10.1242/jcs.110.10.1179. [DOI] [PubMed] [Google Scholar]
- 17.McCreedy DA, Sakiyama-Elbert SE. Neurosci Lett. 2012;519:115. doi: 10.1016/j.neulet.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oddershede LB. Nat Chem Biol. 2012;8:879. doi: 10.1038/nchembio.1082. [DOI] [PubMed] [Google Scholar]
- 19.Kumar A, Shivashankar GV. PLoS One. 2012;7:e33089. doi: 10.1371/journal.pone.0033089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weber GF, Bjerke MA, DeSimone DW. Dev Cell. 2012;22:104. doi: 10.1016/j.devcel.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gleich B, Weizenecker J. Nature. 2005;435:1214. doi: 10.1038/nature03808. [DOI] [PubMed] [Google Scholar]
- 22.Eberbeck D, Dennis CL, Huls NF, Krycka KL, Gruttner C, Westphal F. IEEE Trans Magn. 2013;49:269. [Google Scholar]
- 23.Saville SL, Woodward RC, House MJ, Tokarev A, Hammers J, Qi B, Shaw J, Saunders M, Varsani RR, St Pierre TG, Mefford OT. Nanoscale. 2013;5:2152. doi: 10.1039/c3nr32979h. [DOI] [PubMed] [Google Scholar]
- 24.Dennis CL, Ivkov R. Int J Hyperthermia. 2013;29:715. doi: 10.3109/02656736.2013.836758. [DOI] [PubMed] [Google Scholar]
- 25.Rovner JB, Lapointe CP, Reich DH, Leheny RL. Phys Rev Lett. 2010;105:228301. doi: 10.1103/PhysRevLett.105.228301. [DOI] [PubMed] [Google Scholar]
- 26.Dennis CL, Krycka KL, Borchers JA, Desautels RD, van Lierop J, Huls NF, Jackson AJ, Gruettner C, Ivkov R. Adv Funct Mater. 2015;25:4300. [Google Scholar]
- 27.Dennis CL. In: Biomedical Applications of Magnetic Particles. Anker JN, Mefford OT, editors. Taylor and Francis; New York: 2016. [Google Scholar]
- 28.Blums EJ, Maiorov MM, Cebers AO. Magnetic Fluids, Zinatne, Riga. 1989;24 [Google Scholar]
- 29.Giri AK, Hirsh G, Duncan KJ, Karna SP, Dennis CL. J Appl Phys. 2013;113:173908. [Google Scholar]
- 30.a) Kumari M, Widdrat M, Tompa É, Uebe R, Schüler D, Pósfai M, Faivre D, Hirt AM. J Appl Phys. 2014;116:124304. [Google Scholar]; b) Pike CR, Roberts AP, Verosub KL. J Appl Phys. 1999;85:6660. [Google Scholar]
- 31.Erb RM, Son HS, Samanta B, Rotello VM, Yellen BB. Nature. 2009;457:999. doi: 10.1038/nature07766. [DOI] [PubMed] [Google Scholar]
- 32.Skomski R. Simple Models of Magnetism. Oxford University Press; NY, USA: 2008. pp. 73–104. [Google Scholar]
- 33.Krycka KL, Borchers JA, Booth RA, Ijiri Y, Hasz K, Rhyne JJ, Majetich SA. Phys Rev Lett. 2014;113:147203. doi: 10.1103/PhysRevLett.113.147203. [DOI] [PubMed] [Google Scholar]
- 34.Dennis CL, Jackson AJ, Borchers JA, Hoopes PJ, Strawbridge R, Foreman AR, van Lierop J, Gruttner C, Ivkov R. Nanotechnology. 2009;20:395103. doi: 10.1088/0957-4484/20/39/395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu F, Zhao R, Liu AS, Metz T, Shi Y, Bose P, Reich DH. Lab Chip. 2015;15:2496. doi: 10.1039/c4lc01395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sattel TF, Woywode O, Weizenecker J, Rahmer J, Gleich B, Borgert J. IEEE Trans Magn. 2015;51:1. [Google Scholar]
- 37.a) Mikhaylov G, Mikac U, Magaeva AA, Itin VI, Naiden EP, Psakhye I, Babes L, Reinheckel T, Peters C, Zeiser R, Bogyo M, Turk V, Psakhye SG, Turk B, Vasiljeva O. Nat Nanotechnol. 2011;6:594. doi: 10.1038/nnano.2011.112. [DOI] [PubMed] [Google Scholar]; b) Amstad E, Kohlbrecher J, Muller E, Schweizer T, Textor M, Reimhult E. Nano Lett. 2011;11:1664. doi: 10.1021/nl2001499. [DOI] [PubMed] [Google Scholar]
- 38.Faucher L, Gossuin Y, Hocq A, Fortin MA. Nanotechnology. 2011;22:295103. doi: 10.1088/0957-4484/22/29/295103. [DOI] [PubMed] [Google Scholar]
- 39.Taketomi S, Shull RD. J Appl Phys. 2002;91:8546. [Google Scholar]
- 40.Dennis CL, Borges RP, Buda LD, Ebels U, Gregg JF, Hehn M, Jouguelet E, Ounadjela K, Petej I, Prejbeanu IL, Thornton MJ. J Phys Condens Matter. 2002;14:R1175. [Google Scholar]
- 41.Eggeman AS, Majetich SA, Farrell D, Pankhurst QA. IEEE Trans Magn. 2007;43:2451. [Google Scholar]
- 42.Fields C, Li P, O’Mahony JJ, Lee GU. Biotechnol Bioeng. 2016;113:11. doi: 10.1002/bit.25665. [DOI] [PubMed] [Google Scholar]
- 43.a) O’Mahony JJ, Platt M, Kilinc D, Lee G. Langmuir. 2013;29:2546. doi: 10.1021/la3047565. [DOI] [PubMed] [Google Scholar]; b) Kilinc D, Blasiak A, O’Mahony JJ, Suter DM, Lee GU. Biophys J. 2012;103:1120. doi: 10.1016/j.bpj.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.a) Lin YC, Kramer CM, Chen CS, Reich DH. Nanotechnology. 2012;23:075101. doi: 10.1088/0957-4484/23/7/075101. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Schroeder P, Schotter J, Shoshi A, Eggeling M, Bethge O, Hutten A, Bruckl H. Bioinspir Biomim. 2011;6:046007. doi: 10.1088/1748-3182/6/4/046007. [DOI] [PubMed] [Google Scholar]; c) Zhang Y, DaSilva M, Ashall B, Doyle G, Zerulla D, Sands TD, Lee GU. Langmuir. 2011;27:15292. doi: 10.1021/la203863p. [DOI] [PubMed] [Google Scholar]
- 45.Zhang Y, Wang Q, Ashall B, Zerulla D, Lee GU. Adv Mater. 2012;24:2485. doi: 10.1002/adma.201103991. [DOI] [PubMed] [Google Scholar]
- 46.Platt M, Willmott GR, Lee GU. Small. 2012;8:2436. doi: 10.1002/smll.201200058. [DOI] [PubMed] [Google Scholar]
- 47.Nicewarner-Pena SR, Freeman RG, Reiss BD, He L, Pena DJ, Walton ID, Cromer R, Keating CD, Natan MJ. Science. 2001;294:137. doi: 10.1126/science.294.5540.137. [DOI] [PubMed] [Google Scholar]
- 48.Vogel V. Annu Rev Biophys Biomol Struct. 2006;35:459. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- 49.Vogel V, Sheetz M. Nat Rev Mol Cell Biol. 2006;7:265. doi: 10.1038/nrm1890. [DOI] [PubMed] [Google Scholar]
- 50.a) Jiang G, Huang AH, Cai Y, Tanase M, Sheetz MP. Biophys J. 2006;90:1804. doi: 10.1529/biophysj.105.072462. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez JM, Sheetz MP. Science. 2009;323:638. doi: 10.1126/science.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mack PJ, Kaazempur-Mofrad MR, Karcher H, Lee RT, Kamm RD. Am J Physiol Cell Physiol. 2004;287:C954. doi: 10.1152/ajpcell.00567.2003. [DOI] [PubMed] [Google Scholar]
- 52.Janmey PA, Wells RG, Assoian RK, McCulloch CA. Differentiation. 2013;86:112. doi: 10.1016/j.diff.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hoffman BD, Grashoff C, Schwartz MA. Nature. 2011;475:316. doi: 10.1038/nature10316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grashoff C, Hoffman BD, Brenner MD, Zhou R, Parsons M, Yang MT, McLean MA, Sligar SG, Chen CS, Ha T, Schwartz MA. Nature. 2010;466:263. doi: 10.1038/nature09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thodeti CK, Matthews B, Ravi A, Mammoto A, Ghosh K, Bracha AL, Ingber DE. Circ Res. 2009;104:1123. doi: 10.1161/CIRCRESAHA.108.192930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehrlicher AJ, Nakamura F, Hartwig JH, Weitz DA, Stossel TP. Nature. 2011;478:260. doi: 10.1038/nature10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guilluy C, Swaminathan V, Garcia-Mata R, Timothy O’Brien E, Superfine R, Burridge K. Nat Cell Biol. 2011;13:722. doi: 10.1038/ncb2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ross TD, Coon BG, Yun S, Baeyens N, Tanaka K, Ouyang M, Schwartz MA. Curr Opin Cell Biol. 2013;25:613. doi: 10.1016/j.ceb.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Twiss F, de Rooij J. Cell Mol Life Sci. 2013;70:4101. doi: 10.1007/s00018-013-1329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li S, Leshchyns’ka I, Chernyshova Y, Schachner M, Sytnyk V. J Neurosci. 2013;33:790. doi: 10.1523/JNEUROSCI.1238-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Eyckmans J, Boudou T, Yu X, Chen CS. Dev Cell. 2011;21:35. doi: 10.1016/j.devcel.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Henstock JR, Rotherham M, Rashidi H, Shakesheff KM, El Haj AJ. Stem Cells Transl Med. 2014;3:1363. doi: 10.5966/sctm.2014-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rotherham M, El Haj AJ. PLoS One. 2015;10:e0121761. doi: 10.1371/journal.pone.0121761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gasparski AN, Beningo KA. Arch Biochem Biophys. 2015;586:20. doi: 10.1016/j.abb.2015.07.017. [DOI] [PubMed] [Google Scholar]
- 65.Leckband DE, de Rooij J. Annu Rev Cell Dev Biol. 2014;30:291. doi: 10.1146/annurev-cellbio-100913-013212. [DOI] [PubMed] [Google Scholar]
- 66.Kerstein PC, Nichol RH, Gomez TM. Front Cell Neurosci. 2015;9:244. doi: 10.3389/fncel.2015.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kalil K, Dent EW. Curr Opin Neurobiol. 2005;15:521. doi: 10.1016/j.conb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 68.a) Bray D. Dev Biol. 1984;102:379. doi: 10.1016/0012-1606(84)90202-1. [DOI] [PubMed] [Google Scholar]; b) Fass JN, Odde DJ. Biophys J. 2003;85:623. doi: 10.1016/S0006-3495(03)74506-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lamoureux P, Zheng J, Buxbaum RE, Heidemann SR. J Cell Biol. 1992;118:655. doi: 10.1083/jcb.118.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Lamoureux P, Altun-Gultekin ZF, Lin C, Wagner JA, Heidemann SR. J Cell Sci. 1997;110(Pt 5):635. doi: 10.1242/jcs.110.5.635. [DOI] [PubMed] [Google Scholar]
- 69.Pfister BJ, Iwata A, Meaney DF, Smith DH. J Neurosci. 2004;24:7978. doi: 10.1523/JNEUROSCI.1974-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loverde JR, Pfister BJ. Front Cell Neurosci. 2015;9:308. doi: 10.3389/fncel.2015.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burridge K, Wittchen ES. J Cell Biol. 2013;200:9. doi: 10.1083/jcb.201210090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soiné JRD, Brand CA, Stricker J, Oakes PW, Gardel ML, Schwarz US. PLoS Comput Biol. 2015;11:e1004076. doi: 10.1371/journal.pcbi.1004076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chang CW, Kumar S. J Cell Sci. 2013;126:3021. doi: 10.1242/jcs.119032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Colombelli J, Besser A, Kress H, Reynaud EG, Girard P, Caussinus E, Haselmann U, Small JV, Schwarz US, Stelzer EHK. J Cell Sci. 2009;122:1665. doi: 10.1242/jcs.042986. [DOI] [PubMed] [Google Scholar]
- 75.Odde DJ, Tanaka EM, Hawkins SS, Buettner HM. Biotechnol Bioeng. 1996;50:452. doi: 10.1002/(SICI)1097-0290(19960520)50:4<452::AID-BIT13>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 76.Schaefer AW, Schoonderwoert VT, Ji L, Mederios N, Danuser G, Forscher P. Dev Cell. 2008;15:146. doi: 10.1016/j.devcel.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kirkham GR, Elliot KJ, Keramane A, Salter DM, Dobson JP, El Haj AJ, Cartmell SH. IEEE Trans Nanobioscience. 2010;9:71. doi: 10.1109/TNB.2010.2042065. [DOI] [PubMed] [Google Scholar]
- 78.Mannix RJ, Kumar S, Cassiola F, Montoya-Zavala M, Feinstein E, Prentiss M, Ingber DE. Nat Nanotechnol. 2008;3:36. doi: 10.1038/nnano.2007.418. [DOI] [PubMed] [Google Scholar]
- 79.Hirata H, Gupta M, Vedula SRK, Lim CT, Ladoux B, Sokabe M. EMBO Rep. 2015;16:250. doi: 10.15252/embr.201439140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu D, Wang L, Wang Z, Cuschieri A. Nano Lett. 2012;12:5117. doi: 10.1021/nl301928z. [DOI] [PubMed] [Google Scholar]
- 81.Dasgupta S, Auth T, Gompper G. Nano Lett. 2014;14:687. doi: 10.1021/nl403949h. [DOI] [PubMed] [Google Scholar]