Abstract
Objective
To investigate changes in nucleus pulposus cell expression and secretion of interleukin (IL)-1β and tumour necrosis factor (TNF)-α following stimulation with a low-frequency (LF) pulsed electromagnetic field (PEMF).
Methods
Primary rat nucleus pulposus cells were isolated and cultured in vitro, followed by stimulation with LF-PEMFs at a frequency of 2 Hz and different intensities, ranging from 0.5–3.0 A/m. Cells were observed for morphological changes, and proliferation rates were measured by cell viability counts. Expression of IL-1β and TNF-α within the nucleus pulposus cells was measured using western blotting, and levels of IL-1β and TNF-α secreted in the culture media were measured using enzyme-linked immunosorbent assay.
Results
Stimulation of nucleus pulposus cells with LF-PEMFs did not appear to affect cell morphology or nucleus pulposus cell IL-1β and TNF-α expression levels. LF-PEMFs did not significantly affect cell proliferation, however, levels of IL-1β and TNF-α secreted into the culture media were found to be significantly reduced in an intensity-dependent manner.
Conclusion
Low-frequency PEMF stimulation may inhibit secretion of IL-1β and TNF-α in cultured nucleus pulposus cells.
Keywords: Low-frequency pulsed electromagnetic field, nucleus pulposus cells, interleukin-1β (IL-1β), tumour necrosis factor-α (TNF-α)
Introduction
Interleukin (IL)-1β and tumour necrosis factor (TNF)-α are two key mediators involved in degenerative changes in intervertebral discs, and are synthesized and secreted by monocytes, macrophages, and annulus fibrosus cells.1,2 A range of clinical, in vivo and in vitro studies suggest that excessive secretion of IL-1β and TNF-α may cause a variety of degenerative osteoarthropathies, including osteoarthritis and intervertebral disc degeneration.3–6 The increased levels of inflammatory cytokines, such as TNF-α and IL-1β, associated with degenerative intervertebral disc disease in humans and in animal models, are thought to compromise the water binding capacity and biomechanical properties of the intervertebral disc, thereby promoting the degenerative state.7,8
Electromagnetic field stimulation has complex effects on the physiological status of cultured cells, for example, a low-frequency (LF) pulsed electromagnetic field (PEMF) has been shown to induce the proliferation of stem cells and human chondrocytes.9–11 The present study aimed to determine whether LF-PEMF stimulation affects nucleus pulposus cell function via regulation of IL-1β and TNF-α secretion, providing molecular biological evidence for the use of LF-PEMF stimulation to treat intervertebral disc degeneration.
Materials and methods
Isolation and culture of primary rat nucleus pulposus cells
For isolation and culture of nucleus pulposus cells, this study used male Sprague Dawley rats (age, 10 weeks; weight, 200–250 g). The animals had received care in compliance with the principles of the Association for Assessment and Accreditation of Laboratory Animal Care International (http://www.aaalac.org), and the experimental protocol was approved by the Animal Care and Research Committee of the First Affiliated Hospital of Soochow University, China. All surgical procedures were performed under sterile conditions.
Intervertebral discs from two healthy rats were extracted immediately following sacrifice by inhaled CO2. The entire thoracolumbar spine was removed, and the front disc attached to the muscles was stripped and rinsed twice with 0.01M phosphate buffered saline (pH 7.4; containing 1 g/l streptomycin and 1,000,000 U/l penicillin). The annulus fibrosus was then incised, and the gelatinous nucleus pulposus was removed. The gelatinous nucleus pulposus was then rinsed twice with Dulbecco’s modified Eagle medium supplemented with F12 (DMEM/F12; ThermoFisher Scientific, Asheville, NC, USA) and digested in 0.25% type II collagenase solution (Sigma-Aldrich Corp., St. Louis, MO, USA) at 37℃ for 15–20 min. The digested tissues from the two rats were pooled and pipetted gently, then passed through a mesh filter, and suspended by centrifugation at 200 × g for 8 min. Cells were seeded in DMEM/F12 medium into a 25 cm2 culture flask at a density of 1 × 105 cells/ml, and incubated at 37℃ under 5% CO2 and saturated humidity. When the cells reached 90% confluence, cells were detached using 0.25% trypsin (ThermoFisher Scientific) at 37℃ for 30 min and passaged at a dilution of 1:2 into 25 cm2 culture flasks.
PEMF stimulation
Briefly, 2 × 103 cells/well were seeded into 96-well plates, and 2 × 105 cells/plate were seeded into 100 mm plates. Following incubation for 48 h at 37℃/5% CO2 in DMEM/F12, adherent nucleus pulposus cells were equally divided into five groups (three × 100 mm plates/group for Western blots, and four × 96-well plates/group for cell viability assays) as follows: control group, not subjected to LF-PEMF stimulation; and four experimental groups, stimulated with an LF-PEMF at different intensities (0.5, 1.0, 2.0, and 3.0 A/m) at a frequency of 2 Hz using a YK-2000 pulsed electromagnetic field device (YiKing Company, Guangzhou, China). Cells were exposed to 30 min of LF-PEMF stimulation twice a day for 7 consecutive days. All other culture conditions were identical between the control and experimental groups.
Cell viability assay
At day 7 of LF-PEMF stimulation, cell viability was evaluated using Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technology, Rockville, MD, USA) according to the manufacturer’s instructions. Briefly, CCK-8 solution was added to each well of the 96-well plate and the cells were incubated at 37℃ for 1 h. To quantify cell viability, absorbance was determined at 450 nm using a microplate reader (Beckman Coulter, Brea, CA, USA).
Western blotting
At day 7 of LF-PEMF stimulation, the cells were harvested from 100 mm plates and washed three times with ice-cold phosphate buffered saline (pH 7.4), then lysed in buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 100 µg/ml phenylmethylsulphonyl fluoride, and 1% Triton X-100) for 30 min on ice. After removal of cell debris by centrifugation at 12 000 g for 5 min, the lysate protein concentrations were measured using a NanoDrop™ 2000 spectrophotometer (ThermoFisher Scientific). Next, 50 µg of protein from each experimental group was boiled for 5 min in sample buffer and separated by 12% sodium dodecyl sulphate polyacrylamide-gel electrophoresis and transferred to polyvinylidene fluoride membranes, which were then blocked with 1 × tris-buffered saline (TBS) -Tween-20 buffer (Thermo Fisher Scientific) containing 5% milk for 1 h at room temperature. The membranes were then incubated with goat anti-rat IL-1β monoclonal antibody (1:1000 dilution; Sigma-Aldrich) or goat anti-rat TNF-α monoclonal antibody (1:500 dilution; Sigma-Aldrich) at 4℃ overnight, then washed three times (5 min each) with 1 × tris-buffered saline (TBS). The membranes were then incubated with a horseradish peroxidase (HRP)-coupled rabbit anti-goat IgG monoclonal antibody (1:5000 dilution; Sigma-Aldrich) for 30 min at 37℃, and washed five times (5 min each) with 1 × TBS. The results were visualized using an electrochemiluminescent kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. Membranes were also incubated with rat anti-β-actin antibody (1:1000 dilution; Cell Signalling Technology, Danvers, MA, USA) for use as an internal control.
Sandwich enzyme linked immunosorbent assay (ELISA)
Secreted IL-1β and TNF-α concentrations were measured using sandwich avidin–biotin complex ELISA kits (Rat IL-1β and Rat TNF-α ELISA Kits for cell and tissue lysates; Sigma-Aldrich) according to the manufacturer’s instructions. Briefly, the 96-well plates were loaded with DMEM/F12 blank control, reference standards at different concentrations of target protein, and study sample culture medium (3 wells/sample; 100 µl/well), and were then incubated for 2 h at 37℃. The sample solutions were then discarded, and the wells were washed 4 times with 1 × wash solution prior to adding 100 µl of 1 × prepared detection antibody, followed by incubation for 1 h at 37℃. Absorbance values of the samples were measured at 450 nm using an ELISA plate reader (Model 680; Bio-Rad, Hercules, CA, USA). The sample concentrations were determined by subtracting the OD values of the blank control from the OD values of IL-1β and TNF-α and using the standard curve as a reference.
Statistical analyses
Continuous data are presented as mean ± SD, and all statistical analyses were performed using SPSS software, version 13.0 (SPSS Inc., Chicago, IL, USA). Kolmogorov–Smirnov test was applied to test data normality, and a P value < 0.05 was considered statistically significant. One way analysis of variance was used for between-group comparisons of normally distributed data. Post hoc multiple comparisons were performed in cases of statistically significant difference.
Results
The nucleus pulposus cells exhibited a flat morphology: polygonal cells were the most common, with a small number of spindle-shaped cells. Under visual microscopic examination, cell morphology appeared identical between the control group and the experimental groups stimulated with different LF-PEMF intensities (0.5, 1.0, 2.0, and 3.0 A/m; Figure 1).
Figure 1.
Representative phase-contrast photomicrographs showing morphology of primary rat nucleus pulposus cells (original magnification × 10) exposed to low-frequency pulsed electromagnetic fields at intensities of 0.5, 1.0, 2.0, and 3.0 A/m, and at a frequency of 2 Hz, compared with untreated control cells. Visual analyses showed that cell morphology appeared to be unaffected by exposure to low-frequency pulsed electromagnetic fields.
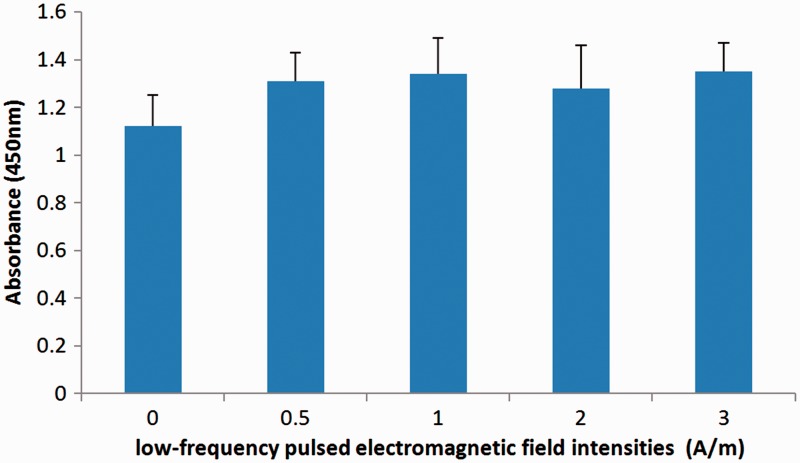
There were no statistically significant differences in cell viability, according to CCK-8 analysis, at day 7 of LF-PEMF stimulation between the control group and the LF-PEMF stimulated groups (Figure 2).
Figure 2.
Cell Counting Kit-8 analysis showing the effect of low-frequency (LF) pulsed electromagnetic field (PEMF) stimulation at intensities of 0, 0.5, 1.0, 2.0, and 3.0 A/m, and at a frequency of 2 Hz, on primary rat nucleus pulposus cell viability. There were no statistically significant differences in cell viability between the control group and experimental groups; data presented as mean ± SD (n = 3 replicates per group).
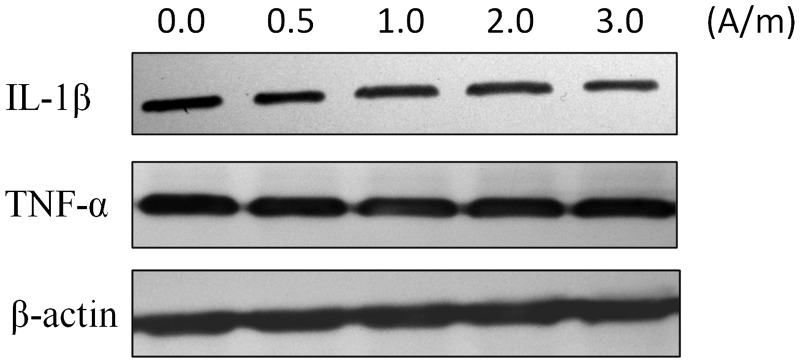
Visual analysis of Western blots showed that IL-1β and TNF-α were expressed in nucleus pulposus cells under all experimental conditions. In addition, there were no obvious visible differences in IL-1β and TNF-α expression between nucleus pulposus cells stimulated with varying intensities of LF-PEMF and unstimulated controls (Figure 3).
Figure 3.
Representative western blots showing the effect of low-frequency (LF) pulsed electromagnetic field (PEMF) stimulation at intensities of 0, 0.5, 1.0, 2.0, and 3.0 A/m, and at a frequency of 2 Hz, on expression of interleukin (IL)-1β and tumour necrosis factor (TNF)-α in primary rat nucleus pulposus cells (n = 3 replicates per group). IL-1β and TNF-α were expressed under all experimental conditions and there were no obvious visible changes in IL-1β and TNF-α levels between the experimental groups.
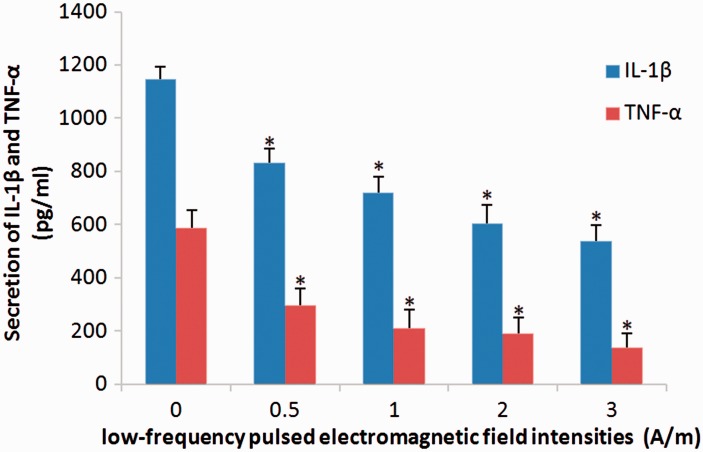
Stimulation with LF-PEMF was shown to significantly decrease the secretion of IL-1β and TNF-α into the culture supernatant of primary rat nucleus pulposus cells in an intensity-dependent manner (P < 0.05 between all groups) (Figure 4).
Figure 4.
Enzyme-linked immunosorbent assay showing the effect of low-frequency (LF) pulsed electromagnetic field (PEMF) stimulation at intensities of 0, 0.5, 1.0, 2.0, and 3.0 A/m, and at a frequency of 2 Hz, on secretion of interleukin (IL)-1β and tumour necrosis factor (TNF)-α by primary rat nucleus pulposus cells. LF-PEMF stimulation was associated with significantly decreased secretion of IL-1β and TNF-α into the cell culture supernatants. Data presented as mean ± SD (n = 3 replicates per group); *P < 0.05, versus other treatment groups (one way analysis of variance).
Discussion
Stimulation with LF-PEMF is reported to have complex biological effects on the physiological and biochemical characteristics of cells.12 Exposure to PEMFs is a therapeutic tool used extensively for the treatment of several pathologies including osteoarthritis, Parkinson’s disease, postsurgical pain and oedema, treatment of chronic wounds, and facilitation of vasodilatation and angiogenesis, producing direct stimulation to excitable cells including nerve and muscle cells.13–16 A moderately intense LF-PEMF stimulation is capable of promoting stem cell proliferation and mammalian cartilage repair, including in humans.17–19 A moderately intense LF-PEMF has also been shown to stimulate the proliferation and differentiation of chondrocytes,20,21 as demonstrated by two different research groups. To the best of the authors’ knowledge, however, the effects of LF-PEMF stimulation have not been explored in nucleus pulposus cells.
In the present study, primary rat nucleus pulposus cells were stimulated with an LF-PEMF at different intensities, and levels of IL-1β and TNF-α were examined using western blot and ELISA techniques. The present results indicated that LF-PEMF stimulation had no visible effects on nucleus pulposus cell morphology or intracellular expression of IL-1β and TNF-α. Cell proliferation was also not significantly affected, however, LF-PEMF stimulation was shown to decrease the secretion of IL-1β and TNF-α in the culture supernatants in an intensity-dependent manner. These data suggest that LF-PEMF stimulation may inhibit extracellular secretion of IL-1β and TNF-α instead of suppressing intracellular synthesis of these inflammatory cytokines, possibly through changes in vesicular transport and membrane dissociation of transmembrane proteins triggered by the EMF-induced membrane potential change.22 The mechanisms by which LF-PEMF stimulation may decrease secretion of IL-1β and TNF-α was not investigated in the present study, thus, further studies are required to elucidate the association between decreased IL-1β and TNF-α secretion and the biological effects on the membrane.
Interleukin-1β and TNF-α are two important cytokines that play critical roles in cell growth, proliferation, and apoptosis.23,24 Following secretion from nucleus pulposus cells, IL-1β and TNF-α can bind to their membrane receptors and induce the down-regulation of B-cell lymphoma 3 protein, a member of the nuclear factor-κB family, promoting nucleus pulposus cell apoptosis.25 Additionally, the production of IL-1β and TNF-α promotes the expression of matrix metalloproteinases and inhibits the synthesis of collagen and proteoglycan and the proliferation of nucleus pulposus cells.26 TNF induces peroxidation in chondrocytes and, together with IL-1β, promotes the absorption of intervertebral discs, thus mediating intervertebral disc destruction.27 In this context, the present authors hypothesize that interrupting the autocrine mechanism of IL-1β and TNF-α may have therapeutic potential for degenerative lumbar spine diseases and could improve the microenvironment of nucleus pulposus cells and promote tissue repair.
Several limitations of the present work should be noted. First, the study included pooled cells isolated from only two animals; secondly, three-dimensional culture systems, rather than the present two-dimensional adherent culture, would provide a more representative model of nucleus pulposus cells;28 thirdly, cell morphology and intracellular IL-1β and TNF-α levels were only visually assessed in the present study, and no semiquantitative analyses were performed – further research should include quantitative or semiquantitative analyses of the effects of LF-PEMF stimulation on IL-1β and TNF-α expression levels; and finally, further research should investigate other biomarkers, in addition to IL-1β and TNF-α, to elucidate the molecular mechanism of LF-PEMF.
In conclusion, the present study suggests that LF-PEMF stimulation may not affect IL-1β and TNF-α expression in primary rat nucleus pulposus cells, but appears to inhibit extracellular secretion of IL-1β and TNF-α in an intensity-dependent manner. These data provide an alternative explanation for PEMF-induced functional improvement in nucleus pulposus cells.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Natural Science Foundation of China (grant No.’s 81472132, 81572183 and 81672220) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
References
- 1.Johnson ZI, Schoepflin ZR, Choi H, et al. Disc in flames: roles of TNF-α and IL-1β in intervertebral disc degeneration. Eur Cell Mater 2015; 30: 104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JH, Studer RK, Sowa GA, et al. Activated macrophage-like THP-1 cells modulate anulus fibrosus cell production of inflammatory mediators in response to cytokines. Spine (Phila Pa 1976) 2008; 33: 2253–2259. [DOI] [PubMed] [Google Scholar]
- 3.Lencel P, Delplace S, Pilet P, et al. Cell-specific effects of TNF-α and IL-1β on alkaline phosphatase: implication for syndesmophyte formation and vascular calcification. Lab Invest 2011; 91: 1434–1442. [DOI] [PubMed] [Google Scholar]
- 4.Gaspari S, Marcovecchio ML, Breda L, et al. Growth in juvenile idiopathic arthritis: the role of inflammation. Clin Exp Rheumatol 2011; 29: 104–110. [PubMed] [Google Scholar]
- 5.Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol 2014; 10: 44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gruber HE, Hoelscher GL, Ingram JA, et al. Autophagy in the degenerating human intervertebral disc: in vivo molecular and morphological evidence, and induction of autophagy in cultured annulus cells exposed to proinflammatory cytokines-implications for disc degeneration. Spine (Phila Pa 1976) 2015; 40: 773–782. [DOI] [PubMed] [Google Scholar]
- 7.Barbir A, Godburn KE, Michalek AJ, et al. Effects of torsion on intervertebral disc gene expression and biomechanics, using a rat tail model. Spine (Phila Pa 1976) 2011; 36: 607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haschtmann D, Stoyanov JV, Gédet P, et al. Vertebral endplate trauma induces disc cell apoptosis and promotes organ degeneration in vitro. Eur Spine J 2008; 17: 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anbarasan S, Baraneedharan U, Paul SF, et al. Low dose short duration pulsed electromagnetic field effects on cultured human chondrocytes: an experimental study. Indian J Orthop 2016; 50: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim K, Hexiu J, Kim J, et al. Effects of electromagnetic fields on osteogenesis of human alveolar bone-derived mesenchymal stem cells. Biomed Res Int 2013; 2013: 296019–296019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang W, Li W, Song M, et al. Effects of electromagnetic fields on the metabolism of lubricin of rat chondrocytes. Connect Tissue Res 2016; 57: 152–160. [DOI] [PubMed] [Google Scholar]
- 12.Falone S, Marchesi N, Osera C, et al. Pulsed electromagnetic field (PEMF) prevents pro-oxidant effects of H2O2 in SK-N-BE(2) human neuroblastoma cells. Int J Radiat Biol 2016; 92: 281–286. [DOI] [PubMed] [Google Scholar]
- 13.Iannitti T, Fistetto G, Esposito A, et al. Pulsed electromagnetic field therapy for management of osteoarthritis-related pain, stiffness and physical function: clinical experience in the elderly. Clin Interv Aging 2013; 8: 1289–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vadalà M, Vallelunga A, Palmieri L, et al. Mechanisms and therapeutic applications of electromagnetic therapy in Parkinson’s disease. Behav Brain Funct 2015; 11: 26–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryang We S, Koog YH, Jeong KI, et al. Effects of pulsed electromagnetic field on knee osteoarthritis: a systematic review. Rheumatology (Oxford) 2013; 52: 815–824. [DOI] [PubMed] [Google Scholar]
- 16.Strauch B, Herman C, Dabb R, et al. Evidence-based use of pulsed electromagnetic field therapy in clinical plastic surgery. Aesthet Surg J 2009; 29: 135–143. [DOI] [PubMed] [Google Scholar]
- 17.Anbarasan S, Baraneedharan U, Paul SF, et al. Low dose short duration pulsed electromagnetic field effects on cultured human chondrocytes: An experimental study. Indian J Orthop 2016; 50: 87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Oliveira Melo M, Pompeo KD, Baroni BM, et al. Effects of neuromuscular electrical stimulation and low-level laser therapy on neuromuscular parameters and health status in elderly women with knee osteoarthritis: a randomized trial. J Rehabil Med 2016; 48: 293–299. [DOI] [PubMed] [Google Scholar]
- 19.Veronesi F, Fini M, Giavaresi G, et al. Experimentally induced cartilage degeneration treated by pulsed electromagnetic field stimulation; an in vitro study on bovine cartilage. BMC Musculoskelet Disord 2015; 16: 308–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito M, Lucariello A, Costanzo C, et al. Differentiation of human umbilical cord-derived mesenchymal stem cells, WJ-MSCs, into chondrogenic cells in the presence of pulsed electromagnetic fields. In Vivo 2013; 27: 495–500. [PubMed] [Google Scholar]
- 21.Vincenzi F, Targa M, Corciulo C, et al. Pulsed electromagnetic fields increased the anti-inflammatory effect of A2A and A3 adenosine receptors in human T/C-28a2 chondrocytes and hFOB 1.19 osteoblasts. PLoS One 2013; 8: e65561–e65561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balcavage WX, Alvager T, Swez J, et al. A mechanism for action of extremely low frequency electromagnetic fields on biological systems. Biochem Biophys Res Commun 1996; 222: 374–378. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Lu Y, Guo A. Platelet-rich plasma protects rat chondrocytes from interleukin-1β-induced apoptosis. Mol Med Rep 2016; 14: 4075–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheng L, Zhang D, Chen B. Tumor necrosis factor α-induced protein-3 protects zinc transporter 8 against proinflammatory cytokine-induced downregulation. Exp Ther Med 2016; 12: 1509–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Yuan W, Jiang S, et al. Prolyl-4-hydroxylase domain protein 2 controls NF-κB/p65 transactivation and enhances the catabolic effects of inflammatory cytokines on cells of the nucleus pulposus. J Biol Chem 2015; 290: 7195–7207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ye W, Zhou J, Markova DZ, et al. Xylosyltransferase-1 expression is refractory to inhibition by the inflammatory cytokines tumor necrosis factor α and IL-1β in nucleus pulposus cells: novel regulation by AP-1, Sp1, and Sp3. Am J Pathol 2015; 185: 485–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim HJ, Yeom JS, Koh YG, et al. Anti-inflammatory effect of platelet-rich plasma on nucleus pulposus cells with response of TNF-α and IL-1. J Orthop Res 2014; 32: 551–556. [DOI] [PubMed] [Google Scholar]
- 28.Goliwas KF, Miller LM, Marshall LE, et al. Preparation and analysis of in vitro three dimensional breast carcinoma surrogates. J Vis Exp 2016111: e54004–e54004. [DOI] [PMC free article] [PubMed] [Google Scholar]