Abstract
Objective
To examine if hypoxia inducible factor-1α (HIF-1α) can induce the upregulation of the purinergic receptor P2Y2 (P2Y2) and thereby promote the viability of human hepatocellular carcinoma (HCC) cells under hypoxic conditions.
Methods
Archival HCC tumour specimens and corresponding non-cancerous tissues were examined immunohistochemically for P2Y2 protein. A series of in vitro experiments were undertaken using HCC cell lines to determine the effect of hypoxia on HIF-1α and P2Y2 levels, the effect of HIF-1α upregulation on P2Y2 levels, and the effect of P2Y2 upregulation on cell viability under hypoxic conditions.
Results
Human HCC specimens were positive for P2Y2. Hypoxia and upregulated HIF-1α both upregulated the P2Y2 levels in HCC cell lines. P2Y2 upregulation using plasmid transfection resulted in enhanced cell viability under hypoxia. Treatment of HepG2 cells with the selective P2Y2 antagonist MRS2312 downregulated P2Y2 and reduced cell viability in five HCC cell lines. P2Y2 knockdown reduced HepG2 cell viability under hypoxia.
Conclusions
These present results suggest that HCC cells upregulate P2Y2 levels during hypoxia, which in turn promotes their growth. P2Y2 could be a potential therapeutic target for treating HCC.
Keywords: Purinergic receptor P2Y2, hypoxia inducible factor-1α, MRS2312, hepatocellular carcinoma
Introduction
The P2Y receptors are G-protein-coupled receptors for extracellular nucleotides. In all, eight P2Y receptor subtypes, specifically P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14, have been identified in humans.1 In addition, most human cells express P2Y receptors.2 P2Y receptors are found in many animal species, indicating their evolutionary conservation.3 Excellent reviews have summarized the roles of this family in physiology and pathophysiology;4–6 for example, ADP-induced platelet aggregation is mediated by the P2Y2 receptor.7
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide and frequently develops in patients with cirrhosis.8,9 Although several treatment options are available for treating HCC,10–12 the long-term prognosis is usually poor. Surgery and living donor liver transplantation are curative treatment options for HCC. However, most patients can receive only palliative treatments, including transarterial chemoembolization (TACE).13,14 The overall recurrence rate of HCC in patients showing initial remission after TACE is high.15 Therefore, the development of anticancer drugs with high efficacy is required for the treatment of HCC.16
Hepatocellular carcinoma cells are found in conditions of hypoxia, which should result in cell death. However, HCC cells develop cellular defence mechanisms to evade cell death that are primarily regulated by transcription factors.17–20 The present study evaluated the possible role of the P2Y2 receptor in HCC cells during hypoxia and resistance to anticancer drugs such as MRS2312.
Materials and methods
Cell culture and hypoxia exposure
Human normal hepatocytes (HepaRG™) obtained from Life Technologies (Carlsbad, CA, USA) were grown in William’s E medium (Life Technologies) with GlutaMAX™-I Supplement (Life Technologies) and HepaRG™ Thaw, Plate & General Purpose Medium (Life Technologies). HCC cell lines purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA; HepG2, SK-Hep1, Huh7, and Hep3B) were grown in Dulbecco's Modified Eagles Medium (Hyclone Laboratories, Logan, UT, USA) supplemented with 10% fetal bovine serum (FBS; Corning Life Sciences, Tewksbury, MA, USA) and 100 units/ml penicillin, and 100 µg/ml streptomycin (both antibiotics from Hyclone Laboratories) and the SNU449 cell line obtained from the Korean Cell Line Bank (KCLB, Seoul, Korea) was grown in RPMI 1640 medium (Hyclone Laboratories) with 10% FBS and antibiotics. For routine culture, the cells were incubated at 37℃ in a humidified normoxic air of 21% O2 and 5% CO2. For hypoxic exposure, the cells were placed in a hypoxia chamber (MCO-18M; Sanyo, Tokyo, Japan) in an atmosphere of 94.9% N2, 5% CO2, and 0.1% O2 for up to 12 h.
Tissue samples from patients with HCC
Human liver tissues were randomly obtained from 68 patients who underwent surgical resection of HCC between February 2015 and December 2015 in the Department of Surgery, Division of Liver Transplantation and Hepatobiliary Surgery, Asan Medical Centre, University of Ulsan College of Medicine, Seoul, Republic of Korea. Paired wedge resection was performed for both HCC and adjacent non-tumour liver tissues immediately after liver specimen delivery from the abdomen and the tissues were stored at −70℃.21,22 Collection and use of patient samples were approved by the Asan Medical Centre Institutional Review Board, Asan Medical Centre, University of Ulsan College of Medicine, Seoul, Republic of Korea (no. 2014-0465). Written informed consent was obtained from all patients who provided tissue samples.
Reverse transcription PCR
Total RNA was isolated from 1.0 × 106 hepatocellular carcinoma cells and 45–50 mg of normal and tumour tissues using a NucleoSpin® RNA II RNA isolation kit (Macherey-Nagel, Dueren, Germany) according to the manufacturer’s instructions. Next, cDNA was synthesized by performing reverse transcription (RT) using an iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA), according to the manufacturer’s instructions. The polymerase chain reaction (PCR) was performed using the following primer sets: glyceraldehyde 3-phosphate dehydrogenase (GAPDH) control forward 5′-GAGTCAACGGATTTGGTCGT-3′, and reverse 5′-TTGATTTTGGAGGGATCTCG-3′; producing a PCR amplification product of 238 base pairs (bp). P2Y2 forward 5′-CCGCTTCAACGAGGACTTCAA-3′, and reverse 5′-GCGGGCGTAGTAATAGACCA-3′; producing a PCR amplification product of 211 bp. The primers were synthesized by Bioneer Corporation (Daejeon, Republic of Korea). Quantitative PCR was performed using the LightCycler® 480 (Roche Diagnostics, Mannheim, Germany) system with AccuPower® 2X Greenstar qPCR Master Mix (Bioneer Corporation). The cycling programme involved preliminary denaturation at 95℃ for 5 min, followed by 40 cycles of denaturation at 95℃ for 15 s, annealing at 60℃ for 30 s, and elongation at 72℃ for 30 s, followed by a final elongation step at 72℃ for 5 min. Levels of and fold changes in GAPDH and P2Y2 mRNAs were determined as described previously.23 Visualization of the PCR products was performed by electrophoresis through 1% agarose gels stained with RedSafe™ Nucleic Acid Staining Solution (iNtRON Biotechnology, Seongnam, Republic of Korea). Images of the gels were captured using a UV transilluminator (GenoSens 1500; Clinx Science Instruments, Shanghai, China).
Western blotting
Protein samples were extracted from 1.5 × 106 HCC cells and approximately 70 mg of frozen normal and tumour liver tissues. For the whole lysate extraction, RIPA Lysis and Extraction Buffer (Biosesang, Sengnam, Republic of Korea) supplemented with a protease inhibitor cocktail (complete Mini, ethylenediaminetetra-acetic acid-free; Roche Diagnostics) was used according to the manufacturer’s instructions. For the nucleus protein isolation kit, NE-PER™ Nuclear and Cytoplasmic Extraction Reagents were used according to the manufacturer’s instructions (catalogue no. 78833; Thermo Fisher Scientific, Rockford, IL, USA). The protein concentration of each sample was determined using a BCA protein assay kit (Thermo Fisher Scientific). Samples containing the same amount of protein were resolved on a 10% polyacrylamide gel and were transferred onto nitrocellulose membranes (Trans-Blot® Turbo™ Nitrocellulose; Bio-Rad). The membranes were blocked using 5% nonfat dry milk (AppliChem, Cheshire, CT, USA) in Tris-buffered saline Tween-20 (TBST; pH 7.6; 20 mM Tris-HCl, 150 mM NaCl and 0.1 % Tween 20) at room temperature (24℃) for 30 min and were incubated overnight at 4℃ with anti-P2Y2 rabbit polyclonal antibody (1:1000 dilution; catalogue no. sc-20124; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and purified mouse anti-human hypoxia inducible factor-1α (HIF-1α) antibody (1:1000 dilution; catalogue no. 610958; BD Bioscience, San Jose, CA, USA). Next, the membranes were rinsed three times with TBST (pH 7.6) for 10 min at room temperature and incubated with horseradish peroxidase (HRP)-conjugated goat anti-mouse antibody (1:1000 dilution; catalogue no. sc-2005; Santa Cruz Biotechnology) and HRP-conjugated goat anti-rabbit IgG (1:10000 dilution; catalogue no. sc-2004; Santa Cruz Biotechnology) at room temperature for 1 h with gentle agitation. Membranes were washed five times with TBST (pH 7.6) for 30 min at room temperature. Protein signals were detected using an enhanced chemiluminescence (ECL) solution (SuperSignal™ West Femto; Thermo Fisher Scientific) and images were captured using an ImageQuant LAS 4000 system (GE Healthcare Biosciences, Piscataway, NJ, USA). The blots were stripped using Restore™ PLUS (Thermo Fisher Scientific) stripping buffer according to the manufacturer’s instructions and were reprobed for β-actin (internal control) using the same methods. Mouse monoclonal anti-human actin antibody (1:20000 dilution; catalogue no. CP01; Calbiochem, Darmstadt, Germany) was used as the primary antibody and HRP-conjugated goat anti-mouse antibody (1:1000 dilution; catalogue no. sc-2005; Santa Cruz Biotechnology) was used as the secondary antibody. The membranes were washed again, and β-actin was detected using the ECL solution.
MTT cell proliferation assay
Cell viability was determined by performing 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assays in 12-well plates (Duchefa, Haarlem, The Netherlands). In brief, the five HCC cell lines (HepG2, SK-Hep1, SNU449, Huh7 and Hep3B) were seeded at a density of 1.0 × 105 cells per well and incubated for 24 h at 37℃. The cells were exposed to different concentrations of MRS2312 (5, 10 and 20 µM) for 24 h, 48 h, 72 h and 96 h. Normal hepatic cells (HepaRG™) with P2Y2 overexpression were placed under hypoxic conditions for up to 96 h for the MTT assay. After incubation, 20 µl of MTT solution (5 mg/ml) was added to each well and the plates were incubated in the dark for 1 h. After removing the culture media, 400 µl of dimethyl sulfoxide (Duchefa) was added to each well in order to solubilize the intracellular formazan crystals. After 10 min, the optical density was assessed at 540 nm using a microplate reader (Sunrise™; Tecan, Männedorf, Switzerland). Cell survival was expressed as the percentage of absorbance of MTT-treated cells relative to that of untreated cells. All treatment groups were measured in triplicate.
Measurement of ATP
The ATP levels were measured using an ATP Bioluminescent Assay Kit (catalogue no. 213-579-1; Sigma-Aldrich, St Louis, MO, USA) according to the manufacturer’s instructions. Normal hepatocyte HepaRG™ cells were seeded at a density of 1.0 × 105 cells per well and incubated for 24 h at 37℃. For normoxic conditions, cells were cultured in normoxic air containing 21% O2 and 5% CO2. For hypoxic conditions, cells were placed in a hypoxia chamber (MCO-18M; Sanyo, Tokyo, Japan) in an atmosphere of 94.9% N2, 5% CO2, and 0.1% O2 for up to 12 h. The ATP was extracted from the cells by the addition of 50 μl of cell extraction reagent to each well. After mixing thoroughly, the microplates were allowed to stand for 20–30 min at room temperature before 50 μl medium from each well was transferred to a white plate from the kit. Then, 50 μl luciferin-luciferase counting reagent was added. Luminescence was measured using a Synergy H1 multi-mode reader (BioTek, Winooski, VT, USA) and normalized to the protein concentration that was measured using the BCA protein assay kit. All experiments were performed in triplicate.
Plasmid overexpression of HIF-1α and P2Y2
HepaRG™ and HepG2 cells were seeded into 6-well plates at a density of 2.0 × 105 cells and were incubated for 24 h at 37℃. The cells were transfected with HIF-1-pcDNA3.1-expressing plasmid using Lipofectamine® 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. HIF-1α and P2Y2 overexpression was confirmed by Western blot analysis. The negative control plasmid pcDNA3.1(+), and the HIF-1-pcDNA3.1 and P2Y2-pc-DNA3.1 expressing plasmids were purchased from Addgene (Cambridge, MA, USA).
RNA interference
Small interfering RNAs (siRNA) specific to either P2Y2 (P2Y2 siRNA) or control sequences (cont siRNA) were prepared by Bioneer Corporation. The sequences were as follows: P2Y2 siRNA, sense: 5′-GAGGAAGGUGGCUUACCAA(dTdT)-3′, anti-sense: 5′-UUGGUAAGCCACCUUCCUC(dTdT)-3′; and cont siRNA, sense: 5′-CCUACGCCACCAAUUUCGU(dTdT)-3′, anti-sense: 5′-ACGAAAUUGGUGGCGUAGG (dTdT)-3′. Transfection of siRNA was carried out using Lipofectamine® 2000 reagent (Invitrogen) according to the manufacturer’s instructions.
Liver histology
Liver sections were deparaffinized with xylene and were serially rehydrated in 100%, 95%, 90% and 80% ethanol. Antigen retrieval was performed by heating the tissue sections in a 10 mM sodium citrate buffer (pH 6.0; Sigma-Aldrich) at 95℃ for 20 min on a hot plate stirrer and the sections were then cooled to room temperature. Slides were incubated overnight at 4℃ with a polyclonal antibody against P2Y2 (1:500 dilution; Santa Cruz Biotechnology). After incubation, the slides were washed three times for 5 min with phosphate-buffered saline containing 0.05% Tween 80 (PBST; pH 7.4) and were incubated with a biotinylated anti-mouse IgG rabbit antibody that was diluted in a diluent buffer included in the VECTASTAIN® ABC kit (Vector Laboratories, Burlingame, CA, USA) for 1 h at room temperature. After being washed three times for 5 min with PBST (pH 7.4), the slides were incubated for 30 min with VECTASTAIN® ABC Reagent at room temperature and washed three times with PBST (pH 7.4) for 5 min. The specimens were covered with 3,3'-diaminobenzidine solution (Liquid DAB+ substrate chromogen system; Dako, Glostrup, Denmark) for 1 min at room temperature and then rinsed gently with distilled water. The sections were counterstained with haematoxylin and eosin to confirm their structural integrity. Results were interpreted by two pathologists who were blinded to the specific diagnosis and prognosis for each patient. The degree of P2Y2 immunoreactivity on the tumour tissues and corresponding non-cancerous tissues was assessed semi-quantitatively. The intensity of staining of tissue sections was scored using a low-power magnification (×40) light microscope (Axioskop 2 plus with AxioCam; Carl Zeiss, Thornwood, NY, USA) and the following four-grade system: 0, no staining; 1, weak staining appearing as light yellow; 2, moderate staining appearing as yellowish-brown; 3, strong staining appearing as brown. The proportion of positively stained cells (i.e. at least grade 1, weak staining appearing as light yellow) was scored at a high-power magnification (×400) using an Axioskop 2 plus with AxioCam light microscope and the following four grades: 0, none; 1, <10%; 2, 10–50%; 3, >50%).24 The final immunoreactivity score for each section was obtained by multiplying the ‘score for staining intensity’ and the ‘score for positive cell proportion’. A scale from ‘–’ to ‘+++’ was used for categorize the results of the scoring system, where ‘–’ represents absolutely no immunoreactivity (score 0; negative), ‘+’ represents weak immunoreactive cells (score 1–3), ‘++’ represents moderate immunoreactive cells (score 4–6), and ‘+++’ represents strong immunoreactive cells (score 7–9). Only sections showing at least grade ‘++’ immunoreactivity were considered positive.
Statistical analyses
All statistical analyses were performed using GraphPad Prism 5.0 software for Windows XP (GraphPad Software, La Jolla, CA, USA). All data are expressed as mean ± SD of 3–8 samples per experimental condition. Each Western blotting experiment was repeated three times. For comparisons of two groups, Student’s t-test was used. For comparisons of three or more groups, one-way analysis of variance (ANOVA) followed by Bonferroni’s multiple comparison test was used. A P-value < 0.05 was considered statistically significant.
Results
To assess the clinical relevance of the P2Y2 receptor, the levels were measured immunohistochemically in pathologically-confirmed HCC tissue specimens and corresponding non-cancerous tissue specimens. Forty-three out of 68 patients (63.2%) with HCC showed moderate (‘++’) to strong (‘+++’) immunoreactivity for the P2Y2 receptor in the HCC tissue specimens (Table 1). In contrast, negative (‘–’) or weak (‘+’) immunoreactivity for the P2Y2 receptor was observed in the corresponding non-cancerous tissue specimens. P2Y2 immunoreactivity levels did not appear to be correlated with tumour grade. A Western blot analysis of tissue samples from five patients demonstrated upregulation of P2Y2 protein levels in the HCC tissue specimens (Figure 1A) and upregulation of P2Y2 mRNA as measured by RT–PCR (Figure 1B). Representative photomicrographs of the immunohistochemical staining patterns of P2Y2 in HCC specimens and corresponding non-cancerous tissue specimens are shown in Figure 1C.
Table 1.
Histopathological features and immunoreactivity levels of P2Y2 protein in hepatocellular carcinoma (HCC) specimens taken from patients (n = 68).
| Tumour grade | Sex | P2Y2 immunoreactivity levels in HCC tumour specimens n = 68 |
|||
|---|---|---|---|---|---|
| _ | + | ++ | +++ | ||
| Grade 1 | Male | 0 | 2 | 4 | 0 |
| Female | 0 | 1 | 1 | 1 | |
| Grade 2 | Male | 2 | 3 | 4 | 1 |
| Female | 2 | 4 | 2 | 1 | |
| Grade 3 | Male | 0 | 10 | 15 | 6 |
| Female | 0 | 0 | 7 | 0 | |
| Grade 4 | Male | 0 | 1 | 1 | 0 |
| Female | 0 | 0 | 0 | 0 | |
Data presented as n of patients.
Figure 1.
The levels of P2Y2 protein and mRNA were measured in hepatocellular carcinoma (HCC) tissues and corresponding non-cancerous tissue specimens. (A) Western blot analysis of P2Y2 protein levels in five patients (P1–P5) with HCC who underwent liver resection and their corresponding non-cancerous tissue specimens (Nor); β-actin was used as a loading control. (B) Real-time reverse transcription–polymerase chain reaction analysis of P2Y2 mRNA levels in the HCC tissues and corresponding non-cancerous tissue specimens (Nor) from five patients. Levels shown relative to the control housekeeping gene glyceraldehyde 3-phosphate dehydrogenase. Data are presented as mean ± SD; P < 0.05, Student’s t-test. (C) Representative photomicrographs of histological specimens of the HCC tissues and corresponding non-cancerous tissue specimens (Nor) stained with haematoxylin and eosin (H&E) and immunohistochemically labelled for P2Y2. Scale bar: 50 µm. One of three representative slides is shown. The colour version of this figure is available at: http://imr.sagepub.com.
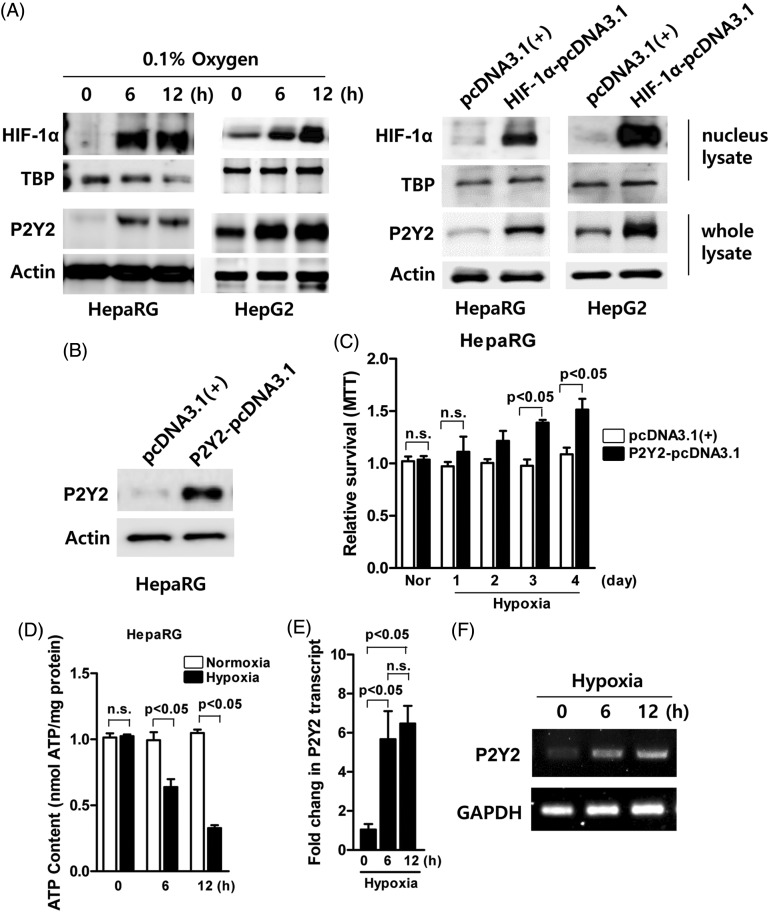
To determine whether hypoxia upregulated P2Y2 levels, normal HepaRG™ and cancerous HepG2 cells were exposed to hypoxia for up to 12 h. Western blot analyses demonstrated that both P2Y2 and HIF-1α levels increased in both cell lines in a time-dependent manner (Figure 2A, left hand image). To investigate whether the increases in P2Y2 were dependent upon the upregulation of HIF-1α, HepaRG™ and HepG2 cells were transiently transfected with the negative control plasmid pcDNA3.1(+) or the HIF-1-pcDNA3.1-expressing plasmid and incubated for 12 h under normoxic culture conditions. P2Y2 levels were upregulated when HIF-1α levels were also upregulated in both cell lines (Figure 2A, right hand image).
Figure 2.
Analysis of the effects of hypoxia on the protein levels of hypoxia inducible factor-1α (HIF-1α) and P2Y2 in cell lines. (A) Western blot analysis of HIF1-α and P2Y2 levels in normal HepaRG™ and cancerous HepG2 cells cultured under hypoxic conditions for up to 12 h (left hand image). Western blot analysis of HepaRG™ and HepG2 cells that were transiently transfected with the negative control plasmid pcDNA3.1(+) or the HIF-1-pcDNA3.1-expressing plasmid and incubated for 12 h under normoxic culture conditions showing that P2Y2 levels were upregulated when HIF-1α levels were also upregulated in both cell lines (right hand image). β-actin was used as a loading control. (B) Western blot analysis to confirm upregulation of P2Y2 levels in HepaRG™ cells transiently transfected with the P2Y2-pcDNA.3.1 plasmid compared with HepaRG™ cells transfected with the negative control pcDNA3.1(+) plasmid. β-actin was used as a loading control. (C) The effect of the upregulation of P2Y2 levels on cell viability under hypoxic conditions (up to 4 days) as determined using the MTT assay in HepaRG™ cells transiently transfected with the negative control plasmid pcDNA3.1(+) or the P2Y2-pcDNA.3.1 plasmid. Data are presented as mean ± SD; P < 0.05, Student’s t-test. (D) ATP levels in HepaRG™ cells cultured under normoxic or hypoxic conditions for up to 12 h. Data are presented as mean ± SD; P < 0.05, Student’s t-test. (E–F) Real-time reverse transcription–polymerase chain reaction analysis to confirm the upregulation of P2Y2 mRNA levels in HepG2 cells under hypoxic conditions for up to 12 h. The control housekeeping gene was glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are presented as mean ± SD; P < 0.05, one-way analysis of variance followed by Bonferroni’s multiple comparison test. MTT, 3 -(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide n.s., no significant between-group difference (P ≥ 0.05).
To investigate the role of overexpressed P2Y2 under hypoxic conditions, HepaRG™ cells were transiently transfected with the negative control pcDNA3.1(+) plasmid or the P2Y2-pcDNA3.1-expressing plasmid. Western blot analysis confirmed the upregulation of P2Y2 levels in HepaRG™ cells transfected with the P2Y2-pcDNA3.1-expressing plasmid (Figure 2B). Using the same transfected cells, the effect of the upregulation of P2Y2 levels on cell viability under hypoxic conditions was examined using the MTT assay. Upregulation of P2Y2 levels in transfected HepaRG™ cells significantly increased cell viability on days 3 and 4 of hypoxic conditions compared with HepaRG™ cells transfected with the negative control pcDNA3.1(+) plasmid (P < 0.05 for both comparisons) (Figure 2C). For the measurement of ATP content, HepaRG™ cells were exposed to normoxic and hypoxic conditions for up to 12 h. The data showed that the cellular ATP content was significantly decreased after 6 and 12 h of hypoxic conditions compared with normoxic conditions (P < 0.05 for both comparisons) (Figure 2D). RT–PCR confirmed the upregulation of P2Y2 levels in HepG2 cells under hypoxic conditions compared with normoxic conditions (Figures 2E & 2F).
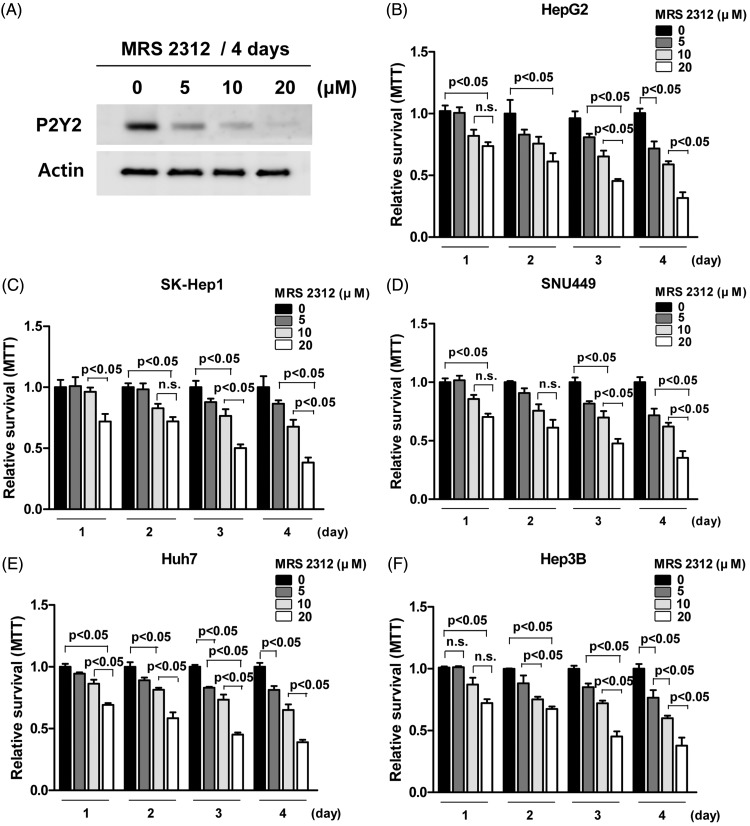
Investigations were undertaken to test if the specific P2Y2 inhibitor, MRS2312, works in a concentration-dependent manner in HepG2 cells to reduce cell viability. A Western blot analysis showed that P2Y2 levels in HepG2 cells were decreased following incubation with MRS2312 (5, 10 and 20 µM) for 4 days under normoxic conditions in a concentration-dependent manner (Figure 3A). As P2Y2 levels were upregulated in HCC cells under hypoxic conditions and in order to examine the effect of P2Y2 inhibition on cell proliferation, cell viability was measured using the MTT assay in five cell lines (HepG2, SK-Hep1, SNU449, Huh7 and Hep3B) exposed to different concentrations of MRS2312 (5, 10 and 20 µM) for up to 4 days under normoxic conditions (Figures 3B–3F). The MRS2312-induced inhibition of P2Y2 reduced the viability of HCC cell lines in a concentration-dependent manner (P < 0.05 for individual comparisons as shown on Figures 3B–3F).
Figure 3.
Investigations into the effects of the P2Y2-selective antagonist MRS2312 on P2Y2 mRNA levels and HCC cell line viability. (A) Western blot analysis of P2Y2 mRNA levels in HepG2 cells treated with different concentrations of MRS2312 (5, 10 and 20 µM) for 4 days under normoxic conditions. β-actin was used as a loading control. (B–F) The effect of the selective inhibition of P2Y2 on cell viability of five HCC cell lines (HepG2, SK-Hep1, SNU449, Huh7 and Hep3B) treated with different concentrations of MRS2312 (5, 10 and 20 µM) for up to 4 days under normoxic conditions as determined using the MTT assay. Data presented as mean ± SD of 8 wells per group; P < 0.05, one-way analysis of variance followed by Bonferroni’s multiple comparison test. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; n.s., no significant between-group difference (P ≥ 0.05).
Investigations were undertaken to test whether specific siRNA targeted to knockdown P2Y2 could increase HepG2 cell death under hypoxic conditions using the MTT assay. Western blot analysis demonstrated that the levels of P2Y2 protein were reduced in HepG2 cells transiently treated with P2Y2 siRNA compared with HepG2 cells treated with cont siRNA (Figure 4A). The results of the MTT assay demonstrated that culture in hypoxic conditions for up to 4 days was more toxic to HepG2 cells transiently treated with P2Y2 siRNA compared with HepG2 cells treated with cont siRNA (Figure 4B). The differences in cell viability between the two groups were significant on days 3 and 4 of hypoxia (P < 0.05 for both comparisons).
Figure 4.
Investigations into the effect of P2Y2 knockdown using a small interfering RNA (siRNA) in HepG2 cells. (A) Western blot analysis of HepG2 cells transiently treated with cont siRNA and P2Y2 siRNA for 12 h. β-actin was used as a loading control. (B) The effect of the selective knockdown of P2Y2 using P2Y2 siRNA on cell viability of HepG2 cells cultured under hypoxic conditions for up to 4 days as determined using the MTT assay. Data are presented as mean ± SD; P < 0.05, Student’s t-test. MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; n.s., no significant between-group difference (P ≥ 0.05).
Discussion
This present study demonstrated the immunohistochemical localization of P2Y2 protein in human HCC specimens and the levels of P2Y2 were consistently higher in these specimens compared with corresponding non-cancerous tissue specimens. When the HepG2 cell line was exposed to hypoxic culture conditions, the levels of P2Y2 protein and mRNA increased. The selective pharmacological inhibition of P2Y2 using MRS2312 reduced cell viability in a concentration-dependent manner in five HCC cell lines. These findings suggest the cancer-promoting properties of P2Y2.25,26 Recent studies suggest that P2Y2 performs important roles during cell proliferation and differentiation.27–29 However, the mechanism underlying the P2Y2-induced proliferation of HCC cells is not completely understood. Adenosine activates P2Y2 in human retinal endothelial cells, which may lead to neovascularization because of an increase in the expression of angiogenic growth factors.25,26,30–33
Hypoxia produces toxic conditions of oxidative stress from the mitochondrial electron transport chain complex III and promotes hypoxia-induced cell death.34 The rapidly growing HCC cells are usually exposed to hypoxic conditions especially in the central region of the tumour. Growth under hypoxic conditions eventually leads to HCC cell death. However, hypoxia stabilizes HIF-1α and prohibits hypoxic cell death by increasing the levels of anti-apoptotic molecules, including Bcl-2, Bax and IAP-2.35 Moreover, HIF-1α enhances the blood supply to the tumour by regulating the transcription of several genes involved in angiogenesis.36,37 As a result, HCC cells are able to escape cell death and continue to proliferate even under hypoxic conditions.36,37 Indeed, it has been suggested that hypoxia enhances cell proliferation, suppresses apoptotic cell death, and consequently leads to enhanced tumour malignancy in HCC.38,39 Usually under hypoxic conditions, ATP is generated in exchange for ADP by the adenosine nucleotide translocases.40 ATP is utilized by a variety of proteins such as P2Y2, adenosine receptors, and NA-K-ATPase, which regenerates ADP pools.41,42 In this present study, P2Y2 was transcriptionally upregulated by HIF-1α and appeared to play an important role in converting cellular ATP into ADP for the homeostatic adaption to the hypoxic culture conditions. These current results demonstrated that HIF-1α upregulates P2Y2, and thereby attenuates cell death under hypoxic conditions. In contrast, treatment of HCC cell lines with the selective P2Y2 antagonist MRS2312 resulted in reduced cell viability as measured by the MTT assay. These findings suggest that the inhibition of P2Y2 might be a novel target for the development of an anticancer treatment for HCC.
Hepatocellular carcinoma is highly resistant to various therapeutic modalities, including combination therapy with TACE, anticancer drugs, and antiangiogenic agents.43–46 Therefore, development of more effective treatment modalities and new anticancer drugs is urgently required.25 These present results suggest that downregulation of P2Y2 levels or inhibition of its enzymatic activity increased the sensitivity of HCC cells to MRS2312 because of an increase in oxidative stress. Therefore, therapeutic agents targeting P2Y2 levels or its enzymatic activity may be effective for treating HCC.47–49 Furthermore, these current results showed that knockdown of P2Y2 using siRNA decreased the viability of HepG2 cells when cultured under hypoxic conditions. Immunohistochemical analysis showed that P2Y2 was overexpressed in 43 out of 68 patients (63.2%) with HCC, suggesting that P2Y2 plays an important role in the tumorigenesis of HCC and its resistance to anticancer therapies. More convincing evidence of the role of P2Y2 in tumour malignancy should be obtained by performing large studies involving more patients with HCC and other cancers.
In conclusion, this present study showed that P2Y2 was upregulated in HCC cells under hypoxic conditions. These current results showed that enhanced P2Y2 levels play an important role in the proliferation of HCC cells, suggesting that the development of an effective P2Y2 inhibitor would be beneficial for treating HCC.
Acknowledgement
The authors thank JH Park and YJ Kim for providing the pathology results and valuable discussions.
Author contributions
E.T. and G.W.S. designed the study; D.Y.J., J.L., G.C.P. and S.H.K. performed the research; S.H. and S.G.L. contributed new reagents and analytic tools; G.C.P. and S.G.L. analysed the data; and E.T. and G.W.S. wrote the manuscript.
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
Funding
This research was partially supported by funds from the Asan Institute for Life Sciences (Tak EY, 15-662), the National Research Foundation of Korea (NRF-2015K1A4A3046807), Mitsubishi Tanabe Pharma Korea Co., Ltd. (Lee SG, 2015-1390), and Yuhan Corporation (Lee SG, 2015-0908).
References
- 1.Abbracchio MP, Burnstock G, Boeynaems JM, et al. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 2006; 58: 281–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajit D, Woods LT, Camden JM, et al. Loss of P2Y2 nucleotide receptors enhances early pathology in the TgCRND8 mouse model of Alzheimer’s disease. Mol Neurobiol 2014; 49: 1031–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic signalling: pathophysiology and therapeutic potential. Keio J Med 2013; 62: 63–73. [DOI] [PubMed] [Google Scholar]
- 4.Burnstock G. Purinergic signalling: from discovery to current developments. Exp Physiol 2014; 99: 16–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jacobson KA, Paoletta S, Katritch V, et al. Nucleotides acting at P2Y receptors: connecting structure and function. Mol Pharmacol 2015; 88: 220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Virgilio F. Purines, purinergic receptors, and cancer. Cancer Res 2012; 72: 5441–5447. [DOI] [PubMed] [Google Scholar]
- 7.Ayata CK, Ganal SC, Hockenjos B, et al. Purinergic P2Y2 receptors promote neutrophil infiltration and hepatocyte death in mice with acute liver injury. Gastroenterology 2012; 143: 1620–1629. [DOI] [PubMed] [Google Scholar]
- 8.Kumar Y, Sharma P, Bhatt N, et al. Transarterial therapies for hepatocellular carcinoma: a comprehensive review with current updates and future directions. Asian Pac J Cancer Prev 2016; 17: 473–478. [DOI] [PubMed] [Google Scholar]
- 9.Hong YM, Yoon KT, Cho M, et al. Trends and patterns of hepatocellular carcinoma treatment in Korea. J Korean Med Sci 2016; 31: 403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jung DH, Hwang S, Song GW, et al. An interim safety analysis of hepatocellular carcinoma patients administrating oral vitamin K with or without sorafenib. Korean J Hepatobiliary Pancreat Surg 2015; 19: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ha TY, Hwang S, Hong HN, et al. Synergistic effect of sorafenib and vitamin K on suppression of hepatocellular carcinoma cell migration and metastasis. Anticancer Res 2015; 35: 1985–1995. [PubMed] [Google Scholar]
- 12.Ha TY, Hwang S, Moon KM, et al. Sorafenib inhibits migration and invasion of hepatocellular carcinoma cells through suppression of matrix metalloproteinase expression. Anticancer Res 2015; 35: 1967–1976. [PubMed] [Google Scholar]
- 13.Chegai F, Orlacchio A, Merolla S, et al. Intermediate hepatocellular carcinoma: the role of transarterial therapy. Hepat Oncol 2015; 2: 399–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma X, Li RS, Wang J, et al. The therapeutic efficacy and safety of compound kushen injection combined with transarterial chemoembolization in unresectable hepatocellular carcinoma: an update systematic review and meta-analysis. Front Pharmacol 2016; 7: 70–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sangiovanni A, Colombo M. Treatment of hepatocellular carcinoma: beyond international guidelines. Liver Int 2016; 36(Suppl 1): 124–129. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Fan W, Wang Y, et al. Sorafenib with and without transarterial chemoembolization for advanced hepatocellular carcinoma with main portal vein tumor thrombosis: a retrospective analysis. Oncologist 2015; 20: 1417–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gessi S, Merighi S, Sacchetto V, et al. Adenosine receptors and cancer. Biochim Biophys Acta 2011; 1808: 1400–1412. [DOI] [PubMed] [Google Scholar]
- 18.Liu K, Min XL, Peng J, et al. The changes of HIF-1α and VEGF expression after TACE in patients with hepatocellular carcinoma. J Clin Med Res 2016; 8: 297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iezzi R, Pompili M, Posa A, et al. Combined locoregional treatment of patients with hepatocellular carcinoma: state of the art. World J Gastroenterol 2016; 22: 1935–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou Y, Guo CG, Zhang MM. Inhibition of human hepatocellular carcinomatumor angiogenesis by siRNA silencing of VEGF via hepatic artery perfusion. Eur Rev Med Pharmacol Sci 2015; 19: 4751–4761. [PubMed] [Google Scholar]
- 21.Zimmerman MA, Grenz A, Tak E, et al. Signaling through hepatocellular A2B adenosine receptors dampens ischemia and reperfusion injury of the liver. Proc Natl Acad Sci USA 2013; 110: 12012–12017. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Zimmerman MA, Tak E, Ehrentraut SF, et al. Equilibrative nucleoside transporter (ENT)-1-dependent elevation of extracellular adenosine protects the liver during ischemia and reperfusion. Hepatology 2013; 58: 1766–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Liu W, Jia W, et al. Overexpression of Mortalin in hepatocellular carcinoma and its relationship with angiogenesis and epithelial to mesenchymal transition. Int J Oncol 2014; 44: 247–255. [DOI] [PubMed] [Google Scholar]
- 25.Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 2006; 58: 58–86. [DOI] [PubMed] [Google Scholar]
- 26.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev 1998; 50: 413–492. [PubMed] [Google Scholar]
- 27.Xie R, Xu J, Wen G, et al. The P2Y2 nucleotide receptor mediates the proliferation and migration of human hepatocellular carcinoma cells induced by ATP. J Biol Chem 2014; 289: 19137–19149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burnstock G, Di Virgilio F. Purinergic signalling and cancer. Purinergic Signal 2013; 9: 491–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tackett BC, Sun H, Mei Y, et al. P2Y2 purinergic receptor activation is essential for efficient hepatocyte proliferation in response to partial hepatectomy. Am J Physiol Gastrointest Liver Physiol 2014; 307: G1073–G1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fredholm B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ 2007; 14: 1315–1323. [DOI] [PubMed] [Google Scholar]
- 31.Burnstock G. Introduction to purinergic signalling in the brain. Adv Exp Med Biol 2013; 986: 1–12. [DOI] [PubMed] [Google Scholar]
- 32.Burnstock G. Introductory overview of purinergic signalling. Front Biosci (Elite Ed) 2011; 3: 896–900. [DOI] [PubMed] [Google Scholar]
- 33.Burnstock G, Fredholm BB, Verkhratsky A. Adenosine and ATP receptors in the brain. Curr Top Med Chem 2011; 11: 973–1011. [DOI] [PubMed] [Google Scholar]
- 34.Kallio PJ, Pongratz I, Gradin K, et al. Activation of hypoxia-inducible factor 1alpha: posttranscriptional regulation and conformational change by recruitment of the Arnt transcription factor. Proc Natl Acad Sci USA 1997; 94: 5667–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong Z, Venkatachalam MA, Wang J, et al. Up-regulation of apoptosis inhibitory protein IAP-2 by hypoxia. Hif-1-dependent mechanisms. J Biol Chem 2001; 276: 18702–18709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carmeliet P, Dor Y, Herbert JM, et al. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature 1998; 394: 485–490. [DOI] [PubMed] [Google Scholar]
- 37.Hirota K, Semenza GL. Regulation of angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol 2006; 59: 15–26. [DOI] [PubMed] [Google Scholar]
- 38.Gwak GY, Yoon JH, Kim KM, et al. Hypoxia stimulates proliferation of human hepatoma cells through the induction of hexokinase II expression. J Hepatol 2005; 42: 358–364. [DOI] [PubMed] [Google Scholar]
- 39.Tak E, Lee S, Lee J, et al. Human carbonyl reductase 1 upregulated by hypoxia renders resistance to apoptosis in hepatocellular carcinoma cells. J Hepatol 2011; 54: 328–339. [DOI] [PubMed] [Google Scholar]
- 40.Abu-Amara M, Yang SY, Tapuria N, et al. Liver ischemia/reperfusion injury: processes in inflammatory networks – a review. Liver Transpl 2010; 16: 1016–1032. [DOI] [PubMed] [Google Scholar]
- 41.Stagg J, Smyth MJ. Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 2010; 29: 5346–5358. [DOI] [PubMed] [Google Scholar]
- 42.Tamiya S, Okafor MC, Delamere NA. Purinergic agonists stimulate lens Na-K-ATPase-mediated transport via a Src tyrosine kinase-dependent pathway. Am J Physiol Cell Physiol 2007; 293: C790–C796. [DOI] [PubMed] [Google Scholar]
- 43.Whittaker S, Marais R, Zhu AX. The role of signaling pathways in the development and treatment of hepatocellular carcinoma. Oncogene 2010; 29: 4989–5005. [DOI] [PubMed] [Google Scholar]
- 44.Mulcahy MF. Management of hepatocellular cancer. Curr Treat Options Oncol 2005; 6: 423–435. [DOI] [PubMed] [Google Scholar]
- 45.El-Serag HB, Marrero JA, Rudolph L, et al. Diagnosis and treatment of hepatocellular carcinoma. Gastroenterology 2008; 134: 1752–1763. [DOI] [PubMed] [Google Scholar]
- 46.Bruix J, Gores GJ, Mazzaferro V. Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 2014; 63: 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alnouri MW, Jepards S, Casari A, et al. Selectivity is species-dependent: characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal 2015; 11: 389–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thimm D, Schiedel AC, Sherbiny FF, et al. Ligand-specific binding and activation of the human adenosine A(2B) receptor. Biochemistry 2013; 52: 726–740. [DOI] [PubMed] [Google Scholar]
- 49.Schiedel AC, Lacher SK, Linnemann C, et al. Antiproliferative effects of selective adenosine receptor agonists and antagonists on human lymphocytes: evidence for receptor-independent mechanisms. Purinergic Signal 2013; 9: 351–365. [DOI] [PMC free article] [PubMed] [Google Scholar]