Abstract
The pattern of vascular remodelling in relation to recovery after stroke remains largely unclear. We used steady-state contrast-enhanced magnetic resonance imaging to assess the development of cerebral blood volume and microvascular density in perilesional and exofocal areas from (sub)acutely to chronically after transient stroke in rats. Microvascular density was verified histologically after infusion with Evans Blue dye. At day 1, microvascular cerebral blood volume and microvascular density were reduced in and around the ischemic lesion (intralesional borderzone: microvascular cerebral blood volume = 72 ± 8%; microvascular density = 76 ± 8%) (P < 0.05), while total cerebral blood volume remained relatively unchanged. Perilesional microvascular cerebral blood volume and microvascular density subsequently normalized (day 7) and remained relatively stable (day 70). In remote ipsilateral areas in the thalamus and substantia nigra – not part of the ischemic lesion – microvascular density gradually increased between days 1 and 70 (thalamic ventral posterior nucleus: microvascular density = 119 ± 9%; substantia nigra: microvascular density = 122 ± 8% (P < 0.05)), which was confirmed histologically. Our data indicate that initial microvascular collapse, with maintained collateral flow in larger vessels, is followed by dynamic revascularization in perilesional tissue. Furthermore, progressive neovascularization in non-ischemic connected areas may offset secondary neuronal degeneration and/or contribute to non-neuronal tissue remodelling. The complex spatiotemporal pattern of vascular remodelling, involving regions outside the lesion territory, may be a critical endogenous process to promote post-stroke brain reorganization.
Keywords: Angiogenesis, magnetic resonance imaging, stroke recovery, substantia nigra, thalamus
Introduction
Despite considerable advances in understanding of pathophysiological mechanisms after stroke, acute clinical treatment options remain limited, underscoring the need for discovering new effective targets and strategies to improve post-stroke therapy. Beside focal tissue injury in the ischemic lesion, secondary degeneration and remodelling of neuronal and vascular structures may occur in and around the lesion, as well as at remote connected sites.1 Multiple gradual endogenous mechanisms contribute to tissue restoration, particularly in the lesion borderzone and exofocal areas, where intact tissue is functionally compromised but capable of recovering through plasticity of neuronal networks.2 Vascular modifications, such as angiogenesis, may be critical for neuroplasticity by supporting neurogenesis and synaptogenesis.3–5 Angiogenesis and increased microvascular density (MVD) have been identified in perilesional regions of stroke patients.6–8 Multiple studies in rodent stroke models have shown that neovascularization in lesion borderzones contributes to restoration of perfusion and recovery of tissue, which may be therapeutically enhanced.5,9–16
In addition to perilesional alterations, secondary tissue changes, including selective neuronal loss, gliosis and microglial activation, occur in remote non-ischemic areas that are connected to the primary lesion.17–19 For example, magnetic resonance imaging (MRI) studies in patients with a middle cerebral artery (MCA) territory infarct in cortical tissue have demonstrated progressive degeneration of the ipsilateral thalamus, which is located outside the ischemic area.20–23 In animal MCA occlusion (MCAO) models, these changes, presumably caused by retrograde degeneration of thalamocortical fibres, are mostly localized within the ventral posterior nucleus (VPN) of the thalamus.24,25 In patients and animals with ischemic damage in the striatum, delayed tissue injury has also been observed in the substantia nigra, which may be explained by retrograde, anterograde or transsynaptic degeneration through striatonigral and nigrostriatal pathways.26–29
Most studies on post-stroke secondary changes in the thalamus and substantia nigra have described parenchymal pathology. However, signs of tissue plasticity have also been reported. Ling et al.30 found newly formed neuronal cells and blood vessels in the non-ischemic ipsilateral VPN with secondary injury after cortical infarction in rats. Furthermore, Hayward et al.31 identified increased vascular density in the thalamus that was accompanied by significant hyperperfusion chronically after transient MCAO in rats.
Changes in vascularity and haemodynamics after stroke may contribute to pathophysiology as well as plasticity. In vivo elucidation of the spatiotemporal profile of these changes, ideally assessed with imaging techniques,32 may provide important insights into the nature of cerebrovascular remodelling and its possible role in tissue outcome after stroke. Therefore, the goal of this study was to characterize the pattern of vascular reorganization in and around the ischemic lesion and in secondarily affected areas (i.e. the thalamus and substantia nigra) from subacute to chronic time-points after transient focal cerebral ischemia. To this aim, we applied steady-state contrast-enhanced (ssCE-)MRI with a long-circulating blood pool agent to quantify various properties of the vascular network, including total and microvascular cerebral blood volume (CBV) and MVD,33 in relation to tissue damage, from acute to chronic phases after experimental stroke in rats.
Materials and methods
All animal procedures were approved by the Ethical Committee on Animal Experiments of the University Medical Center Utrecht and Utrecht University, and conducted in accordance with the guidelines set by the European Community Council Directives 86/609/EEC. Experiments are reported in compliance with the ARRIVE guidelines (Animal Research: Reporting in Vivo Experiments).
Animal model
Focal cerebral ischemia was induced by 60-min MCAO with an intraluminal filament in rats as described previously.34
Adult male Wistar rats (300–330 g) (Harlan, Horst, The Netherlands) were endotracheally intubated and mechanically ventilated with a mixture of air/oxygen (7/1) and 2% isoflurane. The right common carotid artery was exposed through a cervical incision and carefully separated from the adjacent sympathetic nerves. The right common carotid artery and the internal carotid artery were clamped with microvascular clips. The external carotid artery was ligated distally with a nylon suture and electrocoagulated. A 4–0 surgical nylon suture coated with silicon (Doccol, Redlands, CA, USA) was advanced into the internal carotid artery until resistance was felt. The filament was held in place for 60 min by tightening a suture around the internal carotid artery.
During animal surgery, core temperature was maintained at 37.5℃ by a temperature-controlled heating pad. Preoperatively, animals received a subcutaneous injection of 0.05 mg/kg buprenorphin (Reckitt & Coman, Kingston-Upton-Hill, UK) for pain relief. To compensate for water and mineral loss, 2.5 mL Ringer’s lactate solution (Baxter, Utrecht, The Netherlands) was administered subcutaneously immediately before and after operation, and subsequently 5 mL was given once daily for the first 3–5 days post-surgery. All rats were given ad libitum access to food and water, and were pair-housed in a temperature-controlled environment (20 ± 1℃) with lights on from 07:00 to 19:00 h. Body weight and overall animal well-being were monitored on a daily basis. If weight loss continued for more than 5 days, or if weight decreased below 80% of original body weight, the animal was euthanized to avoid further suffering.
Sixteen rats were included in this study. Rats were scanned at 1 (n = 5), 7 (n = 5), 21 (n = 9) and 70 days (n = 9) after stroke, of which 4 animals were scanned serially at all time-points, while 12 animals were scanned at single time-points.
MRI
MRI measurements were performed using a 4.7 T/40 cm horizontal bore MR system (Agilent Technologies Inc., Palo Alto, CA, USA). A custom-built (2.5-cm diameter) surface coil was used for signal reception, while a Helmholtz volume coil was used for radio-frequency transmission.
Rats were secured in a MR-compatible cradle using ear bars and a bite bar, and anaesthesia was maintained in the same manner as described for the MCAO procedure. Blood oxygenation, expired CO2 and heart rate were continuously measured throughout imaging, and kept within physiological range. Core temperature was maintained at 37.5℃.
For ssCE-MRI, we used a multi-slice multi-spin-echo (SE) (repetition time (TR)/first echo time (TE1)/echo time interval (ΔTE) = 3500/13/13 ms; 12 echoes), and a multi-slice multi-gradient echo (GE) (TR/TE1/ΔTE = 1800/4/4 ms; 25 echoes) sequence with 19 slices of 1-mm slice thickness, field-of-view (FOV) of 30 × 30 mm2, data matrix size of 256 × 128 (frequency encoding × phase encoding) and two averages. SE and GE images were acquired before and after intravenous bolus injection of long circulating ultrasmall particles of iron oxide (USPIO), with a hydrodynamic diameter of 25–30 nm (P904, Guerbet Research, Aulnay-sous-Bois, France). USPIO was introduced via a catheter in the tail vein to achieve a total dose of 300 µmol Fe/kg (∼16.8 mg Fe/kg). Post-contrast SE and GE acquisition was started 5 min after contrast agent injection to reach a steady state. Total scan duration was approximately 135 min.
Image processing and analysis
All parametric maps were obtained on a voxel-by-voxel level, using MATLAB (MathWorks, Natick, MA, USA). Transverse relaxation rate changes ΔR2 and ΔR2*, were calculated as ΔR2 = 1/T2 post − 1/T2 pre and ΔR2* = 1/T2* post − 1/T2* pre, where T2 pre, T2 post and T2* pre, T2* post are the pre- and post-contrast T2 and T2* values obtained from the SE and GE acquisitions, respectively. ΔR2 is a measure of primarily microvascular blood volume (e.g. in capillaries and venules), whereas ΔR2* reflects total blood volume, including large vessels.35,36 The parameter Q, an estimate of MVD, was calculated as ΔR2/(ΔR2*)2/3.37,38 Voxels with negative ΔR2 and ΔR2* values were assigned a Q value equal to zero to eliminate non-biological values.
T2 maps, calculated from the pre-contrast SE acquisition, were spatially aligned to a reference image template created from six control rat brains, using non-rigid coregistration.39 Brain parenchyma was automatically segmented from surrounding tissue using a brain extraction tool.40 T2 maps from all animals were averaged, and the mean T2 value and its standard deviation (SD) in normal grey matter were calculated. For each individual animal, voxels with T2 values above the mean plus 4.5 SD were identified as cerebrospinal fluid and excluded from further analyses.
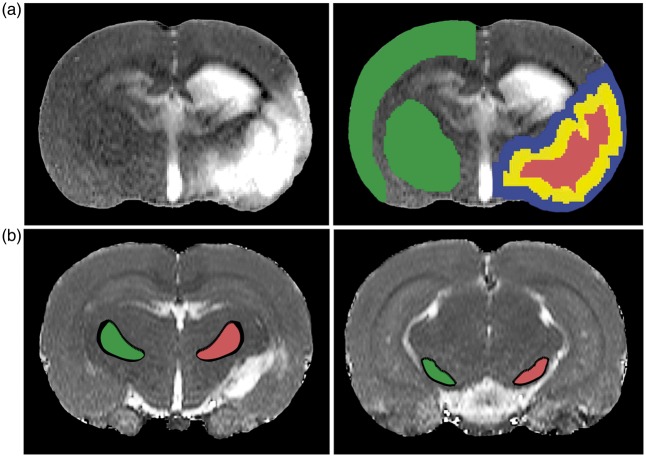
A contralateral region-of-interest (ROI) encompassing cortical and subcortical grey matter tissue in the unaffected hemisphere was defined based on the coregistered image template guided by an anatomical atlas41 (Figure 1(a)). The lesion volume was automatically determined on pre-contrast T2 maps, defined as tissue with T2 values above the mean plus 2SD of contralateral tissue values. The resulting volume was median-filtered, binarized and eroded radially by 9 voxels using a 2D kernel, which provided a lesion core ROI (Figure 1(a)). An intralesional borderzone ROI was created by subtracting the lesion core volume from the total lesion volume (Figure 1(a)). Dilating the total lesion volume by 9 voxels (2D kernel), and subtracting the resultant volume with the total lesion volume provided an extralesional borderzone ROI (Figure 1(a)). In addition, the ipsilateral thalamic VPN and the substantia nigra were manually delineated on pre-contrast T2 maps (Figure 1(b)), guided by a superimposed anatomical atlas41 using FMRIB Software Library (FSL, http://www.fmrib.ox.ac.uk/fsl). Homologous ROIs in the contralateral VPN and substantia nigra were automatically generated, with corrections for midline shift and tissue shrinkage. The processing algorithm was compiled in MATLAB (MathWorks, Natick, MA, USA). In each ipsi- and contralateral ROI we calculated the mean value of T2 pre, T2* pre, ΔR2, ΔR2* and Q.
Figure 1.
T2 maps of coronal brain slices depicting the regions-of-interest (ROIs). (a) Lesioned tissue was indentified by elevated T2 values (left image). Total lesion volume (red and yellow ROIs combined) was calculated from ipsilateral tissue voxels with T2 values 2SD above the mean of values in contralateral grey matter (superimposed on normalized T2 maps) (right image). Subsequent automated image processing (i.e. erosion and dilation) generated three ROIs: lesion core (red), intralesional borderzone (yellow) and extralesional borderzone (blue). A contralateral ROI (green) encompassing cortical and subcortical tissue was manually selected. (b) Ipsi- (red) and contralateral ROIs (green) of the VPN (left image) and SN (right image) were manually outlined on posterior slices.
Evans Blue perfusion of vessels
After the final MRI scan at day 70, in 8 rats vascular density was assessed as described by Gertz et al. in detail previously.16,42 Briefly, anaesthesia was maintained as during MRI. Endovascular dye Evans Blue (Sigma-Aldrich; 1 mL of 2% solution in saline) was infused through the same tail vain catheter as used for contrast agent injection. 5 min after infusion, animals were decapitated and brains were snap-frozen. Brain tissue was cut in 10 µm coronal cryosections. Digitized fluorescence microscopy images were acquired with a cooled-CCD camera (CoolSNAP EZ, Photometrics, Tucson, AZ, USA), attached to a fluorescence microscope. Microscopic resolution images of whole brain sections were acquired by joining together single images using tiled-field mapping software (MCID 7.0 Elite; Interfocus Imaging, Linton, UK). The technique of density slicing was used for ROI specification including the setting of target acceptance criteria.16,42,43 Microvessel counts were performed by G.K. who was blinded for the MRI findings.
Statistical analysis
An ANOVA was performed across time points, and differences between corresponding ROIs in the ipsi- and contralateral hemispheres were evaluated with a paired t-test. Values are shown as mean ± SD. P < 0.05 was considered significant.
Results
Transient unilateral MCAO resulted in an ipsilateral lesion, characterized by tissue with clearly elevated T2 values, which included the striatum and cortex, without involvement of the thalamus and substantia nigra (Figure 1).
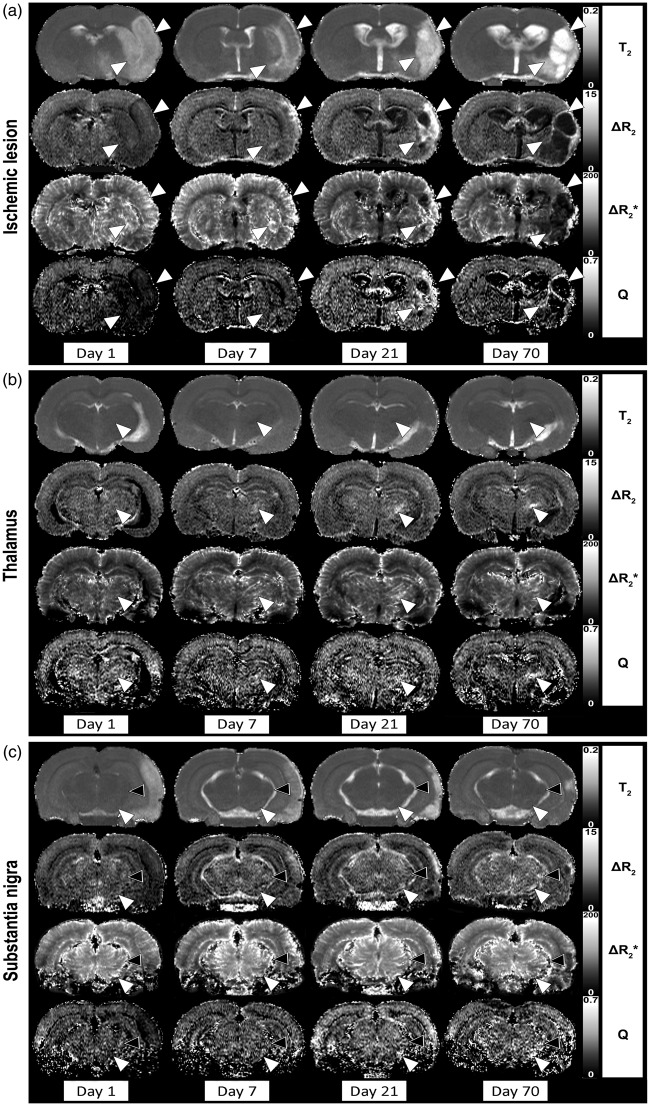
Figure 2 shows representative maps of the T2 (tissue integrity), ΔR2 (microvascular CBV), ΔR2* (total CBV) and Q (MVD) in brain slices encompassing the primary lesion (Figure 2(a)), the thalamus (Figure 2(b)) and the substantia nigra (Figure 2(c)) of a single rat at 1, 7, 21 and 70 days after MCAO. ΔR2* and ΔR2 maps revealed substantial temporal alterations in- and outside the lesion territory, as well as in remote ipsilateral subcortical areas, i.e. the thalamus and substantia nigra. No significant temporal changes in T2, T2*, ΔR2*, ΔR2 or Q were observed in the contralateral ROIs (see also Figure 3).
Figure 2.
Quantitative maps of pre-contrast T2, ΔR2 (microvascular CBV), ΔR2* (total CBV) and Q (MVD) in rat brain at different time-points after 60-min unilateral MCAO. Maps of coronal rat brain slices are shown at the level of the primary lesion site (cortical and striatal borderzones depicted by white arrowheads) (a), thalamus (VPN depicted by white arrowheads) (b) and substantia nigra (white arrowheads) and medial geniculate nucleus (black arrowheads) (c).
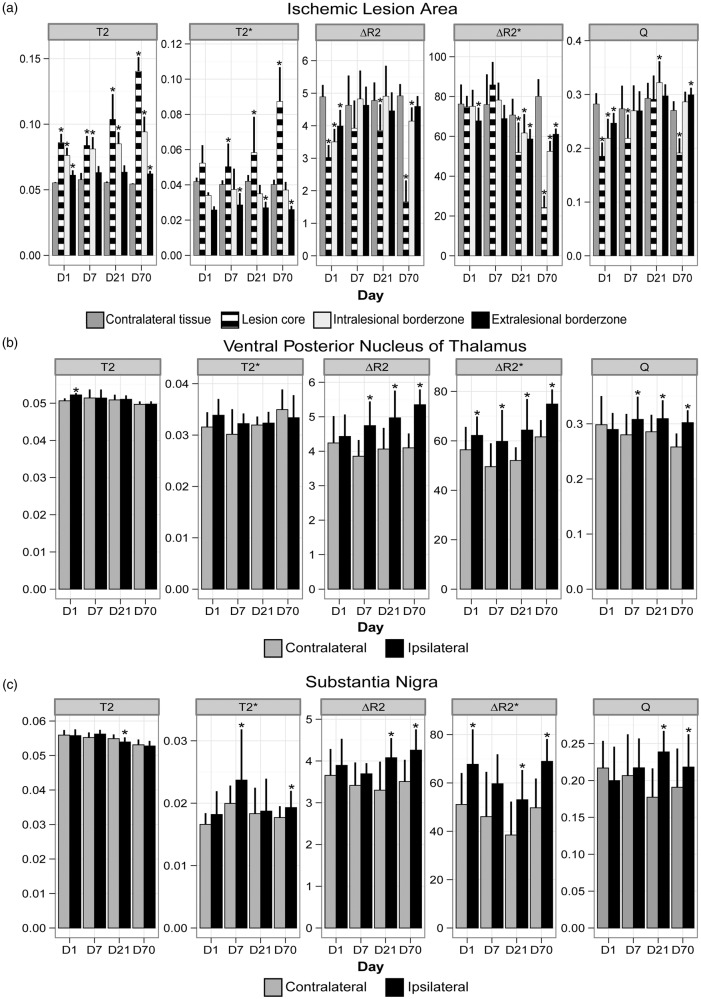
Figure 3.
Pre-contrast T2 (s), pre-contrast T2* (s), ΔR2 (s−1), ΔR2* (s−1) and Q (s−1/3) (mean ± SD) in lesional ROIs (a), VPN (b) and substantia nigra (c) at different post-stroke time-points. *P < 0.05 versus contralateral ROI.
Spatiotemporal alterations in the primary lesion area
During the course of 70 days after stroke, T2 changes in the primary lesion reflected oedema formation (day 1), pseudo-normalization in grey matter accompanied by white matter oedema (day 7) and ultimately liquefaction and tissue shrinkage (days 21 and 70) (Figure 2(a)). Lesion volumes were 165 ± 71 mm3 at day 1, 164 ± 100 mm3 at day 7, 115 ± 96 mm3 at day 21 and 146 ± 84 mm3 at day 70. Figure 3(a), which displays the quantitative results from ROI analysis, shows that T2 was significantly prolonged at day 1 in all lesional ROIs, particularly in the lesion core and intralesional borderzone, where values continued to further increase until day 70. T2* was also prolonged in the lesion core, which progressed over time, whereas T2* was shortened in the extralesional borderzone at post-stroke days 7 to 70.
At day 1 after stroke, ΔR2 (microvascular CBV) and Q (MVD) were significantly decreased in the entire lesion area. However, ΔR2* (total CBV) was relatively unchanged compared with corresponding areas in the contralateral hemisphere, except for the extralesional borderzone. At day 7, ΔR2 was largely restored in intra- and extralesional areas. Normalization of Q was also apparent around the lesion territory, except for in the core where it remained low. ΔR2* was relatively unaffected at this time point. After 21 days, increased ΔR2 and Q were observed in perilesional regions. ΔR2* values in perilesional tissue were more variable and included elevated as well as lowered values. On average, ΔR2* was reduced in all lesional ROIs. At day 70, ΔR2*, ΔR2 and Q were significantly reduced in the lesion core. ΔR2*, ΔR2 and Q in lesion borderzones remained relatively higher, although to a lesser extent as compared with day 21 (e.g. intralesional ΔR2 was significantly lower at this stage).
To confirm the observed temporal patterns, we separately analysed the four rats that were scanned serially at all time points. Results are shown in Supplementary Fig. 1, which clearly demonstrate the same trends for all parameters.
Spatiotemporal alterations in remote regions: Thalamus and substantia nigra
Beside the clear alterations in and around the primary lesion site, mild to moderate temporal changes on the parameteric MRI maps were detected in remote subcortical regions, including the thalamus (Figure 2(b)) and substantia nigra (Figure 2(c)). These changes were most evident in the VPN. Occasionally, similar effects were observed in the ipsilateral medial geniculate nucleus (Figure 2(c), black arrowheads), particularly in animals with substantial damage in the auditory cortex. At post-stroke day 1, a minor but significant prolongation of T2 was detected in the ipsilateral VPN, which slowly declined thereafter (Figure 3(b)). To lesser extent, this was also observed in the ipsilateral substantia nigra (Figure 3(c)), where we also measured significant T2* prolongation at days 7 and 70.
Vascular parameters revealed substantial changes in the ipsilateral VPN and substantia nigra over time (Figure 3(b) and (c)). ΔR2* was significantly increased in the VPN and substantia nigra from day 1 to 70, except for day 7 in the substantia nigra. Significant elevations of ΔR2 and Q were evident from day 7 onwards in the VPN, and from day 21 onwards in the substantia nigra.
Separate analysis of the serially measured cohort corroborated the observed temporal trends (Supplementary Fig. 1).
Evaluation of vascularity in the VPN and substantia nigra
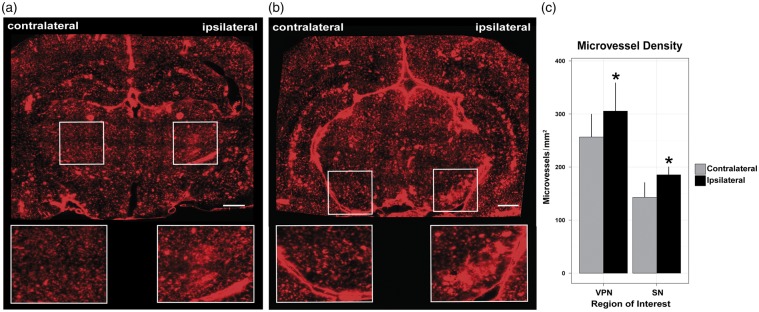
Fluorescence microscopy images of brain sections from Evans Blue-perfused animals after the last MRI time-point, revealed increased vascularity in the ipsilateral VPN (Figure 4(a)) and substantia nigra (Figure 4(b)). Results from vessel counting showed that the density of microvessels was significantly increased in the ipsilateral VPN (116 ± 5%) and substantia nigra (119 ± 10%), as compared with the contralateral homologous area (P < 0.05) (Figure 4(c)).
Figure 4.
Respective micrographs of coronal sections showing Evans Blue-perfused microvessels at the level of the thalamic VPN (a), and substantia nigra (b) of a rat at 70 days after 60-min unilateral MCAO. ROIs selected for vessel counting are depicted by the boxes. Scale bar: (a,b) = 1000 µm. (c) Density of Evans Blue-filled microvessels (n/mm2) in ipsi- and contralateral ROIs. *P < 0.05 versus contralateral.
Discussion
We employed ssCE-MRI to quantitatively assess the effects of transient focal cerebral ischemia on the perfused vasculature during long-term recovery from stroke. Using a 60-min MCAO model in rats, we identified temporally characteristic changes in total CBV (ΔR2*), microvascular CBV and MVD (Q) in different ipsilateral areas. These vascular alterations were not restricted to the lesion site and its borderzones, however, but also included remote regions, i.e. the ipsilateral thalamus and the substantia nigra. Our data demonstrate a gradual increase in (total and microvascular) CBV and MVD, which persisted for at least 3 months after stroke and was confirmed histologically.
Vascular remodelling in and around the ischemic lesion
One day after transient ischemia, the lesion area (characterized by prolonged tissue T2 due to vasogenic oedema44) displayed decreased microvascular CBV and MVD, while total CBV was largely unchanged. Increased pressure due to oedema may cause microvessels to collapse, leading to the observed reduction in microvascular CBV and MVD. Higher total CBV could occur due to early vasodilation or arteriogenesis of collateral vessels,9 which compensate for the lower cerebral blood flow (CBF) in the ischemic territory. Correspondingly, improved collateral flow by an increase in total CBV, as opposed to unchanged or lowered microvascular CBV, has been observed in the rat cortex early after ischemia–reperfusion in an earlier ssCE-MRI study.45
Extensive initial oedema in grey matter, as depicted on T2 maps, largely subsided by post-stroke day 7, consistent with other reports on transient T2 normalization in lesioned tissue after stroke.46–48 This was accompanied by normalization of microvascular CBV and MVD in the lesion border areas. Levels of these parameters remained significantly lowered in the lesion core, except for a transient elevation of the MVD in the core region at day 21, which may be (partly) explained by methodological issues, as described below. Earlier serial ssCE-MRI studies in rats reported a gradual increase of microvascular CBV and/or MVD, following initial reductions in the ischemic lesion territory.40,45,49,50 Still, the pattern of vascular changes strongly depends on the extent to which tissue is damaged after stroke. While the ischemic core is predominantly necrotic, particularly at chronic stages, the surrounding area is a mixture of necrotic, apoptotic, rearranging and normal parenchyma.48,51
Precise lesion segmentation on MR images, however, is difficult as there are no uniform signal differences between the lesion core, the lesion borderzones and unaffected tissue. Therefore, we applied an automated region growing and shrinking approach to differentiate between the lesion core, and extra- and intralesional borderzones, respectively. In the intralesional borderzone, we observed a dynamic temporal development of post-ischemic CBV and MVD changes accompanied by continuously elevated tissue T2. This reflects an active and heterogeneous tissue experiencing the formation and subsequent regression of new undeveloped (leaky) vessels, in line with findings in another study.47 The extralesional borderzone, on the other hand, displayed a more gradual and steady increase in microvascular CBV and MVD, which suggests angiogenesis leading to a stable vascular network. Moreover, this area exhibited a significantly lower T2*, which has been associated with newly developed mature vasculature.52 However, other factors, such as enhanced macrophage activity, may also explain T2* shortening in perilesional tissue.53
Neovascularization in post-stroke brain may indicate a higher demand for vascular support in areas undergoing active neural tissue reorganization. Correspondingly, earlier studies have shown a close association of the dynamics of angiogenesis with neurogenesis (evidenced from specific migration of neuroblasts through areas that exhibited vascular remodelling and increased vessel density3,4), as well as dendritic plasticity (evidenced from paralleled orientation of remodelling dendritic and vascular segments54) in perilesional areas. Furthermore, therapeutic interventions, e.g. cell-based therapies, that concurrently enhance vascular and neuronal remodelling, have shown to be effective in improving functional recovery in preclinical stroke models.5 On the other hand, neovascularization has also been shown to occur in concomitance with astrogliosis,55 which suggests that post-stroke vascular remodelling may also contribute to formation of non-neuronal scar tissue. Future studies should provide further insights in the mechanisms and role of the formation of new vasculature after stroke.
Stroke-induced vascular remodelling in remote areas
Beside the intra- and perilesional vascular alterations, we detected significant changes in remote brain regions that were not part of the primary ischemic stroke lesion. Particularly, the ipsilateral VPN of the thalamus and the substantia nigra had chronically elevated CBV and MVD levels. Microscopic examination of histological brain sections revealed a greater number of microvessels in these regions.
Secondary exofocal neuronal damage in regions remote from a primary ischemic lesion, due to retrograde, anterograde or transsynaptic degeneration of connected tissue, is a well-known phenomenon.18 Histopathological studies in rodent stroke models have shown neuronal loss, axonal injury and glial activation in the non-ischemic thalamus and substantia nigra following cortical or striatal infarction, respectively.19,24,25,34,56–61 The transient T2 and T2* changes that we detected in the VPN and substantia nigra could reflect ongoing neurodegeneration and neuroinflammation, in line with previous observations from MRI in stroke patients23,28 and animal models.34,56–58 Yet, the occurrence of vascular remodelling in remote regions anatomically connected to the primary lesion site is a relatively unknown phenomenon.
Ling et al.30 found that post-stroke secondary degeneration in the thalamus may be accompanied by angiogenesis and neurogenesis. In addition to neuronal loss and microglial activation in the ipsilateral VPN at days 7 and 14 after cortical infarction in rats, immunohistochemical analysis revealed proliferating neural and endothelial cells as well as increased vessel density in the VPN. In a perfusion MRI study by Hayward et al.,31 it was shown that CBF progressively increases in the ipsilateral thalamus, while cortical CBF remained lowered, between 2 days and 3 months after transient MCAO in rats. Furthermore, these authors showed that the number of branching vessel points after 3 months was significantly higher in the ipsilateral thalamus as compared with contralateral. Our present study provides further evidence of progressive microvascularization in the thalamus, specifically in the VPN, after ischemic damage to the sensorimotor cortex and striatum. The rise in total CBV may suggest that recruitment or reinforcement of larger vessels (arteriogenesis) may also be part of the vascular remodelling. Importantly, we detected similar changes in the ipsilateral substantia nigra (and in the medial geniculate nucleus in animals with ischemic injury to the auditory cortex), which indicates that post-stroke angiogenesis and/or arteriogenesis may be a common process in remote connected brain regions with secondary tissue degeneration.
Advantages and limitations of ssCE-MRI for vascular imaging
Using MRI to assess cerebrovascular remodelling offers the advantage of in vivo, serial and whole-brain monitoring with simultaneous measurement of brain tissue injury. Because ssCE-MRI is based on signal from a circulating intravascular contrast agent, only perfused (functional) vessels are detected. This is in contrast to histological assessment by post mortem staining of vascular structures, which may include non-functional vessels. Furthermore, different traits of the cerebrovasculature, such as microvascular and total CBV, as well as MVD, can be characterized. However, there are also limitations to the ssCE-MRI-based approach. In the current study, we used the parameter Q as a measure of MVD,37,38 but more accurate quantification of MVD values may be feasible with additional input of the local tissue diffusion coefficient.33 Since diffusion depends on tissue microstructure, values can vary considerably in different tissue types and under pathological conditions. In a recent article by Boehm-Sturm et al.,49 it was shown that MVD measures may be overestimated when diffusion is high, such as in a cystic lesion chronically after stroke. This could explain the high ΔR2 and Q values that we observed in some regions inside the lesion core at chronic stages. Furthermore, Boehm-Sturm et al. reported that ΔR2 calculations may vary between single- and multi-echo MRI protocols. Future ssCE-MRI studies should further evaluate how different MRI acquisition schemes affect the quantification of different vascular measures to determine the most optimal imaging protocol for assessing vascularity in tissues.
To verify the reliability of our ssCE-MRI data, we directly compared MRI-based MVD findings with vessel counts. Intravenous injections of Evans Blue dye after the final MRI session on post-stroke day 70, allowed the specific detection of perfused vessels similar to ssCE-MRI. We limited vessel quantification to the thalamus and substantia nigra, which were structurally intact after tissue processing. The greater number of microvessels in the ipsilateral thalamus and substantia nigra is in agreement with our ssCE-MRI-based MVD measurements, corroborating Q as a measure of the density of perfused microvasculature.
Conclusions
While early recovery from acute stroke is associated with oedema resolution and re-establishment of circulation in perilesional tissue, ongoing structural and functional reorganization in extended ipsilateral and contralateral neuronal networks is believed to contribute to long-term restoration of functions.62–66 Our data indicate that large-scale vascular remodelling plays a dynamic, long-term role in the reorganizational processes in the brain after stroke. Vascular reorganization follows a complex spatiotemporal pattern in and around the lesion, as well as remote associated areas, namely the substantia nigra and thalamus. At early time points, vasodilation and arteriogenesis of larger vessels may temporarily compensate for microvascular compression in the ischemic territory. The subsequent transient increase in microvascular perfusion in perilesional areas may reflect the formation of new vessels, which could locally contribute to neuronal survival, clearance of debris67 and scar formation. Furthermore, a gradual increase in microvessel densities in the extralesional borderzone and remote areas with secondary tissue degeneration suggests that angiogenesis could support reorganization and restoration of neural networks. Since vascular remodelling after stroke may play a critical role in endogenous neuroprotection and neurorepair, it should be considered as a factor in advancing therapeutic strategies to improve neurological outcome in stroke patients, which may be effectively monitored with ssCE-MRI.
Supplementary Material
Acknowledgements
The authors thank Dr. Ann Stowe for the valuable contributions regarding the improvement of quality and coherence of the manuscript.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The European Union’s Seventh Framework Programme (FP7/2007-2013) under grant agreements no. 201024 and no. 202213 (European Stroke Network to R.M.D. and M.E.), the Netherlands Organization for Scientific Research (NWO) (VIDI 917.76.347; VICI 016.130.662 to R.M.D.), the Deutsche Forschungsgemeinschaft (SFB TRR43 and Exc257 to M.E.; KR 2956/4-1 to G.K.; GE 2576/2-1 to K.G.), the Bundesministerium für Bildung und Forschung (CSB to M.E. K.G. and G.K.), the German Center for Neurodegenerative Diseases (DZNE to M.E.), and the German Center for Cardiovascular Research (DZHK to M.E.); Transatlantic Networks of Excellence Program from the Foundation Leducq (to M.E.).
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Authors' contributions
PY and RMD designed the studies, interpreted data and drafted the manuscript; PY, RMD, PRS, USR, KG, GK, ME and GAT executed experiments and/or analysed data; USR, MJB and AT provided analytical software support. All authors revised and approved the final submission.
Supplementary material
Supplementary material for this paper can be found at http://jcbfm.sagepub.com/content/by/supplemental-data
References
- 1.Stowe AM, Plautz EJ, Eisner-Janowicz I, et al. VEGF protein associates to neurons in remote regions following cortical infarct. J Cereb Blood Flow Metab 2007; 27: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dijkhuizen RM. Imaging neuronal loss and recovery in compromised but viable brain tissue. Brain 2013; 136: 1689–1691. [DOI] [PubMed] [Google Scholar]
- 3.Ohab JJ, Fleming S, Blesch A, et al. A neurovascular niche for neurogenesis after stroke. J Neurosci 2006; 26: 13007–13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thored P, Wood J, Arvidsson A, et al. Long-term neuroblast migration along blood vessels in an area with transient angiogenesis and increased vascularization after stroke. Stroke 2007; 38: 3032–3039. [DOI] [PubMed] [Google Scholar]
- 5.Xiong Y, Mahmood A, Chopp M. Angiogenesis, neurogenesis and brain recovery of function following injury. Curr Opin Investig Drugs 2010; 11: 298–308. [PMC free article] [PubMed] [Google Scholar]
- 6.Krupinski J, Kaluza J, Kumar P, et al. Role of angiogenesis in patients with cerebral ischemic stroke. Stroke 1994; 25: 1794–1798. [DOI] [PubMed] [Google Scholar]
- 7.Slevin M, Krupinski J, Slowik A, et al. Serial measurement of vascular endothelial growth factor and transforming growth factor-beta1 in serum of patients with acute ischemic stroke. Stroke 2000; 31: 1863–1870. [DOI] [PubMed] [Google Scholar]
- 8.Szpak GM, Lechowicz W, Lewandowska E, et al. Border zone neovascularization in cerebral ischemic infarct. Folia Neuropathol 1999; 37: 264–268. [PubMed] [Google Scholar]
- 9.Liu J, Wang Y, Akamatsu Y, et al. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog Neurobiol 2014; 115: 138–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayashi T, Deguchi K, Nagotani S, et al. Cerebral ischemia and angiogenesis. Curr Neurovasc Res 2006; 3: 119–129. [DOI] [PubMed] [Google Scholar]
- 11.Slevin M, Kumar P, Gaffney J, et al. Can angiogenesis be exploited to improve stroke outcome? Mechanisms and therapeutic potential. Clin Sci 2006; 111: 171–183. [DOI] [PubMed] [Google Scholar]
- 12.Arai K, Jin G, Navaratna D, et al. Brain angiogenesis in developmental and pathological processes: neurovascular injury and angiogenic recovery after stroke. FEBS J 2009; 276: 4644–4652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beck H, Plate KH. Angiogenesis after cerebral ischemia. Acta Neuropathol 2009; 117: 481–496. [DOI] [PubMed] [Google Scholar]
- 14.Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke 2012; 43: 2270–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liman TG, Endres M. New vessels after stroke: postischemic neovascularization and regeneration. Cerebrovasc Dis 2012; 33: 492–499. [DOI] [PubMed] [Google Scholar]
- 16.Gertz K, Priller J, Kronenberg G, et al. Physical activity improves long-term stroke outcome via endothelial nitric oxide synthase-dependent augmentation of neovascularization and cerebral blood flow. Circ Res 2006; 99: 1132–1140. [DOI] [PubMed] [Google Scholar]
- 17.Baron JC, Yamauchi H, Fujioka M, et al. Selective neuronal loss in ischemic stroke and cerebrovascular disease. J Cereb Blood Flow Metab 2014; 34: 2–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Zhang Y, Xing S, et al. Secondary neurodegeneration in remote regions after focal cerebral infarction: a new target for stroke management? Stroke 2012; 43: 1700–1705. [DOI] [PubMed] [Google Scholar]
- 19.Kronenberg G, Balkaya M, Prinz V, et al. Exofocal dopaminergic degeneration as antidepressant target in mouse model of poststroke depression. Biol Psychiatry 2012; 72: 273–281. [DOI] [PubMed] [Google Scholar]
- 20.Tamura A, Tahira Y, Nagashima H, et al. Thalamic atrophy following cerebral infarction in the territory of the middle cerebral artery. Stroke 1991; 22: 615–618. [DOI] [PubMed] [Google Scholar]
- 21.De Reuck J, Decoo D, Lemahieu I, et al. Ipsilateral thalamic diaschisis after middle cerebral artery infarction. J Neurol Sci 1995; 134: 130–135. [DOI] [PubMed] [Google Scholar]
- 22.Nakane M, Tamura A, Sasaki Y, et al. MRI of secondary changes in the thalamus following a cerebral infarct. Neuroradiology 2002; 44: 915–920. [DOI] [PubMed] [Google Scholar]
- 23.Ogawa T, Yoshida Y, Okudera T, et al. Secondary thalamic degeneration after cerebral infarction in the middle cerebral artery distribution: evaluation with MR imaging. Radiology 1997; 204: 255–262. [DOI] [PubMed] [Google Scholar]
- 24.Iizuka H, Sakatani K, Young W. Neural damage in the rat thalamus after cortical infarcts. Stroke 1990; 21: 790–794. [DOI] [PubMed] [Google Scholar]
- 25.Dihne M, Grommes C, Lutzenburg M, et al. Different mechanisms of secondary neuronal damage in thalamic nuclei after focal cerebral ischemia in rats. Stroke 2002; 33: 3006–3011. [DOI] [PubMed] [Google Scholar]
- 26.Forno LS. Reaction of the substantia nigra to massive basal ganglia infarction. Acta Neuropathol 1983; 62: 96–102. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa T, Okudera T, Inugami A, et al. Degeneration of the ipsilateral substantia nigra after striatal infarction: evaluation with MR imaging. Radiology 1997; 204: 847–851. [DOI] [PubMed] [Google Scholar]
- 28.Nakane M, Teraoka A, Asato R, et al. Degeneration of the ipsilateral substantia nigra following cerebral infarction in the striatum. Stroke 1992; 23: 328–332. [DOI] [PubMed] [Google Scholar]
- 29.Winter B, Brunecker P, Fiebach JB, et al. Striatal infarction elicits secondary extrafocal MRI changes in ipsilateral substantia nigra. PLoS One 2015; 10: e0136483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ling L, Zeng J, Pei Z, et al. Neurogenesis and angiogenesis within the ipsilateral thalamus with secondary damage after focal cortical infarction in hypertensive rats. J Cereb Blood Flow Metab 2009; 29: 1538–1546. [DOI] [PubMed] [Google Scholar]
- 31.Hayward NM, Yanev P, Haapasalo A, et al. Chronic hyperperfusion and angiogenesis follow subacute hypoperfusion in the thalamus of rats with focal cerebral ischemia. J Cereb Blood Flow Metab 2011; 31: 1119–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanev P, Dijkhuizen RM. In vivo imaging of neurovascular remodeling after stroke. Stroke 2012; 43: 3436–3441. [DOI] [PubMed] [Google Scholar]
- 33.Seevinck PR, Deddens LH, Dijkhuizen RM. Magnetic resonance imaging of brain angiogenesis after stroke. Angiogenesis 2010; 13: 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Longa EZ, Weinstein PR, Carlson S, et al. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 1989; 20: 84–91. [DOI] [PubMed] [Google Scholar]
- 35.Boxerman JL, Hamberg LM, Rosen BR, et al. MR contrast due to intravascular magnetic susceptibility perturbations. Magn Reson Med 1995; 34: 555–566. [DOI] [PubMed] [Google Scholar]
- 36.Kiselev VG, Strecker R, Ziyeh S, et al. Vessel size imaging in humans. Magn Reson Med 2005; 53: 553–563. [DOI] [PubMed] [Google Scholar]
- 37.Jensen JH, Chandra R. MR imaging of microvasculature. Magn Reson Med 2000; 44: 224–230. [DOI] [PubMed] [Google Scholar]
- 38.Wu EX, Tang HY, Jensen JH. High-resolution MR imaging of mouse brain microvasculature using the relaxation rate shift index Q. NMR Biomed 2004; 17: 507–512. [DOI] [PubMed] [Google Scholar]
- 39.Klein S, Staring M, Murphy K, et al. elastix: a toolbox for intensity-based medical image registration. IEEE Trans Med Imaging 2010; 29: 196–205. [DOI] [PubMed] [Google Scholar]
- 40.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp 2002; 17: 143–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, 5th ed San Diego: Elsevier Academic Press, 2005. [Google Scholar]
- 42.Gertz K, Kronenberg G, Kalin RE, et al. Essential role of interleukin-6 in post-stroke angiogenesis. Brain 2012; 135: 1964–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gobel U, Theilen H, Kuschinsky W. Congruence of total and perfused capillary network in rat brains. Circ Res 1990; 66: 271–281. [DOI] [PubMed] [Google Scholar]
- 44.Dijkhuizen RM, Nicolay K. Magnetic resonance imaging in experimental models of brain disorders. J Cereb Blood Flow Metab 2003; 23: 1383–1402. [DOI] [PubMed] [Google Scholar]
- 45.Lin CY, Chang C, Cheung WM, et al. Dynamic changes in vascular permeability, cerebral blood volume, vascular density, and size after transient focal cerebral ischemia in rats: evaluation with contrast-enhanced magnetic resonance imaging. J Cereb Blood Flow Metab 2008; 28: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 46.Lin SP, Schmidt RE, McKinstry RC, et al. Investigation of mechanisms underlying transient T2 normalization in longitudinal studies of ischemic stroke. J Magn Reson Imaging 2002; 15: 130–136. [DOI] [PubMed] [Google Scholar]
- 47.Wagner DC, Deten A, Hartig W, et al. Changes in T2 relaxation time after stroke reflect clearing processes. Neuroimage 2012; 61: 780–785. [DOI] [PubMed] [Google Scholar]
- 48.Wegener S, Weber R, Ramos-Cabrer P, et al. Temporal profile of T2-weighted MRI distinguishes between pannecrosis and selective neuronal death after transient focal cerebral ischemia in the rat. J Cereb Blood Flow Metab 2006; 26: 38–47. [DOI] [PubMed] [Google Scholar]
- 49.Boehm-Sturm P, Farr TD, Adamczak J, et al. Vascular changes after stroke in the rat: a longitudinal study using optimized magnetic resonance imaging. Contrast Media Mol Imaging 2013; 8: 383–392. [DOI] [PubMed] [Google Scholar]
- 50.Moisan A, Favre IM, Rome C, et al. Microvascular plasticity after experimental stroke: a molecular and MRI study. Cerebrovasc Dis 2014; 38: 344–353. [DOI] [PubMed] [Google Scholar]
- 51.Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med 2008; 14: 497–500. [DOI] [PubMed] [Google Scholar]
- 52.Ding G, Jiang Q, Li L, et al. Angiogenesis detected after embolic stroke in rat brain using magnetic resonance T2*WI. Stroke 2008; 39: 1563–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weber R, Wegener S, Ramos-Cabrer P, et al. MRI detection of macrophage activity after experimental stroke in rats: new indicators for late appearance of vascular degradation? Magn Reson Med 2005; 54: 59–66. [DOI] [PubMed] [Google Scholar]
- 54.Brown CE, Li P, Boyd JD, et al. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci 2007; 27: 4101–4109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014; 81: 229–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakane M, Tamura A, Nagaoka T, et al. MR detection of secondary changes remote from ischemia: preliminary observations after occlusion of the middle cerebral artery in rats. AJNR Am J Neuroradiol 1997; 18: 945–950. [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao F, Kuroiwa T, Miyasaka N, et al. Ultrastructural and MRI study of the substantia nigra evolving exofocal post-ischemic neuronal death in the rat. Neuropathology 2002; 22: 91–105. [DOI] [PubMed] [Google Scholar]
- 58.Justicia C, Ramos-Cabrer P, Hoehn M. MRI detection of secondary damage after stroke: chronic iron accumulation in the thalamus of the rat brain. Stroke 2008; 39: 1541–1547. [DOI] [PubMed] [Google Scholar]
- 59.Kataoka K, Hayakawa T, Yamada K, et al. Neuronal network disturbance after focal ischemia in rats. Stroke 1989; 20: 1226–1235. [DOI] [PubMed] [Google Scholar]
- 60.Nagasawa H, Kogure K. Exo-focal postischemic neuronal death in the rat brain. Brain Res 1990; 524: 196–202. [DOI] [PubMed] [Google Scholar]
- 61.Prinz V, Hetzer AM, Muller S, et al. MRI heralds secondary nigral lesion after brain ischemia in mice: a secondary time window for neuroprotection. J Cereb Blood Flow Metab 2015; 35: 1903–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol 1999; 9: 740–747. [DOI] [PubMed] [Google Scholar]
- 63.Ward NS. Neural plasticity and recovery of function. Prog Brain Res 2005; 150: 527–535. [DOI] [PubMed] [Google Scholar]
- 64.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol 2006; 59: 735–742. [DOI] [PubMed] [Google Scholar]
- 65.Murphy TH, Corbett D. Plasticity during stroke recovery: from synapse to behaviour. Nat Rev Neurosci 2009; 10: 861–872. [DOI] [PubMed] [Google Scholar]
- 66.Dijkhuizen RM, Zaharchuk G, Otte WM. Assessment and modulation of resting-state neural networks after stroke. Curr Opin Neurol 2014; 27: 637–643. [DOI] [PubMed] [Google Scholar]
- 67.Manoonkitiwongsa PS, Jackson-Friedman C, McMillan PJ, et al. Angiogenesis after stroke is correlated with increased numbers of macrophages: the clean-up hypothesis. J Cereb Blood Flow Metab 2001; 21: 1223–1231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.