Abstract
Feeding and sleep are highly conserved, interconnected behaviors essential for survival. Starvation has been shown to potently suppress sleep across species; however, whether satiety promotes sleep is still unclear. Here we use the fruit fly, Drosophila melanogaster, as a model organism to address the interactions between feeding and sleep. We first monitored the sleep of flies that had been starved for 24 h and found that sleep amount increased in the first 4 h after flies were given food. Increased sleep after starvation was due to an increase in sleep bout number and average sleep bout length. Mutants of translin or adipokinetic hormone, which fail to suppress sleep during starvation, still exhibited a sleep increase after starvation, suggesting that sleep increase after starvation is not a consequence of sleep loss during starvation. We also found that feeding activity and food consumption were higher in the first 10‒30 min after starvation. Restricting food consumption in starved flies to 30 min was sufficient to increase sleep for 1 h. Although flies ingested a comparable amount of food at differing sucrose concentrations, sleep increase after starvation on a lower sucrose concentration was undetectable. Taken together, our results suggest increased food intake after starvation enhances sleep and reveals a novel relationship between feeding and sleep.
Keywords: Drosophila, feeding, sleep, starvation, satiety
1. Introduction
Organisms maintain homeostasis by tightly regulating numerous biological processes, such as feeding and sleep. Impairments in feeding or sleep are indicators of an organism’s overall health. Poor sleep quality, high sleep loss, and high predisposition to sleep disorders are major symptoms in obesity (Romero-Corral et al., 2010; Beccuti and Pannain, 2011). In addition, chronic partial sleep deprivation can lead to an increased risk of obesity and diabetes (Sharma and Kavuru, 2010). There is also evidence that suggests an acute interaction between feeding and sleep. During periods of starvation, time spent in rapid eye movement (REM) sleep is significantly decreased, suggesting food deprivation alters sleep architecture (MacFadyen et al., 1973). Furthermore, sleep deprivation for limited time periods (a couple of nights) has the ability to alter feelings of hunger and levels of feeding-related hormones (Knutston et al., 2007). Despite substantial evidence of the interaction between feeding and sleep, little is known about the mechanisms that link these two behaviors.
The fruit fly, Drosophila melanogaster, displays several behavioral hallmarks of sleep and feeding, making it an excellent model organism for studying the genetic and neural regulation of these behaviors (Shaw et al., 2000; Hendricks et al., 2000; Sehgal and Mignot, 2011; Itskov et al., 2014). Flies have been increasingly used to study how sleep and feeding interact. For example, during periods of starvation, flies increase their activity and decrease sleep amount (Lee and Park, 2004; Keene et al., 2010; Thimgan et al., 2010). Mutations in core circadian clock genes, clock and cycle, have been shown to amplify starvation-induced sleep suppression, while mutations in translin (trsn), an RNA-DNA binding protein, result in a loss of starvation-induced sleep suppression (Keene et al., 2010; Murakami et al., 2016). Mutations in adipokinetic hormone (akh), the insect analog of glucagon, have been shown to affect starvation-induced hyperactivity (Lee and Park, 2004). The role of akh in starvation-induced sleep suppression, however, remains unknown.
Despite studies on the interaction between starvation and sleep, little is known about the mechanisms by which food intake, especially in high amounts, affect behavior. During periods of high metabolic needs, such as starvation, animals ingest a large amount of food to achieve nutritional homeostasis (Davis and Levine, 1977; Itskov et al., 2014; Yapici et al., 2016). Thus, increased food intake is mediated by prolonged periods of starvation. Increased food intake results in a series of behaviors among different animal species, such as in cessation of food ingestion (Antin et al., 1975; You et al., 2008). In humans, a state of drowsiness after a meal (postprandial somnolence) has been shown to be highly variable across individuals (Stahl et al., 1983). While studies have displayed a link between postprandial sleep and meal size/composition (Danguir, 1979; Danguir and Nicolaidis, 1987, Murphy et al., 2016), the numerous variable characteristics of postprandial sleep have limited the thorough analysis of its mechanistic basis.
To study the effect of increased food intake after starvation on sleep, our current study utilizes starvation to induce food intake and monitors sleep changes in D. melanogaster. We find that after starvation, sleep is profoundly increased for several hours. Furthermore, sleep increase after starvation is not due to sleep loss during starvation. The increased amount of feeding activity and food consumption in the first 30 min after starvation is sufficient to acutely increase sleep. Interestingly, low concentration of sucrose after starvation was not sufficient to increase sleep even though flies ingest an increased amount of food after starvation. Thus, our findings present new evidence to suggest an interaction between feeding and sleep that will lead to new investigations into the mechanisms that promote this interaction.
2. Results
2.1. Drosophila increase sleep after starvation
Although several studies have shown that starvation decreases sleep, the changes in sleep after starvation remain ambiguous (Keene et al., 2010; Thimgan et al., 2010). To address this question, we developed a paradigm to starve individual flies in activity tubes for 24 h and then allow them to feed again while continuously monitoring their sleep with the Drosophila Activity Monitoring System (DAMS) (Fig. 1A) (Pfeiffenberger et al., 2010). After a day of baseline sleep recording on sucrose and agar medium for food, we starved a group of wild-type flies for 24 h by providing them with only non-nutritious agar and compared their sleep with a group of wild-type flies fed ad libitum (“Fed ad lib”; Fig. 1A). Consistent with previous studies, sleep was dramatically decreased in female and male flies in the starved group (Figs. 1B, 1C, S1A and S1B) (Keene et al., 2010; Thimgan et al., 2010). This finding was conserved across several different wild-type backgrounds (Figs. 1C and S1B). After 24 h of starvation, flies were transferred back onto sucrose and agar medium to feed again (Fig. 1A). Sleep was significantly increased in the first 4 h after feeding in female and male flies in the starved > fed group (Figs. 1D, 1E, S1C and S1D). This increase in sleep after starvation was conserved across other wild-type backgrounds (Figs. 1E and S1D). To address what sleep parameters were altered to increase sleep after starvation, we analyzed sleep bout number and average sleep bout length. We found that the sleep bout number and average sleep bout length were increased after starvation in female and male flies (Figs. 1F, 1G, S1E and S1F). We also performed high-resolution video recordings in the first 4 h after feeding to exclude periods of body movements such as grooming (Fig. S2). We found a similar fold increase in the starved > fed group compared to fed ad lib group as our DAMS recordings, suggesting that sleep increase after starvation is not due to feeding or grooming events (Fig. S2).
Fig. 1. Sleep is increased after starvation.
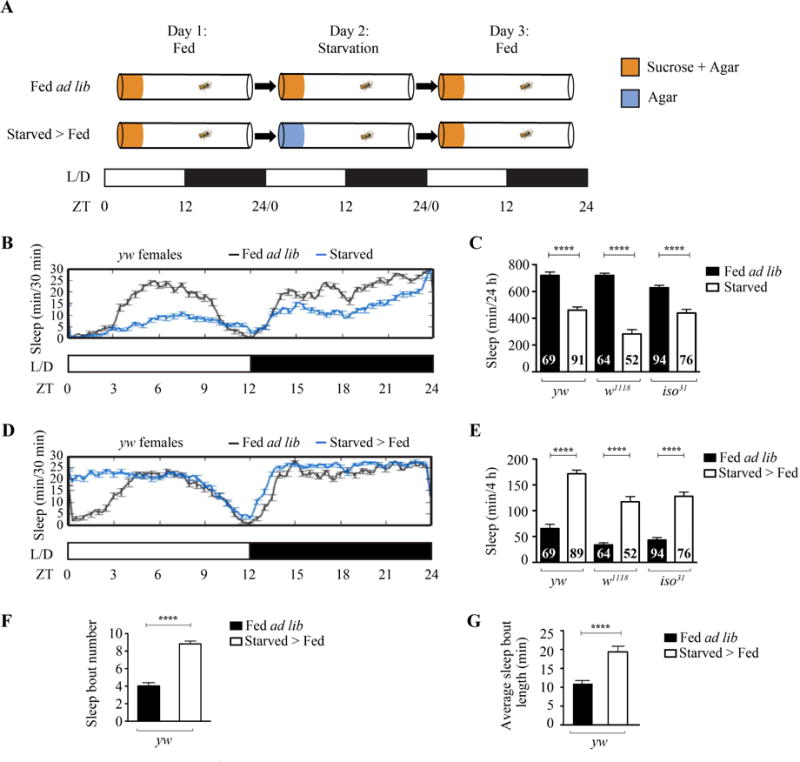
A: Schematic of experimental setup. Two groups of adult flies were placed into Drosophila Activity Monitor tubes with medium containing 5% sucrose and 2% agar on day 1. One group of flies were fed ad libitum throughout the experiment. The other group of flies were transferred onto medium containing 1% agar for starvation on day 2 and then transferred back onto medium containing 5% sucrose and 2% agar on day 3. Sleep was tracked throughout the experiment. L/D indicates the 12h light: 12h dark cycle. ZT0 indicates the time of light on, while ZT12 indicates light off. B: Sleep profile of yw females during 24-h fed ad lib (n = 12) or starved (n = 30). C: Quantification of sleep differences between 24-h fed ad lib and starved female flies in yw, w1118, or iso31 wild-type background. Sleep was significantly suppressed in all starved female flies compared to fed ad lib (P < 0.0001). D: Sleep profile of yw females during 24-h fed ad lib (n = 12) or starved > fed (n = 30). E: Quantification of sleep differences between 4-h fed ad lib and starved > fed female flies in yw, w1118, or iso31 wild-type background. Sleep was significantly increased in the first 4 h in all starved > fed female flies compared to fed ad lib (P < 0.0001). F: Sleep bout number in the first 4 h in fed ad lib (n = 69) and starved > fed (n = 89) yw female flies. The sleep bout number was higher in the starved > fed yw female flies compared to fed ad lib (P < 0.0001). G: Average sleep bout length in the first 4 h in fed ad lib (n = 69) and starved > fed (n = 89) yw female flies. The average sleep bout length was higher in the starved > fed yw female flies compared to fed ad lib (P < 0.0001).
2.2. Sleep increase after starvation does not depend on sleep loss during starvation
After sleep deprivation, animals use ill-defined homeostatic strategies to recover from sleep loss by increasing their sleep (Borbely, 1982). Since animals suppress sleep during starvation, sleep increase after starvation might be due to a homeostatic strategy to recover from sleep loss. To test this hypothesis, we studied fly mutants, which fail to suppress sleep during starvation. Previous studies have identified the akh gene, the fly ortholog of glucagon, as a crucial mediator of starvation-induced hyperactivity (Lee and Park, 2004). However, akh’s role in starvation-induced sleep suppression is unknown. We tested akh mutants for sleep changes during starvation and found that, unlike their wild-type controls, they do not suppress sleep (Fig. 2A and C). Remarkably, akh mutants continue to increase sleep after starvation (Fig. 2A and D). These results suggest that the sleep increase in flies after starvation is independent of previous sleep loss.
Fig. 2. Sleep increase after starvation does not depend on sleep loss.
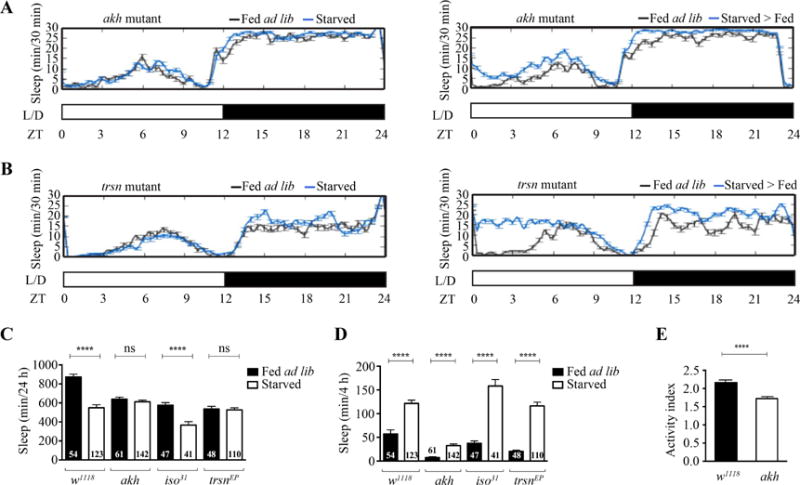
A: Sleep profile of akh mutants during 24-h fed ad lib (n = 16) or starved (n = 31) (left panel) and during 24-h fed ad lib (n = 16) or starved > fed (n = 31) (right panel). B: Sleep profile of trsn mutants during 24-h fed ad lib (n = 16) or starved (n = 30) (left panel) and during 24-h fed ad lib (n = 16) or starved > fed (n = 30) (right panel). C: Quantification of sleep differences between 24-h fed ad lib and starved groups of akh, trsn and their respective wild-type controls. Sleep was suppressed in all wild-type starved groups, but not in akh and trsn mutants (P < 0.0001 for wild type; P > 0.3167 for akh or trsn mutants). D: Quantification of sleep differences between 4-h fed ad lib and starved > fed groups of akh, trsn, and their respective wild-type controls. Sleep was increased in the first 4 h in all starved > fed groups (P < 0.0001). E: Activity index for akh mutants (n = 64) and wild-type control (n = 64) under fed ad lib conditions. Activity is lowered in akh mutants compared to wild-type control (P < 0.0001).
A recent study has shown that trsn fly mutants fail to show starvation-induced sleep suppression while preserving homeostatic feeding strategies, such as increased food intake, after starvation (Murakami et al., 2016). Thus, we used the trsn mutants to further test whether the sleep increase after starvation might be due to sleep loss during starvation. In support of the study of Murakami et al. (2016), we found that trsn mutants, unlike their wild-type controls, fail to decrease their sleep during starvation (Fig. 2B and C). After starvation, trsn mutants show an increase in sleep similar to wild-type controls (Fig. 2B and D). Together with akh mutants, these findings suggest that sleep increase after starvation does not depend on sleep loss during starvation.
Since akh has been associated with mediating starvation-induced hyperactivity, we tested the activity of akh mutants under fed ad lib conditions (Lee and Park, 2004). We found that akh mutants have a lower activity index (average beam crosses per waking minute) (Fig. 2E). Since akh mutants do not suppress sleep during starvation and have lower overall activity, akh may play a role in the connection between sleep and activity.
2.3. Starved flies acutely increase feeding for 30 min
Since sleep loss during starvation is not the sole driver of sleep increase after starvation, we examined whether fly feeding behavior after starvation regulates sleep. We video recorded individual wild-type flies in activity tubes after 24-h starvation, and tracked the percentage of flies feeding after starvation (see Materials and methods). The percentage of flies feeding was increased in starved flies within the first 30 min. After 30 min, this proportion decreased to match control levels, suggesting that a high amount of feeding behavior occurs in the first 30 min after starvation. (Fig. 3A and C).
Fig. 3. Feeding is increased in the first 30 min after starvation.
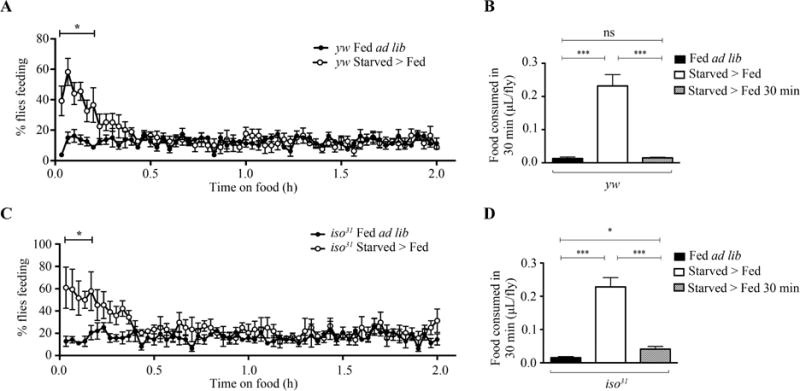
A: Proportion of fed ad lib and starved > fed yw flies feeding in individual activity tubes (N = 5). The percentage of starved > fed yw flies feeding was significantly higher in the first 12 min on food compared to fed ad lib yw flies (P < 0.05). B: Quantification of food intake for 30 min in fed ad lib (N = 5), starved > fed (N = 9), or starved > fed 30 min (N = 5) yw flies. Food intake in starved > fed yw flies was significantly higher than that in both fed ad lib (P = 0.0006) and starved > fed 30 min (P = 0.0006) yw flies. Food intake was not significantly different between fed ad lib and starved > fed 30 min yw flies (P = 0.6761). C: Proportion of fed ad lib and starved > fed iso31 flies feeding in individual activity tubes (N = 4). The percentage of starved > fed iso31 flies feeding was significantly higher in the first 10 min on food compared to fed ad lib iso31 flies (P < 0.005). D: Quantification of food intake for 30 min in fed ad lib (N = 5), starved > fed (N = 9), or starved > fed 30 min (N = 6) iso31 flies. Food intake in starved > fed iso31 flies was significantly higher than that in both fed ad lib (P = 0.0001) and starved > fed 30 min (P = 0.0001) iso31 flies. Food intake was significantly higher in starved > fed 30 min iso31 flies compared to fed ad lib iso31 flies (P = 0.0245). N represents the amount of experiments performed.
To determine if homeostatic feeding in the first 30 min was sufficient to promote a satiated feeding state, we quantified food intake per fly via the Capillary Feeding (CAFE) assay (Ja et al., 2007). We determined the differences in food intake between fed ad lib, starved > fed, or starved > fed 30 min (flies that were starved for 23.5 h and then fed for 30 min prior to the experiment; see Materials and methods). As indicated by previous studies, starved > fed flies increase food intake within 30 min compared to their fed controls (Fig. 3B and D) (Itskov et al., 2014; Yapici et al 2016). Interestingly, we found that food intake is remarkably reduced in starved > fed 30 min flies in comparison to starved > fed flies (Fig. 3B and D). Furthermore, starved > fed 30 min yw flies showed no significant difference in food intake compared to yw flies fed ad lib (Fig. 3B). In contrast, starved > fed 30 min iso31 flies continued to show an increase in food consumption compared to iso31 flies fed ad lib (Fig. 3D). Together with our video recording results, our findings suggest that after starvation flies increase their feeding behavior and food intake predominantly in the first 30 min.
2.4. Acute food intake after starvation increases sleep
Since feeding activity and food consumption is highest within the first 30 min in starved flies, we wondered if 30 min of feeding after starvation was sufficient to drive sleep increase. To study this, we developed an experimental paradigm where two groups of flies were starved on non-nutritious agar medium (Fig. 4A). One group of flies “starved > fed” was starved for 24 h and then returned onto sucrose and agar medium to observe increase in sleep in the first 4 h after starvation, as previously identified (Fig. 1). The other group of flies “starved > fed 30 min” was starved for 23.5 h, given a sucrose and agar medium for only 30 min, and returned to non-nutritious agar medium to observe sleep changes (Fig. 4A). A group of flies “fed ad lib” was used as a control to compare sleep changes in starved groups. Interestingly, sleep analysis revealed that sleep was increased for 1 h in the “starved > fed 30 min” group (Fig. 4B and C). Despite this increase in sleep for 1 h in the “starved > fed 30 min” group, sleep was decreased overall for 4 h (Fig. 4B and D). This suggests that 30 min of feeding after starvation can acutely increase sleep for 1 h, but is insufficient to increase sleep for 4 h.
Fig. 4. Thirty minutes of feeding after starvation acutely increases sleep.
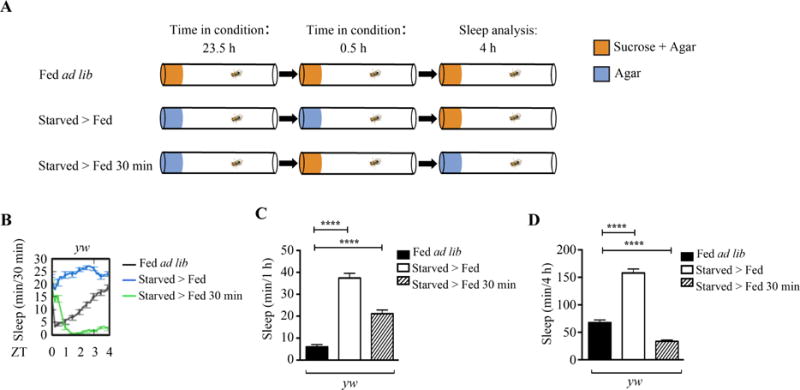
A: Schematic of experimental setup. One group of adult flies “fed ad lib” were placed into Drosophila Activity Monitor tubes on 5% sucrose and 2% agar and fed ad libitum throughout the experiment. One group of adult flies “starved > fed” was starved on 1% agar for 24 h and then transferred back onto 5% sucrose and 2% agar. One group of adult flies “starved > fed 30 min” were starved on 1% agar for 23.5 h, allowed to feed on 5% sucrose and 2% agar for 30 min, and transferred back onto 1% agar. Sleep changes were measured for 4 h after starved flies were allowed to feed for 30 min. B: Sleep profile of fed ad lib (n = 15), starved > fed (n = 30), or starved > fed 30 min (n = 31) yw females. C: Quantification of sleep for 1 h in fed ad lib (n = 89), starved > fed (n = 59), or starved > fed 30 min (n = 101) yw flies. Sleep was increased in starved > fed and starved > fed 30 min yw flies compared to fed ad lib (P < 0.0001). D: Quantification of sleep for 4 h in fed ad lib (n = 89), starved > fed (n = 59), or starved > fed 30 min (n = 101) yw flies. Sleep was increased in starved > fed yw flies compared to fed ad lib (P < 0.0001), but decreased in starved > fed 30 min yw flies compared to fed ad lib (P < 0.0001).
2.5. Sucrose concentration drives sleep increase after starvation
Food consumption may influence behavior through the volume or the quality of food ingested. To address whether the volume or sucrose concentration drives sleep increase after starvation, we modified the paradigm shown in Fig. 1 to study fly sleep on two different sucrose concentrations (10 mM and 100 mM; Fig. 5A). After 24 h of starvation on day 2, flies were broken up into two groups and transferred to either 10 mM or 100 mM sucrose (Fig. 5A). The fed ad lib flies were also transferred to 10 mM or 100 mM sucrose as a control (Fig. 5A). Sleep analysis showed that flies on 10 mM sucrose failed to increase sleep after starvation, while flies on 100 mM sucrose did (Fig. 5B‒D).
Fig. 5. Lower concentration of sucrose does not drive sleep increase after starvation.
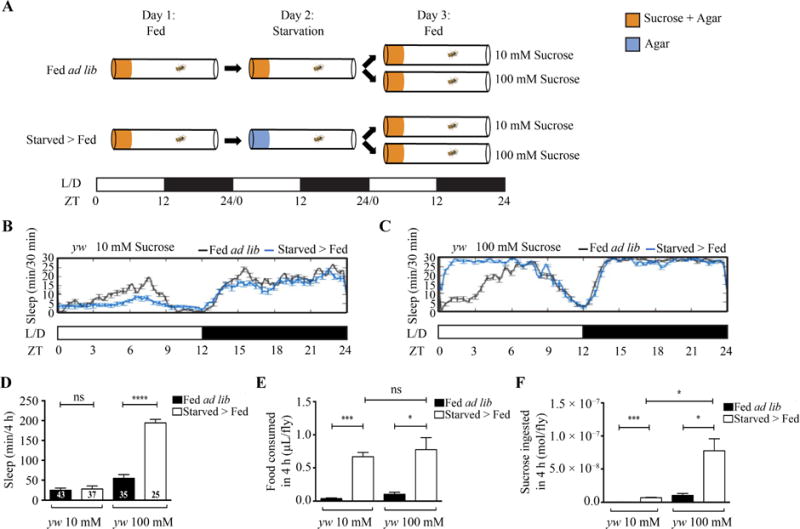
A: Schematic of experimental setup. Two groups of adult flies were placed into Drosophila Activity Monitor tubes with medium containing 5% sucrose and 2% agar on day 1. One group of flies were fed ad libitum throughout the experiment. The other group of flies were transferred onto medium containing 1% agar for starvation on day 2 and then transferred back onto medium containing 2% agar and 10 mM or 100 mM sucrose. The fed ad lib flies were also transferred onto medium containing 2% agar and 10 mM or 100 mM sucrose as controls. Sleep was tracked throughout the experiment. B: Sleep profile of fed ad lib (n = 12) and starved > fed (n = 14) yw flies on 10 mM sucrose medium. C: Sleep profile of fed ad lib (n = 12) and starved > fed (n = 16) yw flies on 100 mM sucrose medium. D: Quantification of sleep differences between the 4-h fed ad lib and starved > fed yw flies. The starved > fed yw flies increased sleep on 100 mM sucrose medium (P < 0.0001) but not on 10 mM sucrose medium (P = 0.7278) compared to their relevant fed ad lib controls. E: Quantification of food intake for 4 h in fed ad lib or starved > fed yw flies (fed ad lib on 10 mM sucrose, N = 3; starved > fed on 10 mM sucrose, N = 4; fed ad lib on 100 mM sucrose, N= 4; starved > fed on 100 mM sucrose, N = 5). Sucrose intake was significantly higher in starved > fed yw flies compared to fed ad lib yw flies on both 10 mM (P = 0.0004) and 100 mM sucrose solution P < 0.0141). No difference was observed between starved > fed yw flies on 10 mM and 100 mM sucrose solution (P = 0.6311). F: Quantification of sucrose ingested for 4 h in fed ad lib and starved > fed yw flies (fed ad lib on 10 mM sucrose, N = 3; starved > fed on 10 mM sucrose, N = 4; fed ad lib on 100 mM sucrose, N= 4; starved > fed on 100 mM sucrose, N = 5). Sucrose intake was significantly higher in starved > fed yw flies compared to fed ad lib yw flies on both 10 mM (P = 0.0004) and 100 mM sucrose solution (P < 0.0141). Sucrose intake was higher in starved > fed flies on 100 mM sucrose solution compared to those on 10 mM sucrose solution (P = 0.0107). N represents the amount of experiments performed.
We then quantified the food intake per fly via the CAFE assay to compare the volume ingested by flies on 10 mM or 100 mM sucrose (Ja et al., 2007). We found that volume ingested was higher in starved > fed flies on both 10 mM and 100 mM sucrose solution compared to their relevant fed ad lib controls (Fig. 5E). However, the volume ingested did not differ between the starved >fed groups on 10 mM and 100 mM sucrose solution (Fig. 5E). We further quantified the amount of moles of sucrose ingested per fly (10 mM or 100 mM; see Materials and methods). We found that sucrose ingested was higher in starved >fed flies on both 10 mM and 100 mM sucrose solution compared to their relevant fed ad lib controls (Fig. 5F). Interestingly, starved > fed flies on 100 mM sucrose solution ingested more moles of sucrose than those on 10 mM (Fig. 5F). This difference in sucrose ingested per fly may increase sleep after starvation.
3. Discussion
In this study, we examined the effects of increased food intake on sleep. We utilized starvation to induce food intake while simultaneously monitoring sleep changes (Fig. 1A). We found that sleep is increased after starvation due to an increase in sleep bout number and average sleep bout length (Fig. 1). Since sleep increase after starvation might be due to sleep loss during starvation, we tested trsn fly mutants, which do not show sleep suppression during starvation (Murakami et al., 2016; Fig. 2). In addition, we found akh mutants also fail to suppress their sleep during starvation (Fig. 2). Our results that akh and trsn mutants continue to increase sleep after starvation suggest that food intake likely plays a role in increased sleep (Fig. 2). We further identified that in the first 30 min after starvation a high percentage of flies consume food, and that this amount of feeding is sufficient to increase sleep for 1h (Figs. 3 and 4). Lastly, our finding that a lower sucrose concentration does not drive sleep increase after starvation suggests that food quality plays an important role in behavioral changes in response to food intake (Fig. 5).
3.1. Impact of food intake on sleep architecture
In accordance with other studies, our results show that starvation is a potent driver of food intake (Fig. 3; Davis and Levine, 1977; Itskov et al., 2014; Yapici et al., 2016). Moreover, our results display that increased food intake after starvation drives an important behavioral switch to increase time spent asleep and suppress time spent active (Fig. 1). In a high-fed state, an organism may require increased energy expenditure on food digestion, which drives the organism to initiate sleep rather than spend limited energy reserves on activity. Additionally, the quality of the food ingested may enhance sleep consolidation, leading to longer sleep episodes. Our result that feeding on a lower concentration of sucrose does not increase sleep after starvation reflects the importance of food quality to initiate sleep episodes and consolidate these episodes for longer periods (Fig. 5).
3.2. Homeostatic versus circadian feeding regulation and sleep changes
A recent study identified that sleep after a single meal was positively correlated with meal volume, protein and salt concentration (Murphy et al., 2016). While their experimental setup allows for the study of postprandial sleep over the course of a day, we use an experimental setup that uses starvation to induce food intake (Fig. 3). Both scenarios are of physiological relevance because, like sleep, feeding is under both circadian and homeostatic control (Borbely, 1982; Xu et al., 2008). Therefore, distinct mechanisms by which each feeding process affects sleep are plausible. For example, we see an increase in sleep after starvation using sucrose and agar medium in all of our experiments while another study identifies sucrose as irrelevant to postprandial sleep (Fig. 1D and E; Murphy et al., 2016). Moreover, our finding that 10 mM sucrose and agar medium does not increase sleep, but 100 mM can, suggests that feeding after starvation modulates sleep via sucrose concentration. Overall, we believe that our experimental setup, utilizing starvation to drive food intake, will shed light on how homeostatic feeding regulation alters behaviors.
3.3. Dissecting the communication between gut and brain
The ability for food to effect behavior reflects the importance of the bidirectional communication between the peripheral intestinal functions (e.g., the gut) and the central nervous system (Carabotti et al., 2015). This crosstalk, referred to as the “gut-brain axis”, has been shown to affect emotion, cognition, and sleep/wakefulness (Carabotti et al., 2015). Disruptions in the gut-brain axis have been used to explain why patients with gastrointestinal disorders have a higher propensity to develop mood disorders (Galland, 2014; Carabotti et al., 2015). It’s also possible that the gut-brain axis mediates the relationship between feeding disorders (e.g., obesity) and sleep disorders (Turnbaugh and Gordon, 2009). However, the genetic and neural mechanisms of the connection between feeding and sleep remain a mystery. To uncover the molecular mechanisms of the crosstalk between the gut and the brain, genetically tractable organisms are invaluable.
In several species, the end of a meal is met with a series of behaviors, including rest or sleep (Antin et al., 1975; You et al., 2008). Furthermore, postprandial sleep has been linked to the satiety-promoting hormone, cholecystokinin (CCK; Shemyakin and Kapas, 2001). However in humans, sleep changes in response to a meal are highly variable (Stahl et al., 1983). This discrepancy in humans may be better understood by using a model organism with a more consistent and prominent phenotype in which food intake can be driven by a period of starvation to induce sleep. Here we showed that sleep increase is an important effect of high food intake and that by using the genetically tractable fly as a model organism we can begin to uncover the underlying mechanisms by which food intake modulates sleep.
4. Materials and methods
4.1. Drosophila maintenance and stocks
Flies stocks were maintained on Jazz-Mix Drosophila Food (Fisherbrand, Thermo Fisher Scientific, USA) and reared on a 12:12 light-dark cycle. Flies are maintained and tested in humidified incubators at 25 °C and 65% humidity for sleep behavior recording (I-36, Percival Scientific, USA). translin mutant flies were provided by Dr. Pedro Miura. akh mutant flies were obtained from Dr. Zhangwu Zhao.
4.2. Sleep analysis
Fly activity is monitored using the DAMS (Trikinetics). Female flies were briefly anesthetized using CO2 at least before 12 h of lights on at Zeitgeber Time 0 (ZT0) and placed into glass tubes containing 2% agar and 5% sucrose. DAMS detects activity by recording infrared beam crossings for each animal. These data were used to calculate sleep information by extracting immobility bouts of 5 min using the pySolo software (Gilestro and Cirelli, 2009). Multiple variables of sleep and activity were analyzed, including total sleep amount and activity index (average beam crosses per waking minute) as previously described (Gilestro and Cirelli, 2009). Sleep bout number and average sleep bout length were analyzed using an excel algorithm which allowed manual scoring of the amount of sleep bouts and the length of each sleep bout for each individual fly.
Baseline sleep was recorded on day 1 on 2% agar and 5% sucrose (Fig1A; Sigma Aldrich, USA). For experiments examining the effects of starvation on sleep, flies were transferred into tubes containing 1% agar 30 min before ZT0 on day 2 and sleep was recorded (Fig. 1A). To examine sleep changes after starvation, starved flies were transferred to tubes containing 2% agar and 5% sucrose 30 min before ZT0 on day 3 (Fig. 1A). Sleep was then analyzed for the first 4 h on day 3 and compared to the fed ad lib control group. For experiments examining restricted feeding on day 3, we transferred starved flies to tubes containing 2% agar and 5% sucrose 45 min before ZT0 on day 3, and then transferred flies back to tube containing 1% agar 15 min before ZT0 (Fig. 4A). For experiments examining the effect of different sucrose concentrations on sleep increase after starvation, we transferred fed ad lib and starved flies to tubes containing 2% agar and 10 mM or 100 mM sucrose medium 30 min before ZT0 on day 3 (Fig. 5A).
4.3. Feeding activity video assay
To monitor fly feeding behavior in DAMS activity tubes, videos were recorded of flies in DAMS activity tubes. Groups of 15‒16 flies were fed or starved for 24 h prior to video recording. Flies were then flipped into activity tubes containing 2% agar and 5% sucrose and placed in a custom built humidified chamber with LED lights. Feeding behavior was recorded using a camcorder (HDR-CX405, Sony). To calculate the percentage of flies feeding, we manually counted the amount of flies on food with proboscis extended at 2-min time intervals and divided the amount of flies feeding by the total amount of flies per group. Counts were taken every 2 min over the span of 4 h. Only the first 2 h are shown in Fig. 3.
4.4. Capillary feeding assay
To measure food consumption, a modified capillary feeding assay was used as previously described (Ja et al., 2007). Groups of 10‒40 flies were fed for 24 h and 30 min, starved for 24 h and 30min, or starved for 24h and fed for 30 min prior to the experiment. Flies were then flipped into an empty plastic food vial capped with parafilm. Capillary micropipettes were created out of Pasteur pipettes (VWR #14673-043) by cutting 8 cm off of the ends. Capillaries were filled with 5% sucrose solution by capillary action and inserted through the parafilm with shortened 200-μL pipette tips. For experiments testing food consumption of different sucrose concentrations we filled capillaries with 10 mM or 100 mM sucrose solution by capillary action. Food consumption after 30 min and 4 h was calculated by measuring the length of liquid missing from the capillary and multiplying the length by the inner area of the capillaries (Masek et al., 2014). After the experiment flies were anesthetized and counted. Food consumption per fly was calculated by dividing the food consumed by the amount of flies. Sucrose ingested was calculated by multiplying the volume of food consumed per fly (L) by the molarity of the solution.
4.5. High-resolution video capture
Videos were captured using a USB 2.0 or FireWire monochrome camera (Point Grey Research, CMLN-13S2M-CS or GS2-FW-14S5M, respectively) with either a 12.5 mm or 25 mm focal length C-mount lens (Fujinon, HF12.5HA-1B or HF25HA-1B, respectively). All experiments were performed in programmable temperature and light-controlled incubators (Percival Scientific, model I-36VL). Videos were recorded using StreamPix 6 (Norpix) and recorded at 3 frames per second (fps) in the form of a QuickTime (H.264) movie format for 4 h from the moment lights turned on. About 8 flies were individually placed in DAMS activity tubes, and their movement was recorded and video captured. Analysis of activity was performed using custom-written image analysis software within the Volumetry application environment as previously described (Winbush et al., 2015). Briefly, movies were background subtracted leaving only individual flies; an intensity threshold was then applied to adequately outline each fly. Flies were then flood-filled and saved as graphic particle files (GPF) that were used for subsequent analysis. Simple velocities were extracted from particle files and periods of inactivity were identified. Periods of inactivity, assumed to be sleep, were defined as bouts greater than or equal to 5 min in length in which no movement greater than 15% of total body length occurred. Additionally, all feeding and grooming events were omitted. Each data set was individually normalized to 1 to compare fold-increase of starved > fed sleep and fed ad lib sleep in the first 4 h after starvation between the high resolution video recordings and the DAMS. Normalization was calculated by dividing the starved > fed sleep and fed ad lib sleep values by the average amount of sleep (min) in the first 4 h after starvation of the fed ad lib group (Fig. S2).
4.6. Statistics
Statistical analyses are performed using InStat software (GraphPad Software 5.0 Inc.). We employed two-way unpaired t-test for most comparative analysis (Figs. 1, 2, 3B, 3D, 4, 5, S1, and S2) or two-way ANOVA (Fig. 3A and C). In figures, graph bars are mean values and error bars represent the standard error of the mean.
Supplementary Material
Acknowledgments
We would like to thank the Bloomington Drosophila Stock Center, as well as Drs. Pedro Miura, Zhangwu Zhao and Tom Kidd for providing fly stocks. We are grateful to members of Zhang lab for advice and discussions. This work was supported by National Institutes of Health COBRE Grant P20 GM103650.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol. 1975;89:784–790. doi: 10.1037/h0077040. [DOI] [PubMed] [Google Scholar]
- Beccuti G, Pannain S. Sleep and obesity. Curr Opin Clin Nutr Metab Care. 2011;14:402–412. doi: 10.1097/MCO.0b013e3283479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbély AA. A two-process model of sleep regulation. Hum Neurobiol. 1982;1:195–204. [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- Danguir J. Cafeteria diet promotes sleep in rats. Appetite. 1987;8:49–53. doi: 10.1016/s0195-6663(87)80026-0. [DOI] [PubMed] [Google Scholar]
- Danguir J, Nicolaidis S. Dependence of sleep on nutrients availability. Physiol Behav. 1979;22:735–740. doi: 10.1016/0031-9384(79)90240-3. [DOI] [PubMed] [Google Scholar]
- Davis JD, Levine MW. A model for the control of ingestion. Psychol Rev. 1977;84:379–412. [PubMed] [Google Scholar]
- Galland L. The gut microbiome and the brain. J Med Food. 2014;17:1261–1272. doi: 10.1089/jmf.2014.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilestro GF, Cirelli C. pySolo: a complete suite for sleep analysis in Drosophila. Bioinformatics. 2009;25:1466–1467. doi: 10.1093/bioinformatics/btp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25:129–138. doi: 10.1016/s0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Itskov PM, Moreira JM, Vinnik E, Lopes G, Safarik S, Dickinson MH, Ribiero C. Automated monitoring and quantitative analysis of feeding behaviour in Drosophila. Nat Commun. 2014;5:4560. doi: 10.1038/ncomms5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ja WW, Carvalho GB, Mak EM, de la Rosa NN, Fang AY, Liong JC, Brummel T, Benzer S. Prandiology of Drosophila and the CAFE assay. Proc Natl Acad Sci USA. 2007;104:8253–8256. doi: 10.1073/pnas.0702726104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Duboué ER, McDonald DM, Dus M, Suh GS, Waddell S, Blau J. Clock and cycle limit starvation-induced sleep loss in Drosophila. Curr Biol. 2010;20:1209–1215. doi: 10.1016/j.cub.2010.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFadyen UM, Oswald I, Lewis SAJ. Starvation and human slow-wave sleep. J Appl Physiol. 1973;35:391–394. doi: 10.1152/jappl.1973.35.3.391. [DOI] [PubMed] [Google Scholar]
- Masek P, Reynolds LA, Bollinger WL, Moody C, Mehta A, Murakami K, Yoshizawa M, Gibbs AG, Keene AC. Altered regulation of sleep and feeding contributes to starvation resistance in Drosophila melanogaster. J Exp Biol. 2014;217:3122–3132. doi: 10.1242/jeb.103309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami K, Yurgel ME, Stahl BA, Masek P, Mehta A, Heidker R, Bollinger W, Gingras RM, Young-Joon Kim Y, Ja WW, Suter B, DiAngelo JR, Keene AC. Translin is required for metabolic regulation of sleep. Curr Biol. 2016;26:972–980. doi: 10.1016/j.cub.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy KR, Deshpande SA, Yurgel ME, Quinn JP, Weissbach JL, Keene AC, Dawson-Scully K, Huber R, Tomchik SM, Ja WW. Postprandial sleep mechanics in Drosophila. eLife. 2016;5:e19334. doi: 10.7554/eLife.19334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Locomotor activity level monitoring using the Drosophila activity monitoring (DAM) system. Cold Spring Harb Protoc. 2010;5 doi: 10.1101/pdb.prot5518. [DOI] [PubMed] [Google Scholar]
- Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea. Chest. 2010;137:711–9. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Kavuru M. Sleep and metabolism: an overview. Int J Endocrinol. 2010;2010:1–12. doi: 10.1155/2010/270832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster. Science. 2000;287:1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- Shemyakin A, Kapas L. L-364,718, a cholecystokinin-A receptor antagonist, suppresses feeding-induced sleep in rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:1420–1426. doi: 10.1152/ajpregu.2001.280.5.R1420. [DOI] [PubMed] [Google Scholar]
- Sehgal A, Mignot E. Genetics of sleep and sleep disorders. Cell. 2011;146:194–207. doi: 10.1016/j.cell.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl ML, Orr WC, Bollinger C. Postprandial sleepiness: objective documentation via polysomnography. Sleep. 1983;6:29–35. doi: 10.1093/sleep/6.1.29. [DOI] [PubMed] [Google Scholar]
- Thimgan MS, Suzuki Y, Seugnet L, Gottschalk L, Shaw PJ. The perilipin homologue, lipid storage droplet 2, regulates sleep homeostasis and prevents learning impairments following sleep loss. PLoS Biol. 2010;8:e1000466. doi: 10.1371/journal.pbio.1000466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Gordon JI. The core gut microbiome, energy balance and obesity. J Physiol. 2009;587:4153–4158. doi: 10.1113/jphysiol.2009.174136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winbush A, Gruner M, Hennig GW, van der Linden AM. Long-term imaging of circadian locomotor rhythms of a freely crawling C. elegans population. J Neurosci Methods. 2015;249:66–74. doi: 10.1016/j.jneumeth.2015.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu K, Zheng X, Sehgal A. Regulation of feeding and metabolism by neuronal and peripheral clocks in Drosophila. Cell Metab. 2008;8:289–300. doi: 10.1016/j.cmet.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yapici N, Cohn R, Schusterreiter C, Ruta V, Vosshall LB. A taste circuit that regulates ingestion by integrating food and hunger signals. Cell. 2016;165:715–729. doi: 10.1016/j.cell.2016.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You YJ, Kim J, Raizen DM, Avery L. Insulin, cGMP, and TGF-beta signals regulate food intake and quiescence in C. elegans: A model for satiety. Cell Metab. 2008;7:249–257. doi: 10.1016/j.cmet.2008.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


