Abstract
While much is known about the molecular pathways that regulate embryonic development and adult homeostasis of the endocrine pancreas, little is known about what regulates early postnatal development and maturation of islets. Given that birth marks the first exposure to enteral nutrition, we investigated how nutrient-regulated signaling pathways influence postnatal islet development in mice. We performed loss-of-function studies of mechanistic target of rapamycin (mTOR), a highly conserved kinase within a nutrient-sensing pathway known to regulate cellular growth, morphogenesis and metabolism. Deletion of Mtor in pancreatic endocrine cells had no significant effect on their embryonic development. However, within the first 2 weeks after birth, mTOR-deficient islets became dysmorphic, β-cell maturation and function were impaired, and animals lost islet mass. Moreover, we discovered that these distinct functions of mTOR are mediated by separate downstream branches of the pathway, in that mTORC1 (with adaptor protein Raptor) is the main complex mediating the maturation and function of islets, whereas mTORC2 (with adaptor protein Rictor) impacts islet mass and architecture. Taken together, these findings suggest that nutrient sensing may be an essential trigger for postnatal β-cell maturation and islet development.
KEY WORDS: Islet, Pancreas, Mtor, Postnatal, Diabetes, Mouse
Highlighted article: The nutrient-sensing pathway mTOR and its downstream effectors are required in the first two weeks of life for the morphogenesis, maturation and function of pancreatic islets in mice.
INTRODUCTION
Although making up only about 1-2% of total pancreatic mass, the endocrine pancreas is part of a vital system controlling nutrient and metabolic homeostasis. To do this, β cells, α cells, δ cells and pancreatic polypeptide (PP) cells secrete insulin, glucagon, somatostatin and PP in a coordinated fashion in response to nutrient and metabolic demands. During embryonic and postnatal development, these endocrine cells are specified and undergo maturation, migration, and morphogenesis to form clusters known as the islets of Langerhans.
Extensive studies in model organisms have identified numerous signaling pathways and responding transcriptional networks that regulate key stages of embryonic endocrine pancreas development (reviewed by Mastracci and Sussel, 2012; Pan and Wright, 2011). By contrast, little is known about early postnatal endocrine cell development, where endocrine cells expand in number and mature into fully functional islets that are capable of controlling nutrient homeostasis. Certain regulators of postnatal maturation have been identified that influence the function of the adult endocrine pancreas. Rodent β cells begin as an embryonic Mafb+ population but transition and ultimately mature into Mafa single-positive cells after birth (Artner et al., 2010; Hang et al., 2014; Nishimura et al., 2006). In addition to having significant roles during embryonic development, Mafb and Arx remain required for the α-cell population in mature murine islets (Wilcox et al., 2013). Exclusive to the β-cell population in mice, urocortin 3 (Ucn3) is upregulated in the postnatal pancreas starting at postnatal day (P) 6 (Blum et al., 2012) and potentiates a mature paracrine feedback loop necessary for proper glycemic control (van der Meulen et al., 2015). During this time, expression of Pdx1 within β cells also increases and is necessary for β-cell survival and identity (Ahlgren et al., 1998; Gao et al., 2014). Additional transcription factors have been found to be required for adult endocrine maintenance and subsequent function of the islet. Among other roles, Nkx2.2, Isl1 and Pax6 regulate expression of glucose transporter 2 (Glut2; also known as Slc2a2) (Doyle and Sussel, 2007; Ediger et al., 2014; Gosmain et al., 2013). Glut2 is a necessary carrier protein that is part of the glucose-sensing machinery of a β cell and, as such, is an integral part of glucose-stimulated insulin secretion (GSIS). In mouse models, downregulation or loss of key postnatal maturation factors leads to a loss of β-cell functionality, decreased responsiveness to glucose, and diabetes.
There are vast changes in the gastrointestinal tract after birth, primarily in response to oral feeding (Zhang et al., 1998). Published findings suggest that islets are glucose responsive during this time, but are not able to perform oxidative metabolism nor maintain efficient whole-body glucose homeostasis like adult islets (Bliss and Sharp, 1992). At later postnatal stages, changes in diet, such as milk to chow transition, can drastically affect the maturation and function of the islet (Stolovich-Rain et al., 2015). These studies have shown that timing, weaning and diet composition all play major roles in the final stages of postnatal islet development and glycemic control. In addition, several studies demonstrate the importance of nutrient sensing in adult islet function. By contrast, nothing is known about the impact of nutrients on islet development just after birth.
Mechanistic target of rapamycin (Mtor) is a component of two nutrient-sensitive serine-threonine kinase complexes that have known roles in proliferation, transcription, translation, cytoskeletal rearrangement and cell survival. One key role of mTOR signaling is to modulate cellular metabolism and homeostasis in response to the availability of growth factors and nutrients. The two complexes, mTORC1 and mTORC2, differ in both the adaptor proteins associated with the complex and their sensitivity to the drug rapamycin. mTORC1 utilizes the adaptor protein Raptor, which directly phosphorylates ribosomal protein S6 kinase (S6K) and Eif4ebp1, both of which promote protein synthesis. mTORC2 includes the adaptor protein Rictor, and plays roles in cytoskeletal reorganization and cell size. Whereas mTORC1 is highly sensitive to rapamycin, short-term exposure to the drug has no effect on mTORC2.
Although few studies have detailed the role of mTOR during embryonic or postnatal endocrine pancreas development, the mTOR pathway is known to be involved in several aspects of adult β-cell biology. Aberrant regulation of mTOR has been implicated in obesity and type 2 diabetes, and inhibition of mTOR signaling via rapamycin improves blood glucose levels in some patients with hyperinsulinemic hypoglycemia (Alexandrescu et al., 2010; Senniappan et al., 2014). However, in normal glycemic mice, rapamycin exposure leads to decreased glucose sensitivity and diabetes (Schindler et al., 2014). In addition, mTORC1 activation promotes adult β-cell proliferation via regulation of cyclin D2 synthesis and stability (Balcazar et al., 2009), while mTORC2 has been implicated in the balance between cell size and proliferation in the adult β cell (Gu et al., 2011). Deletion of the negative regulator of mTOR signaling, TSC1, can actually improve glucose-sensing mechanisms and increase insulin production (Mori and Guan, 2012), while upregulation of the pathway via Lkb1 (Stk11) deletion has been shown to enhance β-cell mass, glucose tolerance and insulin content (Fu et al., 2009; Granot et al., 2009). A more detailed description of mTOR signaling in the adult islet is provided elsewhere (Wang et al., 2016); however, these data imply that mTOR signaling plays crucial roles in the function of adult β cells and influences glucose metabolism in both normal and pathological contexts.
Birth marks the start of enteral nutrition in mammals, which then must develop the ability to sense and regulate systemic glucose. Correspondingly, the endocrine pancreas undergoes profound changes, including expansion of endocrine cell mass, the formation of islets and the maturation of β cells. To investigate how nutrient-sensing pathways are involved in these postnatal changes in endocrine pancreas development, we studied the impact of inactivating mTOR on postnatal islet development. We deleted Mtor in the developing endocrine pancreas using a Neurog3Cre;Mtorf/f mouse model and found that mTOR was not required for embryonic specification or differentiation of endocrine cells. By contrast, mTOR was essential in the first weeks of life for the expansion of endocrine mass, β-cell maturation and functionality, as well as islet morphogenesis. Further examination revealed distinct roles for mTORC1 and mTORC2 during postnatal development, in which mTORC1 was the primary complex affecting islet maturation and function. Ultimately, these late developmental defects caused β-cell failure and hyperglycemia in young adult mice.
RESULTS
mTOR is required for normal postnatal islet development
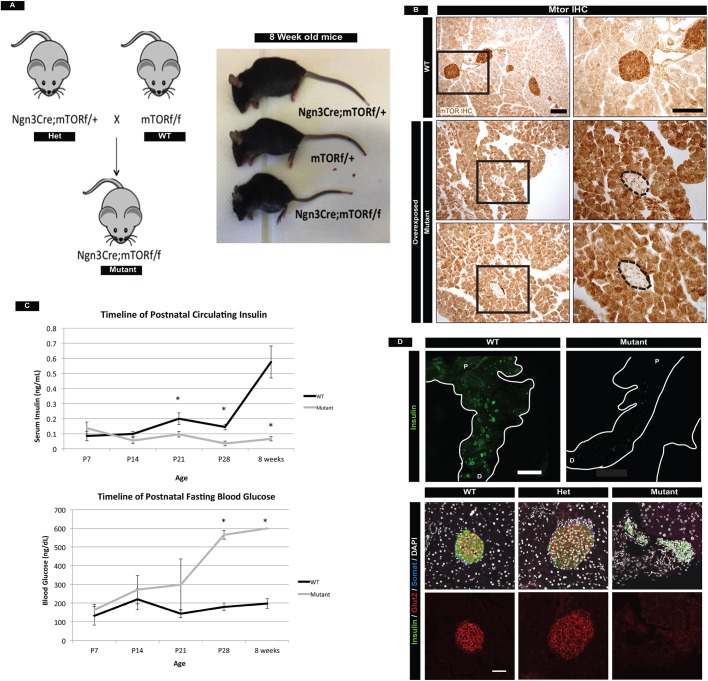
To investigate the role of mTOR in islet development, we used Neurog3Cre to delete Mtor from the developing endocrine pancreas (Fig. 1A). For the first 4 weeks after birth, Neurog3Cre;Mtorf/f (mTOR mutant) mice grew in parallel with their control littermates and, by immunohistochemistry, we confirmed efficient deletion of mTOR within the endocrine compartment (Fig. 1B, Fig. S1A). However, by 7 weeks of age, both male and female mutant mice showed signs of impaired health and most animals perished by 8 weeks. To identify when mutant mice first exhibited an endocrine pancreas phenotype we analyzed animals from P7 to 8 weeks of life for fasted blood glucose levels and circulating insulin levels. Mutant mice first showed signs of impaired glucose regulation at P14 and were fully diabetic at P28 (Fig. 1C). Whole-mount immunofluorescence staining of insulin in the pancreas revealed substantially fewer islets in adult mutant mice (Fig. 1D top, Movies 1 and 2). Moreover, remaining islets were dysmorphic, smaller, and lacked functional markers of mature β cells such as Glut2 (Fig. 1D, bottom). For the first 3 weeks of postnatal life, the growth of mutant mice was the same as that of control littermates (Fig. S1A), suggesting that feeding behavior and nutrient absorption were normal. We also stained for PECAM1 and synaptophysin and found normal vascularization and innervation of islets (Fig. S1B). These data suggested that hyperglycemia and β-cell failure were primarily the result of a cell-autonomous role of mTOR within the postnatal islet, and were not the culmination of intestinal or CNS effects on the β cell. Lastly, we detected a subtle phenotype in mTOR heterozygous mice through slightly reduced β-cell numbers (Fig. S2A,B); however, this phenotype did not culminate in elevated blood glucose levels or lowered glucose-sensing mechanisms, even at 1 year of age (Fig. S2C). Therefore, we focused all remaining analyses on homozygous mutant mice.
Fig. 1.
Deletion of mTOR in the endocrine pancreas leads to loss of islet cell mass and hyperglycemia in adult mice. (A) Schematic of breeding Neurog3Cre;Mtorf/f (mutant) mice. Heterozygous mice were bred to Mtorf/f mice to obtain mutants. At 8 weeks of age, mutant mice are significantly smaller than their wild-type littermates. Both male and female mutants perish just after 8 weeks of age. (B) mTOR protein in wild-type and mutant islets. mTOR is efficiently deleted from islets of mutant mice (outlined) as compared with wild-type control mice. Mutant panels show overexposed immunohistochemistry (IHC) to highlight deletion of mTOR in islets. Boxed regions are magnified to the right. Scale bars: 100 µm. (C) Timeline of fasting blood glucose and circulating insulin levels in wild type and mutants. Increased glucose levels and reduced insulin are first detected at P14, worsen at P21, and all mutants are unable to regulate fasted glucose levels at P28. Mean±s.e.m.; *P<0.05. (D) (Top) Whole-mount immunofluorescence staining of insulin in the adult pancreas, showing reduction in the number of islets in mutant mice. P, proximal; D, distal. Scale bar: 1.5 mm. (Bottom) Analysis of insulin, somatostatin and Glut2 (single channel shown in bottom row) protein in adult islets. Mutant islets are morphologically distorted and have no detectable Glut2 protein. Scale bar: 50 µm. See also Figs S1 and S2, Movies 1 and 2, and Table S3 for the numbers of animals used.
Since Neurog3 is expressed embryonically, we needed to first determine if postnatal effects were due to an earlier role of mTOR during embryonic endocrine development. Analysis of pancreas samples from embryonic day (E) 18.5 of wild-type and mutant animals indicated no statistically significant difference in the relative proportions of α, β and δ cells (Fig. 2A). In addition, analysis of key regulators of endocrine cell migration from the duct, such as Sox9, E-cadherin and Snail2, indicated that islet morphogenesis before birth in mTOR mutant mice was similar to that of control mice (Fig. S3A). Given that mTOR mutant animals became diabetic between 3 and 4 weeks of life, we hypothesized that mTOR primarily affected postnatal islet development. We therefore investigated when mTOR signaling was most active during postnatal pancreas development by analyzing phospho-mTOR levels, a readout of active mTOR signaling. mTOR signaling was highest in the first few weeks after birth in both the endocrine and exocrine compartments, after which the pathway became progressively less active as the mice aged (Fig. S3B). Together, these data indicated that mTOR was dispensable for embryonic development, instead having a prominent role during postnatal stages of islet development.
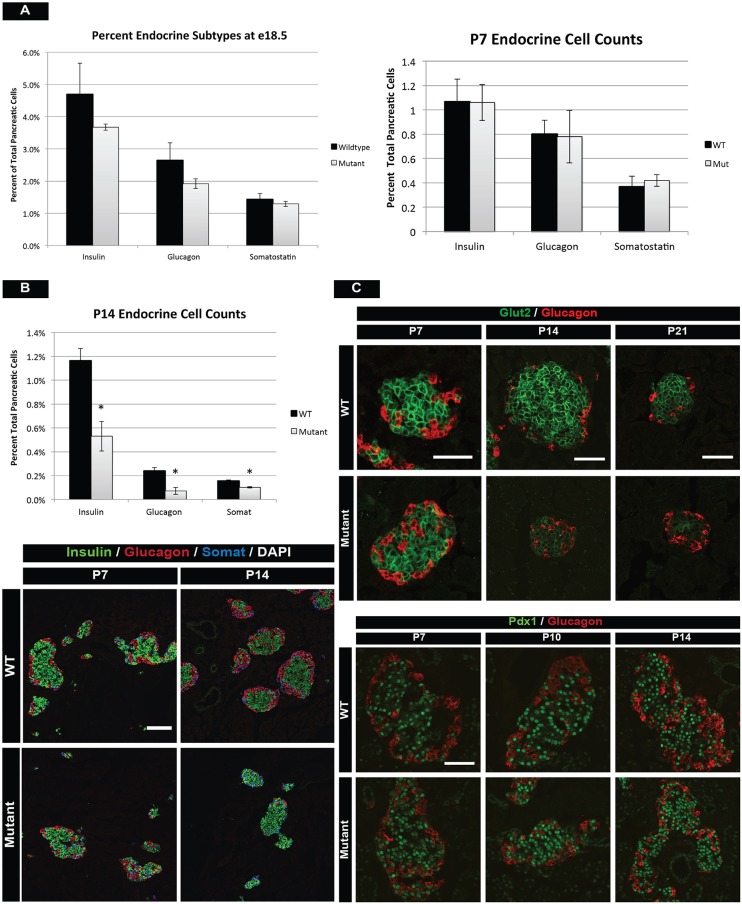
Fig. 2.
mTOR is required after birth for islet development. (A) The percentage of β-, α- and δ-cell populations at E18.5 (left) and P7 (right) in mTOR mutants. No significant difference in islet mass is observed at these stages between wild-type and mutant animals. (B) (Top) However, flow cytometry at P14 reveals reduced cell counts for all mutant islet subtypes as compared with wild-type littermates. (Bottom) Hormone immunofluorescence of wild-type and mTOR mutant islets at P7 and P14. Globally, mutant islets are indistinguishable from wild-type islets at P7. One week later, mutants have noticeably fewer endocrine cells. (C) Immunofluorescence for Glut2 in β cells reveals normal glucose-sensing mechanisms at P7, reduced expression beginning at P14, and near absence of Glut2 protein at P21. Despite lower cell counts and fewer glucose transporters, β cells retain normal Pdx1 protein levels, indicating retention of endocrine identity. Mean±s.e.m.; *P<0.05. Scale bars: 50 µm. See also Figs S3 and S4, and Table S3 for numbers of animals used.
Loss of postnatal endocrine cell mass in mTOR mutant islets
Given the high levels of mTOR signaling in the first few weeks of life, we interrogated mTOR mutant animals at early postnatal stages for alterations in islet mass, β-cell maturation and function, as well as islet architecture. Analysis of islet mass using flow cytometry at P7 indicated no significant differences in endocrine populations within mutant islets (Fig. 2A). However, there were noticeably fewer endocrine cells present at P14, with a decrease in α-, β- and δ-cell populations as compared with controls (Fig. 2B). These reductions culminated in fewer large islets at P14 in mutant mice (Fig. S3C). We investigated if this was due to apoptosis; however, analysis of TUNEL staining from E18.5 through adult stages did not show any significant changes in cell death of endocrine cells (Fig. S4B). We obtained similar results with active Casp3 staining (data not shown), concluding that endocrine cells were not undergoing apoptosis in the absence of mTOR. Finally, lineage tracing of endocrine cells did not uncover transdifferentiation or dedifferentiation events in mTOR mutant mice (data not shown).
We next investigated whether the proliferation of endocrine cells was altered in mTOR mutant islets using BrdU incorporation, as well as Ki67 and phosphohistone (PHH3) staining. By Ki67 immunofluorescence, we found significantly fewer proliferating cells just prior to birth, and a trend of decreased proliferation was also seen with PHH3 immunostaining (Fig. S4C,D). Additionally, we assessed long-term proliferation through daily BrdU injections between 1 and 2 weeks of postnatal life and observed that although there was no statistically significant difference in BrdU incorporation between wild-type and mutant mice, there was a decreasing trend. These data suggested that mTOR mutant animals retained lower levels of postnatal proliferation (Fig. S4C). From these data, we concluded that lower proliferation and numbers of endocrine cells during late embryonic/early postnatal development culminated in the reduced cell counts at P14 in mTOR mutant animals. Together, these findings suggested that mTOR signaling regulates islet mass and expansion during early postnatal islet development, which is consistent with its known role in adult animals in regulating β-cell mass (Balcazar Morales and Aguilar de Plata, 2012; Balcazar et al., 2009; Gu et al., 2011).
Maturation and function are compromised in mTOR mutant islets
Another key process that occurs during early postnatal islet development is the functional maturation of β cells. Glucose sensing and transport are required for β-cell function and we investigated if glucose-sensing mechanisms were impaired in mutant mice by analyzing expression of the glucose transporter Glut2. Glut2 expression was normal at P7, but a striking decrease in Glut2 was apparent beginning at P14, ultimately leading to a complete loss at P21 in mTOR mutant mice (Fig. 2C, top). Despite the loss of Glut2, these islet cells retained β-cell identity, as marked by expression of Pdx1 (Fig. 2C, bottom) (Gao et al., 2014). We concluded from these data that mTOR was affecting both islet cell expansion and function in the first 2 weeks of postnatal life.
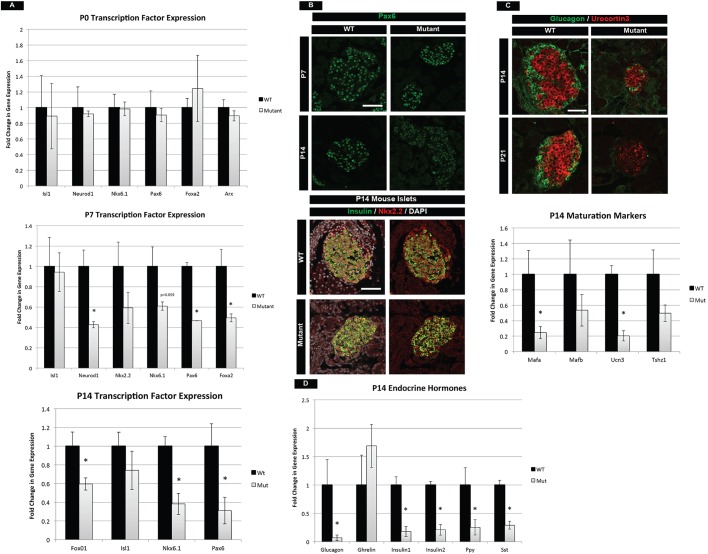
Previous work has identified several transcription factors that are necessary for β-cell maintenance, identity and function postnatally. Alterations in these transcription factors could, in part, contribute to the phenotype in mTOR mutants at P14. We analyzed mRNA levels of a number of transcription factors at different stages ranging from birth to P14. At birth, islet transcription factors and hormones were expressed at normal levels in mTOR mutants, indicating that mTOR was functionally dispensable for embryonic islet development (Fig. 3A, Fig. S5B). However, at P7 and P14 several factors were significantly reduced, including Neurod1, Nkx6.1, Pax6, Foxa2 and Foxo1 (Fig. 3A, Fig. S5B). At the protein level, we also observed a decrease in Pax6 and Nkx2.2 at P14 (Fig. 3B). Pax6 has been shown to be essential for the maintenance of adult islets, while Nkx2.2 is known to regulate β-cell function (Doyle and Sussel, 2007; Hart et al., 2013; Sander et al., 1997). In addition, Nkx6.1 and Foxo1 transcription factors are known to regulate β-cell growth (Okada et al., 2007; Taylor et al., 2015). Therefore, the significant reduction of all of these factors could contribute to the phenotypes observed in mTOR mutant islets.
Fig. 3.
mTOR mutants have compromised postnatal maturation. (A) qPCR for essential transcription factors required for growth and maturation in the postnatal islet. Whereas no difference is seen at birth, loss of these transcription factors occurs in the first 2 weeks of life in mTOR mutants. (B) In conjunction, protein levels of Pax6 and Nkx2.2 are significantly reduced at P14. (C) Maturation markers Ucn3 and Mafa are reduced in β cells at P14. Ucn3 protein continues to decrease at P21, indicative of a continued loss of maturity in mTOR mutant islets. (D) qPCR analysis of P14 pancreatic hormones. Decreased mRNA levels are observed for all islet hormones. A trend of increasing Ghrl expression is also seen, indicative of a maturation defect. See also Fig. S5. Mean±s.e.m.; *P<0.05. Scale bars: 50 µm. See Table S3 for numbers of animals used.
To investigate the molecular basis for maturation defects in mTOR mutant islets, we analyzed the β-cell maturation markers Mafa and Ucn3. Ucn3 protein and mRNA were reduced in P14 islets, as was Mafa mRNA (Fig. 3C). The reduced levels of Mafa and Ucn3 observed in mTOR mutant animals at P14 were not due to a delay in islet maturation, since P21 mutant mice also had further reduced Ucn3 levels, indicating persistent immaturity and loss of function (Fig. 3C). Robust production of hormones is also a measure of islet function and we found that nearly all hormone transcripts were reduced in mTOR mutant animals by P14 (Fig. 3D, Fig. S5B). One exception was a trend towards a transcriptional increase in the hormone ghrelin (Ghrl), which is normally expressed in a subset of embryonic endocrine cells but decreases postnatally (Prado et al., 2004). Despite an increase in Ghrl mRNA, we did not observe increased levels of ghrelin protein (data not shown). Lastly, we investigated the expression of selectively disallowed genes, which are downregulated specifically during the neonatal period in β cells. The low-level expression of these genes ensures that the β cells respond exclusively to glucose within the body and not to other metabolites such as pyruvate (Thorrez et al., 2011). At P14 we did not observe any changes in lactase dehydrogenase A (Ldha) or other selectively disallowed genes in mTOR mutant islets relative to controls (Fig. S5C).
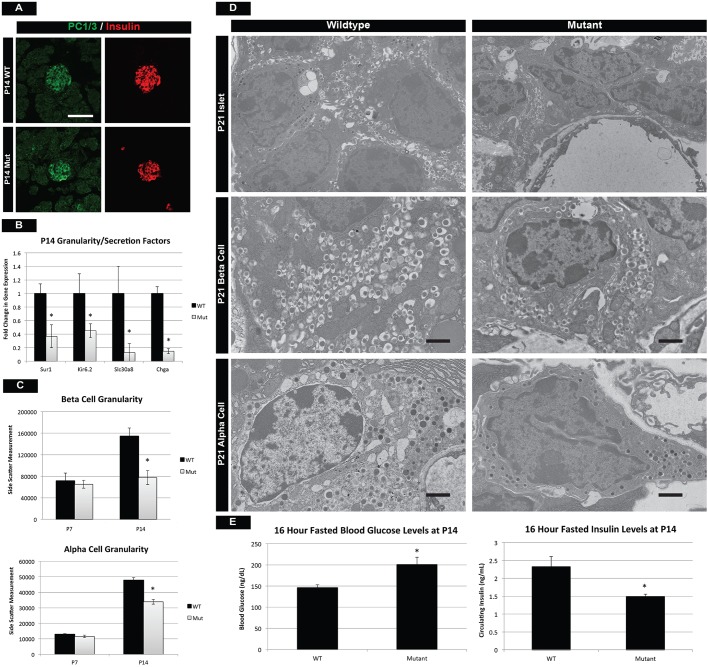
Postnatal maturation results in the formation of functional endocrine cells with the ability to accurately modulate glucose levels when animals transition from milk to a more complex diet. Production, processing, packaging and secretion of insulin are all hallmarks of a functional β cell and we investigated whether these processes were affected in mTOR mutants. Preproinsulin processing by the prohormone convertase 1/3 (PC1/3; also known as Pcsk1) is essential for β-cell function, and immunostaining for PC1/3 was similar between control and mTOR mutant β cells (Fig. 4A). However, at P7 and P14 we observed a significant reduction in Slc30a8 (Znt8), a transporter that is responsible for the influx of zinc into β cells and the subsequent packaging of insulin into mature granules (Fig. 4B, Fig. S5D). Consistent with this, we observed a reduction in granule formation in both α and β cells by two separate measures: flow cytometry at P14 and transmission electron microscopy (TEM) at P21 (Fig. 4C,D). Assessment of β-cell granularity by side scatter during flow cytometry has been reported previously (Katsuta et al., 2012) and while average side-scatter measurements were similar at P7, both α and β cells were less granular at P14 (Fig. 4C, Fig. S5E). In conjunction, TEM analysis at P21 revealed fewer insulin and glucagon granules within endocrine cells, most of which lacked a mature morphology (Fig. 4D). Lastly, expression of Sur1 (Abcc8) and Kir6.2 (Kcnj11), which make up an essential K+ transporter in the β cell, were reduced in mTOR mutants at P14 (Fig. 4B). This would indicate a defect in the depolarization of the membrane and release of insulin granules from the β cell. To assess the functionality of β-cell GSIS at P14, we performed a glucose challenge on mTOR mutant mice. Although mutant mice were able to secrete comparable, wild-type levels of insulin in response to a bolus of glucose (Fig. S5F), these same cells were unable to maintain normal glycemia after a 16-h fast (Fig. 4E). These data suggested that although mTOR mutant β cells were able to respond appropriately to high levels of circulating glucose, they were less sensitive to basal glucose levels, probably owing to the loss of functional channels, transporters and vesicles. Taken together, our data suggested that mTOR signaling is playing fundamental roles in the regulation of postnatal endocrine maturation, formation of mature insulin secretory granules, and expression of transporters necessary for insulin secretion.
Fig. 4.
Mutant islet cells show reduced granularity and cellular function. (A) Prohormone convertase 1/3 (PC1/3) staining reveals proper insulin-processing machinery in β cells of mTOR mutants. Scale bar: 50 µm. (B) However, qPCR for endocrine transporters and granularity factors shows significant reduction in mRNA for the zinc transporter Slc30a8, which is involved in β-cell granule formation. This is also reflected in the decreased transcription of chromogranin A (Chga), a marker of intracellular vesicles. In addition, components (Sur1 and Kir6.2) of a K+ transporter necessary for insulin release are also downregulated in mutants. (C) Assessment of α- and β-cell granularity by average side-scatter measurements reveals normal granule formation at P7 but attenuated granularity at P14. (D) TEM at P21 confirms that mutant β and α cells have reduced numbers of insulin and glucagon granules, respectively. Scale bars: 1 µm. (E) These defects culminate in the inability of mutant β cells to respond to fasting glucose requirements at P14. See also Fig. S5. Mean±s.e.m.; *P<0.05. See Table S3 for numbers of animals used.
Islet morphogenesis is abnormal in mTOR mutants
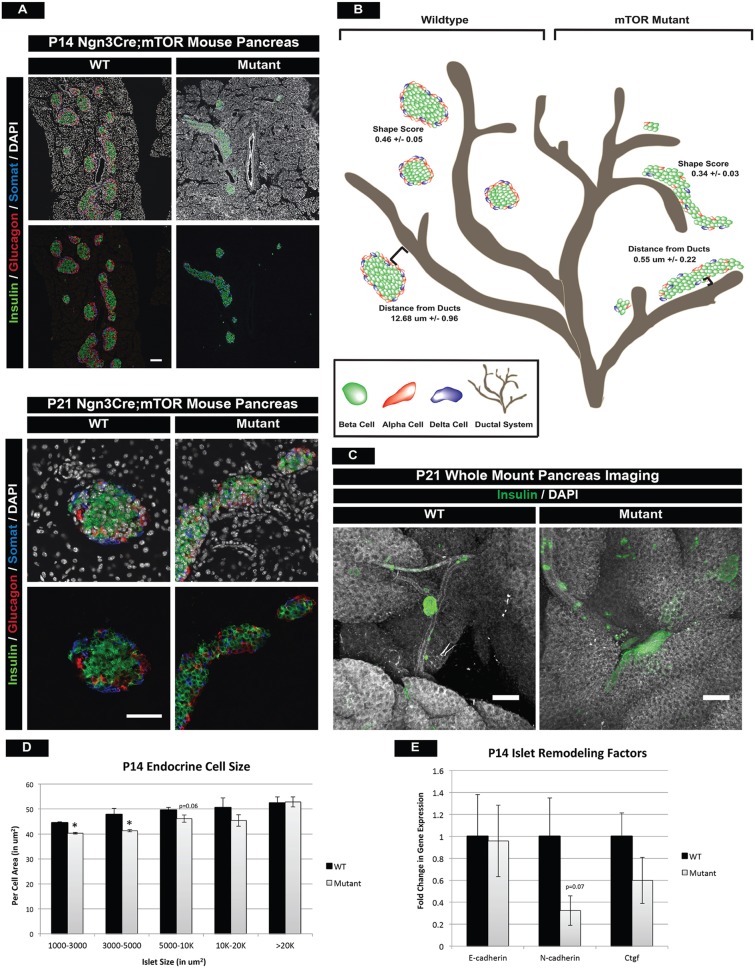
One poorly understood process of postnatal pancreas development is the formation of islets of Langerhans. Just before birth, pancreatic endocrine cells are found along the major pancreatic ducts in large, disorganized clusters (see Fig. S3A). Over the next few weeks, endocrine cells begin to aggregate and organize into islets that are distinct from the ducts. In mTOR mutant mice, these processes appear to be perturbed, resulting in dysmorphic islet architecture and continued close proximity to the duct. This was clearly evident at P14 in mTOR mutants, where we observed long aggregations of endocrine cells that failed to round off into individual islets and remained closely associated with ductal structures (Fig. 5A). In order to thoroughly assess these morphogenesis defects, we took four approaches to characterize these phenotypes.
Fig. 5.
mTOR mutant islets display islet architecture and morphogenesis defects. (A) Hormone immunofluorescence of islets at P14 and P21, highlighting morphological defects in mTOR mutants. Larger mutant islets exhibit abnormally elongated architecture and both small and larger islets remain clustered close to ducts. The morphological defects did not resolve at P21. Scale bars: P14, 100 µm; P21, 50 µm. (B) Schematic of normal islet architecture in P14 wild-type animals (left) and shape score in large islets (>5000 µm2) as compared with that of mTOR mutant islets (right). Also quantified is the distance of large islets (10-20,000 µm2) from ductal structures within wild-type and mutant pancreatic tissue. Large islets remain in close proximity to the ducts in mTOR mutant animals. (C) Whole-mount immunofluorescence staining of P21 wild-type and mutant islets confirms persistent morphological and migration defects in mTOR mutants. Scale bars: 200 µm. (D) Endocrine cell size is also perturbed and correlates with islet size in mTOR mutants. (E) qPCR for cell adhesion and remodeling factors in islets. Whereas no significant difference is seen in E-cadherin expression, slight decreases in N-cadherin and Ctgf might contribute to islet architecture defects. Mean±s.e.m; *P<0.05. See also Fig. S6, Movies 3 and 4, and Table S3 for numbers of animals used.
First, we examined immunofluorescence of serially sectioned wild-type and mTOR mutant islets at P14 and P21 (Fig. 5A). We then calculated a size and shape score (see Materials and Methods) for each of the islets within the P14 samples. This revealed a significant decrease in the shape score of mutant islets, which was most apparent in the larger islets at P14 (Fig. 5B, Fig. S6A).
Second, we used Dolichos biflorus agglutinin (DBA) as a ductal marker and calculated the distance of each islet to the nearest main duct. Islets in mTOR mutants remained closely associated with large ducts, indicating that endocrine cells failed to undergo normal islet morphogenesis (Fig. 5B, Fig. S6A).
Next, we performed whole-mount immunofluorescence staining and confocal analysis of the P21 pancreas to confirm in a 3D setting that large islets retained abnormal architecture and remained in close proximity to large ducts in mTOR mutants (Fig. 5C, Movies 3 and 4). This also confirmed that the phenotype did not resolve with age. We also noted a decrease in the size of individual cells within islets, and this phenotype correlated with the size of the islet (Fig. 5D).
Lastly, we investigated the impact of mTOR deficiency on adhesion molecules known to regulate islet morphogenesis during development. Downregulation of E-cadherin is necessary for endocrine cell delamination (Gouzi et al., 2011) and increased expression is correlated with a loss of endocrine cell migration away from ductal structures (Greiner et al., 2009). We noted a trend of increased E-cadherin protein levels in mTOR mutant duct-associated islets, suggesting improper E- to N-cadherin transition during segregation from ductal structures (Fig. S6B,C). We also noted reduced N-cadherin (Cdh2) expression and a slight decrease in connective tissue growth factor (Ctgf), which has been implicated in islet architecture defects as well (Fig. 5E) (Crawford et al., 2009). From these data, we concluded that mTOR activity is required for normal morphogenesis of the postnatal islet.
mTORC1 and mTORC2 each contribute to different aspects of postnatal islet development
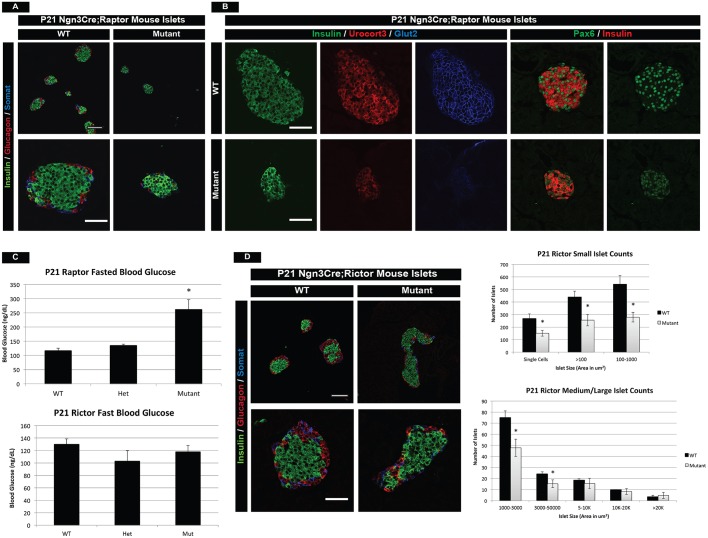
Mtor is a component of two complexes, namely mTORC1 containing Raptor and mTORC2 containing Rictor, each of which mediates different signaling effects of the mTOR pathway. mTORC1 signaling is primarily associated with transcriptional and translational changes within a cell, whereas mTORC2 signaling has been shown to mediate cytoskeletal reorganization processes. Despite their differences, both complexes have been implicated in proliferation and islet mass in the adult pancreas (Balcazar Morales and Aguilar de Plata, 2012; Gu et al., 2011). We therefore investigated whether the different phenotypes observed during early postnatal islet development in the mTOR mutants were complex specific. Analysis of Neurog3Cre;Raptorf/f (Raptor mutant) mice revealed smaller, yet morphologically normal islets within the pancreas (Fig. 6A). Raptor-deficient islets, however, had reduced Ucn3 and Glut2 immunostaining at P21, indicative of maturation and functional defects within the β cells (Fig. 6B). In addition, Raptor mutant mice had reduced Pax6 protein, implying an altered transcriptional network, much like the mTOR mutant model (Fig. 6B). These phenotypes culminated in elevated fasting blood glucose levels and reduced circulating insulin at 3 weeks of age (Fig. 6C, Fig. S7A). Although the mTOR and Raptor phenotypes shared many similarities, such as reduced maturation and impaired function, these effects arose at P21 in Raptor mutants, whereas the mTOR phenotype was present at P14 (Fig. S7B). Consistently, mTOR mutants died within 8 weeks, whereas Raptor mutants perished around 12 weeks of age (data not shown), which indicated latency in the phenotype.
Fig. 6.
mTOR complexes 1 and 2 have distinct roles in postnatal islet development. (A) Islet hormone immunofluorescence highlights smaller and fewer islets in Raptor mutants (complex 1) as compared with wild type at P21. (B) Immunofluorescence for the maturation and functional markers Ucn3 and Glut2 demonstrates reduced Ucn3 and virtually absent Glut2 protein in Raptor mutant β cells. In addition, Raptor mutant β cells have reduced Pax6 expression at P21. (C) Analysis of fasted blood glucose levels identifies increased blood glucose in Raptor mutant animals, yet normal levels in Rictor mutants (complex 2) at 3 weeks of age. (D) (Left) Immunofluorescence for Rictor mutant islets shows a slight morphological defect in larger islets. (Right) Quantification of islet number based on islet size. Lower islet counts are indicative of an islet mass defect. Scale bars: 50 µm. Mean±s.e.m.; *P<0.05. See also Fig. S7, and Table S3 for the number of animals used.
Although a functional and maturation defect was identified in Raptor mutants, we did not observe any morphological islet deformities within the pancreas. However, analysis of Neurog3Cre;Rictorf/f (Rictor mutant) animals at P21 showed that Rictor-deficient islets had architecture defects similar to mTOR mutants (Fig. 6D). Shape score quantification of mutant islets did not reach statistical significance (Fig. S7C), but suggested that both Raptor and Rictor complexes might contribute to morphological defects observed in mTOR mutant islets. Additionally, Rictor mutant islets displayed no changes in the maturation or functional markers Ucn3 or Glut2, respectively (Fig. S7D), and had normal fasted blood glucose levels and circulating insulin at P21 (Fig. 6C, Fig. S7A). However, Rictor mutants did have reduced numbers of small/medium size islets at P21 (Fig. 6D). Although there are no published reports of the impact of Rictor on early postnatal islet development, our data are consistent with known roles of Rictor in β-cell mass in the adult (Gu et al., 2011).
Since a functional defect was not present in Rictor mutants, this implicated a primary role for Raptor in postnatal islet development. From these data, we concluded that mTORC1 signaling is primarily responsible for the maturation and function of postnatal islets, whereas mTORC2 may play a lesser role in postnatal islet architecture events but a consistent role in islet mass. It seems reasonable to conclude that the increased severity of the phenotypes observed in mTOR mutants is due to the additive effects of losing signaling through both mTOR complexes.
DISCUSSION
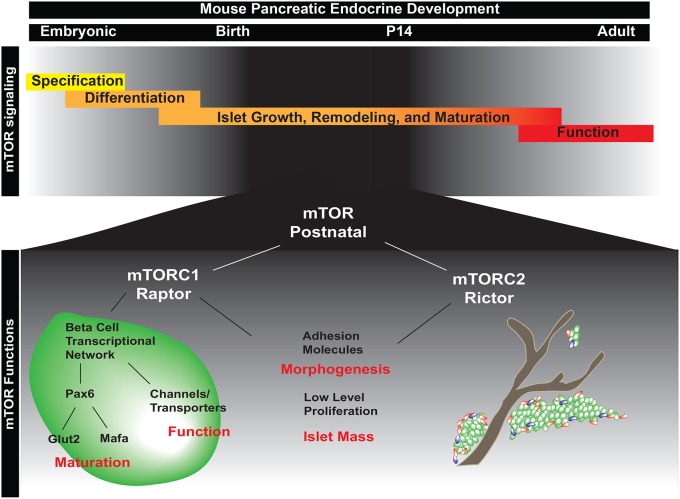
In this study, we have identified several novel and separable functions for the mTOR signaling pathway during postnatal islet development. Whereas previous studies have focused primarily on mTOR signaling in adult β-cell maintenance and function, we show that mTOR is additionally required in the first few weeks after birth. Examination of postnatal animals revealed that mTOR is involved in three distinct aspects of postnatal islet development: (1) β-cell maturation and function; (2) islet mass; and (3) islet morphogenesis (Fig. 7). Moreover, we show that specific complexes, mTORC1 and mTORC2, are required for different aspects of islet maturation/function and together may play an underappreciated role in islet morphogenesis.
Fig. 7.
Model of the role of mTOR in postnatal islet morphogenesis, maturation and function. (Top) Stages of embryonic and postnatal development of the endocrine pancreas. mTOR signaling is essential in the first two weeks of postnatal life, the time during which endocrine cells undergo morphogenesis into islets and mature into fully functional β cells. (Bottom) During postnatal islet development, mTOR signaling is required for islet morphogenesis and β-cell maturation. Our data indicate that morphogenesis is regulated by both mTORC1 (Raptor) and mTORC2 (Rictor), as a robust phenotype is only seen with deletion of both complexes. In addition, both complexes influence islet mass. However, mTORC1 is the main complex influencing postnatal islet development. mTORC1 signaling is required specifically for the maturation and function of endocrine cells within the pancreas and regulates these processes, in part, via Pax6. These signals potentiate cellular maturation and functional responses within the cell.
One key function of the mTOR pathway is to sense the availability of nutrients and coordinate the appropriate cellular response. Interesting, we found that mTOR-deficient islets are normal at birth and do not require mTOR signaling until the beginning of postnatal life. During the 2-week window after birth, it is crucial for β cells to acquire the functional capacity to sense fluctuations in physiological glucose levels. This process, termed maturation (Blum et al., 2012; Pan and Wright, 2011), is not well understood but can be monitored through the expression of key transcription factors and proteins necessary for proper β-cell function (e.g. Ucn3, Glut2, Pax6), and physiologically through GSIS. During the same developmental time period, the entire gastrointestinal tract is undergoing major changes in response to oral nutrient exposure (Zhang et al., 1998). Changes to postnatal diet have been shown to impact islet development and maturation at weaning (P21) (Stolovich-Rain et al., 2015). However, the current study has identified an essential role for mTOR just after birth, possibly by controlling β-cell maturation in response to the introduction of oral nutrients. Beyond the scope of the current study, future experiments will need to identify the key regulators upstream of mTOR that stimulate this pathway in response to enteral nutrition. We hypothesize that there could be many direct and indirect sources of mTOR activation, including components within the mother's milk and amino acids such as leucine (González and Hall, 2017; Haschke et al., 2016; Millward, 2012). In addition, the connection between enteroendocrine cells, the neuroendocrine system, and the endocrine pancreas in regulating global nutrient homeostasis has been well documented (Heijboer et al., 2006). We could therefore use the Neurog3Cre;Mtorf/f system to study mTOR in neuroendocrine and enteroendocrine cells. However, in the first 2 weeks of life, it appears that the predominant function of mTOR is in the endocrine pancreas, since we do not observe any changes in weight or feeding behavior that would be expected if satiety or digestion were grossly abnormal. Overall, our study is one of the first to highlight the importance of early nutrient-sensing mechanisms in postnatal pancreatic endocrine development for the overall function of the adult islet.
From previous studies, we know that mTOR positively regulates Pax6 expression in the CNS and is required for maintenance of neuroepithelial and glial cells (Endo et al., 2009). Pax6 is a transcription factor that is essential in the adult islet for maintenance of cell identity and function, suggesting that the reduced function in mTOR mutant islets is due, in part, to loss of Pax6 expression. Loss of Pax6 in the adult islet results in an increase in ghrelin and severe reductions in α, β and δ cells (Hart et al., 2013), a phenotype that mirrors a loss of mTOR in islets. Pax6 and mTOR mutants additionally share similar maturation and functional defects as measured by reductions in Mafa and Glut2 in β cells (Gosmain et al., 2013). Glut2 has essential roles in GSIS (reviewed by Thorens, 2015) and downregulation marks attenuated function of β cells. We additionally observed this in P14 mTOR mutant mice, where β cells were able to secrete insulin in response to a high bolus of glucose, but were unable to maintain normal basal glucose levels. This correlated with a significant loss of Glut2, most likely accounting for decreased glucose transport and an inability to maintain glucose homeostasis during fasting conditions. In addition, reports have identified Mafa as a factor that promotes β-cell maturation through regulation of genes such as the zinc transporter Slc30a8, which is essential for formation of mature insulin granules (Artner et al., 2010; Hang et al., 2014). In mTOR mutants, Mafa and Slc30a8 were both significantly reduced, as was the granularity of both mutant β and α cells as assessed by side scatter measurements and TEM. β cells from Slc30a8 and mTOR mutants appear strikingly similar in TEM, having fewer highly dense granules and more immature, less compact granules (Wijesekara et al., 2010). Overall, these data support a model whereby mTOR signaling promotes postnatal endocrine maintenance, maturation, and the subsequent function of β cells in part through the regulation of Pax6 and its downstream transcriptional network.
Besides cellular maturation, pancreatic islets additionally change morphology after birth by transitioning from long strings of cells clustered next to ductal structures, to spherical, distinct islets. Surprisingly, we also observed striking morphological defects in mTOR mutants. Mutant islets retained elongated architecture and remained closely associated with ducts, which was reminiscent of earlier stages of development. Using a novel shape-score algorithm, we revealed significant differences at P14 in the overall architecture of large islets and confirmed these findings through a secondary, whole-mount immunofluorescence approach at P21. Using this 3D imaging of the islet, we clearly observed dysmorphic islets and concluded that the phenotype did not resolve with age. We analyzed mTOR mutant islets for expression of Snail2, E-cadherin and N-cadherin, all of which are involved in epithelial remodeling processes in many contexts. For example, Snail2 has known roles in epithelial-mesenchymal transition (EMT) and is expressed most highly at late embryonic/early postnatal stages of pancreatic endocrine development (Gouzi et al., 2011; Rukstalis and Habener, 2007). At E18.5 we found comparable levels of E-cadherin and Snail2 prior to birth between mTOR mutants and control animals. This indicated that mTOR-deficient endocrine cells are still able to delaminate from the ducts and cluster into nascent islets. However, at later postnatal stages we observed a decrease in N-cadherin and a slight increase in E-cadherin levels in the mutant islets, suggesting that a switch from E- to N-cadherin might be impaired (Gouzi et al., 2011). Although these data show that mTOR signaling is required for normal islet morphogenesis, a more detailed description of normal islet morphogenesis postnatally will be essential to uncovering the exact mechanism of mTOR action in this process.
mTOR controls a wide range of cellular effects, some of which can be separated based on mTORC1 or mTORC2 downstream targets. The deletion of mTOR or the individual adaptor proteins Raptor (mTORC1 signaling) and Rictor (mTORC2 signaling) using Neurog3Cre allowed us to examine the function of mTOR and each complex within all pancreatic endocrine cells during postnatal maturation. This is in contrast to previous work that focused on the roles of mTORC1 and mTORC2 specifically in adult β cells (Balcazar et al., 2009; Fu et al., 2009; Granot et al., 2009; Gu et al., 2011; Mori and Guan, 2012). Deletion of mTOR in all islet cells prior to birth allowed us to identify that the kinase is required for the maturation, function and granularity of postnatal endocrine populations. The Rictor and Raptor deletion studies identified that mTOR effects are mediated primarily through mTORC1 signaling, as Raptor mutant islets displayed nearly identical loss of transcription factor Pax6, maturation marker Ucn3, and functional marker Glut2. The morphogenesis defect observed in mTOR mutant islets was partially reproduced with the deletion of Rictor. While consistent with previously reported roles of Rictor in cytoskeletal reorganization, our data suggest that mTORC1 and mTORC2 have overlapping roles in regulating islet architecture and islet mass. We therefore propose a model whereby mTORC1 signaling is the primary inducer of postnatal islet maturation, whereas both complexes are involved in morphogenesis and islet mass. It would be of interest to analyze how the function of the two complexes change from postnatal stages into adulthood and, in turn, as diet composition switches during this time. Moreover, to understand how nutrient-sensing pathways contribute to the overall maturation of individual pancreatic endocrine cell types, a more rigorous analysis of mTOR deletion specifically within the α, β and δ subpopulations will need to be performed.
In conclusion, we have identified essential and separable roles for the mTOR signaling in early postnatal islet development. mTORC1-Raptor is essential for β-cell maturation and acquisition of function, while mTORC2-Rictor contributes to islet morphogenesis. Recently, studies of human juvenile islets determined that they are transcriptionally distinct from adult islets, implying that human islets might also undergo significant postnatal maturation (Arda et al., 2016). Therefore, understanding the molecular basis of islet maturation and morphogenesis in response to enteral nutrients is of clinical importance. Moreover, given the therapeutic possibility of pharmacological manipulation of the mTOR pathway in premature infants, we could conceivably control the timing of maturation to correspond with normal gestational age. Lastly, our findings suggest that manipulation of nutrient-sensing pathways might be crucial for controlling the maturation and functionality of human pluripotent stem cell-derived β cells.
MATERIALS AND METHODS
Mice
Mouse experiments were preapproved by the Committee of Ethics of Animal Experiments at Cincinnati Children's Hospital Research Foundation (CCHRF) and were in accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (IACUC2016-004). All mice in the study were housed in the CCHRF mouse facility and maintained in a normal 12-h light/dark cycle and on regular chow. To obtain mutants, Neurog3Cre; Mtorf/+ mice were mated to Mtorf/f mice. Both male and female mice are represented in all experiments performed. All mouse strains and references are listed in the supplementary Materials and Methods. Genotyping primers are listed in Table S2.
Tissue processing, immunostaining and imaging
In order to assess islet markers by immunofluorescence, pancreatic tissue was isolated, fixed, embedded and sectioned using modified protocols described by Spence et al. (2009) and Jonatan et al. (2014), details of which can be found in the supplementary Materials and Methods. Slides were imaged using a Nikon A1 inverted confocal microscope and analyzed using Nikon NIS-Elements and Imaris (Bitplane) software. Antibodies are listed in Table S1.
qPCR analysis
Whole pancreatic tissue was collected at P0, P7 and P14. RNA was isolated using the RNAqueous micro total RNA isolation kit (Life Technologies). cDNA was made using the SuperScript Vilo cDNA synthesis kit (Invitrogen). qPCR was performed using a predesigned TaqMan Array 96-well fast plate (Life Technologies). Data were normalized to the pan-endocrine transcription factor Neurog3.
Morphogenesis and migration analysis
For shape score analysis, P14 serially sectioned pancreas tissue was stained for insulin, glucagon and somatostatin. Eight sections per slide (per animal) were tiled-scanned using a Nikon A1 inverted confocal microscope. Islet circularity, elongation and convexity were calculated with NIS-Elements software to determine shape score (see the supplementary Materials and Methods).
Whole-mount pancreas staining
Whole pancreas was isolated and processed using a modified version of the passive clarity procedure of Yang et al. (2014) (see the supplementary Materials and Methods for protocol). Tissue was cleared using refractive index matching solution (RIMS) and imaged using a Nikon A1 inverted confocal microscope.
Circulating insulin and glucose
Mice were fasted for 4 h (or for an extended 16 h fast) and blood glucose levels were tested using a TrueTrack glucometer. The Mouse Ultrasensitive Insulin ELISA Kit (ALPCO) was used to determine fasted circulating insulin levels. Data were analyzed using Prism software (GraphPad). For detailed methods and the glucose tolerance test, see the supplementary Materials and Methods.
Flow cytometry analysis and granularity
Whole pancreatic tissue was collected, dissociated and immunostained for insulin, glucagon and somatostatin (see the supplementary Materials and Methods for protocol). Cells were analyzed using an LSR II bench-top flow cytometer (BD Biosciences), and FACS plots were processed using FACSDiva (BD Biosciences) and FlowJo (Tree Star). A full list of antibodies is provided in Table S1.
Granularity via flow cytometry was determined from the mean side-scatter measurement (SSC) of all endocrine cells analyzed per sample. SSC analysis was performed at P7 and P14, with six litters analyzed in total.
Proliferation and apoptosis
BrdU (100 mg/kg) was intraperitoneally injected daily into pups from P7 to P13 and tissue was collected at P14 for analysis. Tissue was processed and analyzed by flow cytometry.
TUNEL staining was performed using an In Situ Cell Death Detection Kit, Fluorescein (Roche). Slides were imaged using a Nikon A1 inverted confocal microscope.
Statistical methods
All quantitated data were assessed for significance using a one-tailed Student's t-test, assuming normal distribution, and are represented as mean±s.e.m. The number of animals in each experiment and significance values are provided in Table S3.
Acknowledgements
We thank members of the J.M.W., A. Zorn and S. Huppert labs as well as the CCHMC PSCF Core for feedback and reagents; and Matt Kofron and Mike Muntifering for their expertise in imaging and analysis quantification. We acknowledge CCHMC Vet Services, CCHMC Pathology Core, the CCHMC Confocal Imaging Core and the Flow Cytometry Core.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: K.L.S., W.J.S., J.V.S., J.M.W.; Methodology: K.L.S., W.J.S., J.V.S., J.M.W.; Software: K.L.S.; Validation: K.L.S., W.J.S., J.V.S.; Formal analysis: K.L.S., W.J.S., J.M.W.; Investigation: K.L.S., W.J.S., J.V.S., J.I.S., L.S., J.M.W.; Resources: K.L.S., L.S., Y.Z., J.M.W.; Data curation: K.L.S.; Writing - original draft: K.L.S., J.M.W.; Writing - review & editing: K.L.S., W.J.S., J.V.S., J.I.S., Y.Z., J.M.W.; Visualization: K.L.S., J.M.W.; Supervision: K.L.S., J.M.W.; Project administration: K.L.S., J.M.W.; Funding acquisition: J.M.W.
Funding
This work was supported by National Institutes of Health (NIH) grants R01DK092456 and U19 AI116491 (J.M.W.) and core support from the Cincinnati Digestive Disease Center Award (NIH grant P30 DK0789392). Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.146316.supplemental
References
- Ahlgren U., Jonsson J., Jonsson L., Simu K. and Edlund H. (1998). beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 12, 1763-1768. 10.1101/gad.12.12.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrescu S., Tatevian N., Olutoye O. and Brown R. E. (2010). Persistent hyperinsulinemic hypoglycemia of infancy: constitutive activation of the mTOR pathway with associated exocrine-islet transdifferentiation and therapeutic implications. Int. J. Clin. Exp. Pathol. 3, 691-705. [PMC free article] [PubMed] [Google Scholar]
- Arda H. E., Li L., Tsai J., Torre E. A., Rosli Y., Peiris H., Spitale R. C., Dai C., Gu X., Qu K. et al. (2016). Age-dependent pancreatic gene regulation reveals mechanisms governing human β cell function. Cell Metab. 23, 909-920. 10.1016/j.cmet.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artner I., Hang Y., Mazur M., Yamamoto T., Guo M., Lindner J., Magnuson M. A. and Stein R. (2010). MafA and MafB regulate genes critical to beta-cells in a unique temporal manner. Diabetes 59, 2530-2539. 10.2337/db10-0190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcazar N., Sathyamurthy A., Elghazi L., Gould A., Weiss A., Shiojima I., Walsh K. and Bernal-Mizrachi E. (2009). mTORC1 activation regulates beta-cell mass and proliferation by modulation of cyclin D2 synthesis and stability. J. Biol. Chem. 284, 7832-7842. 10.1074/jbc.M807458200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balcazar Morales N. and Aguilar de Plata C. (2012). Role of AKT/mTORC1 pathway in pancreatic β-cell proliferation. Colomb. Médica 43, 235-243. [PMC free article] [PubMed] [Google Scholar]
- Bliss C. R. and Sharp G. W. (1992). Glucose-induced insulin release in islets of young rats: time-dependent potentiation and effects of 2-bromostearate. Am. J. Physiol. Endocrinol. Metab. 263, E890-E896. [DOI] [PubMed] [Google Scholar]
- Blum B., Hrvatin S. S. Š., Schuetz C., Bonal C., Rezania A. and Melton D. A. (2012). Functional beta-cell maturation is marked by an increased glucose threshold and by expression of urocortin 3. Nat. Biotechnol. 30, 261-264. 10.1038/nbt.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. A., Guney M. A., Oh Y. A., Deyoung R. A., Valenzuela D. M., Murphy A. J., Yancopoulos G. D., Lyons K. M., Brigstock D. R., Economides A. et al. (2009). Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol. Endocrinol. 23, 324-336. 10.1210/me.2008-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle M. J. and Sussel L. (2007). Nkx2.2 regulates beta-cell function in the mature islet. Diabetes 56, 1999-2007. 10.2337/db06-1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ediger B. N., Du A., Liu J., Hunter C. S., Walp E. R., Schug J., Kaestner K. H., Stein R., Stoffers D. A. and May C. L. (2014). Islet-1 is essential for pancreatic β-cell function. Diabetes 63, 4206-4217. 10.2337/db14-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Antonyak M. A. and Cerione R. A. (2009). Cdc42-mTOR signaling pathway controls Hes5 and Pax6 expression in retinoic acid-dependent neural differentiation. J. Biol. Chem. 284, 5107-5118. 10.1074/jbc.M807745200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu A., Ng A. C.-H., Depatie C., Wijesekara N., He Y., Wang G.-S., Bardeesy N., Scott F. W., Touyz R. M., Wheeler M. B. et al. (2009). Loss of Lkb1 in adult β cells increases β cell mass and enhances glucose tolerance in mice. Cell Metab. 10, 285-295. 10.1016/j.cmet.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Gao T., McKenna B., Li C., Reichert M., Nguyen J., Singh T., Yang C., Pannikar A., Doliba N., Zhang T. et al. (2014). Pdx1 maintains β cell identity and function by repressing an α cell program. Cell Metab. 19, 259-271. 10.1016/j.cmet.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González A. and Hall M. N. (2017). Nutrient sensing and TOR signaling in yeast and mammals. EMBO J. 15, 397-408. 10.15252/embj.201696010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosmain Y., Katz L. S., Masson M. H., Cheyssac C., Poisson C. and Philippe J. (2013). Pax6 is crucial for β-cell function, insulin biosynthesis, and glucose-induced insulin secretion. Mol. Endocrinol. 26, 696-709. 10.1210/me.2011-1256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzi M., Kim Y. H., Katsumoto K., Johansson K. and Grapin-Botton A. (2011). Neurogenin3 initiates stepwise delamination of differentiating endocrine cells during pancreas development. Dev. Dyn. 240, 589-604. 10.1002/dvdy.22544 [DOI] [PubMed] [Google Scholar]
- Granot Z., Swisa A., Magenheim J., Stolovich-Rain M., Fujimoto W., Manduchi E., Miki T., Lennerz J. K., Stoeckert C. J., Meyuhas O. et al. (2009). LKB1 regulates pancreatic β cell size, polarity, and function. Cell Metab. 10, 296-308. 10.1016/j.cmet.2009.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner T. U., Kesavan G., Ståhlberg A. and Semb H. (2009). Rac1 regulates pancreatic islet morphogenesis. BMC Dev. Biol. 9, 2 10.1186/1471-213X-9-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Lindner J., Kumar A., Yuan W. and Magnuson M. A. (2011). Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes 60, 827-837. 10.2337/db10-1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang Y., Yamamoto T., Benninger R. K. P., Brissova M., Guo M., Bush W., Piston D. W., Powers A. C., Magnuson M., Thurmond D. C. et al. (2014). The MafA transcription factor becomes essential to islet β-cells soon after birth. Diabetes 63, 1994-2005. 10.2337/db13-1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart A. W., Mella S., Mendrychowski J., van Heyningen V. and Kleinjan D. A. (2013). The developmental regulator Pax6 is essential for maintenance of islet cell function in the adult mouse pancreas. PLoS ONE 8, e54173 10.1371/journal.pone.0054173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haschke F., Haiden N. and Thakkar S. K. (2016). Nutritive and bioactive proteins in breast milk. Ann. Nutr. Metab. 69, 17-26. 10.1159/000452820 [DOI] [PubMed] [Google Scholar]
- Heijboer A. C., Pijl H., van den Hoek A. M., Havekes L. M., Romijn J. A. and Corssmit E. P. M. (2006). Gut-brain axis: regulation of glucose metabolism. J. Neuroendocrinol. 18, 883-894. 10.1111/j.1365-2826.2006.01492.x [DOI] [PubMed] [Google Scholar]
- Jonatan D., Spence J. R., Method A. M., Kofron M., Sinagoga K., Haataja L., Arvan P., Deutsch G. H. and Wells J. M. (2014). Sox17 regulates insulin secretion in the normal and pathologic mouse β cell. PLoS ONE 9, e104675 10.1371/journal.pone.0104675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsuta H., Aguayo-Mazzucato C., Katsuta R., Akashi T., Hollister-Lock J., Sharma A. J., Bonner-Weir S. and Weir G. C. (2012). Subpopulations of GFP-marked mouse pancreatic β-cells differ in size, granularity, and insulin secretion. Endocrinology 153, 5180-5187. 10.1210/en.2012-1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mastracci T. L. and Sussel L. (2012). The endocrine pancreas: insights into development, differentiation, and diabetes. Wiley Interdiscip. Rev. Dev. Biol. 1, 609-628. 10.1002/wdev.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J. (2012). Knowledge gained from studies of leucine consumption in animals and humans. J. Nutr. 142, 2212S-2219S. 10.3945/jn.111.157370 [DOI] [PubMed] [Google Scholar]
- Mori H. and Guan K.-L. (2012). Tissue-specific ablation of Tsc1 in pancreatic beta-cells. Methods Mol. Biol. 821, 407-419. 10.1007/978-1-61779-430-8_26 [DOI] [PubMed] [Google Scholar]
- Nishimura W., Kondo T., Salameh T., El Khattabi I., Dodge R., Bonner-Weir S. and Sharma A. (2006). A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells. Dev. Biol. 293, 526-539. 10.1016/j.ydbio.2006.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T., Liew C. W., Hu J., Hinault C., Michael M. D., Krtzfeldt J., Yin C., Holzenberger M., Stoffel M. and Kulkarni R. N. (2007). Insulin receptors in beta-cells are critical for islet compensatory growth response to insulin resistance. Proc. Natl. Acad. Sci. USA 104, 8977-8982. 10.1073/pnas.0608703104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan F. C. and Wright C. (2011). Pancreas organogenesis: from bud to plexus to gland. Dev. Dyn. 240, 530-565. 10.1002/dvdy.22584 [DOI] [PubMed] [Google Scholar]
- Prado C. L., Pugh-Bernard A. E., Elghazi L., Sosa-Pineda B. and Sussel L. (2004). Ghrelin cells replace insulin-producing beta cells in two mouse models of pancreas development. Proc. Natl. Acad. Sci. USA 101, 2924-2929. 10.1073/pnas.0308604100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rukstalis J. M. and Habener J. F. (2007). Snail2, a mediator of epithelial-mesenchymal transitions, expressed in progenitor cells of the developing endocrine pancreas. Gene Expr. Patterns 7, 471-479. 10.1016/j.modgep.2006.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander M., Neubuser A., Kalamaras J., Ee H. C., Martin G. R. and German M. S. (1997). Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 11, 1662-1673. 10.1101/gad.11.13.1662 [DOI] [PubMed] [Google Scholar]
- Schindler C. E., Partap U., Patchen B. K. and Swoap S. J. (2014). Chronic rapamycin treatment causes diabetes in male mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 307, R434-R443. 10.1152/ajpregu.00123.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senniappan S., Alexandrescu S., Tatevian N., Shah P., Arya V., Flanagan S., Ellard S., Rampling D., Ashworth M., Brown R. E. et al. (2014). Sirolimus therapy in infants with severe hyperinsulinemic hypoglycemia. N. Engl. J. Med. 370, 1131-1137. 10.1056/NEJMoa1310967 [DOI] [PubMed] [Google Scholar]
- Spence J. R., Lange A. W., Lin S.-C. J., Kaestner K. H., Lowy A. M., Kim I., Whitsett J. A. and Wells J. M. (2009). Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev. Cell 17, 62-74. 10.1016/j.devcel.2009.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolovich-Rain M., Enk J., Vikesa J., Nielsen F. C., Saada A., Glaser B. and Dor Y. (2015). Weaning triggers a maturation step of pancreatic β cells. Dev. Cell 32, 535-545. 10.1016/j.devcel.2015.01.002 [DOI] [PubMed] [Google Scholar]
- Taylor B. L., Benthuysen J. and Sander M. (2015). Postnatal β-cell proliferation and mass expansion is dependent on the transcription factor Nkx6.1. Diabetes 64, 897-903. 10.2337/db14-0684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B. (2015). GLUT2, glucose sensing and glucose homeostasis. Diabetologia 58, 221-232. 10.1007/s00125-014-3451-1 [DOI] [PubMed] [Google Scholar]
- Thorrez L., Laudadio I., Van Deun K., Quintens R., Hendrickx N., Granvik M., Lemaire K., Schraenen A., Van Lommel L., Lehnert S. et al. (2011). Tissue-specific disallowance of housekeeping genes: the other face of cell differentiation. Genome Res. 21, 95-105. 10.1101/gr.109173.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meulen T., Donaldson C. J., Cáceres E., Hunter A. E., Cowing-Zitron C., Pound L. D., Adams M. W., Zembrzycki A., Grove K. L. and Huising M. O. (2015). Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion. Nat. Med. 21, 769-776. 10.1038/nm.3872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Yang X. and Zhang J. (2016). Bridges between mitochondrial oxidative stress, ER stress and mTOR signaling in pancreatic β cells. Cell Signal. 28, 1099-1104. 10.1016/j.cellsig.2016.05.007 [DOI] [PubMed] [Google Scholar]
- Wijesekara N., Dai F. F., Hardy A. B., Giglou P. R., Bhattacharjee A., Koshkin V., Chimienti F., Gaisano H. Y., Rutter G. A. and Wheeler M. B. (2010). Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 53, 1656-1668. 10.1007/s00125-010-1733-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilcox C. L., Terry N. A., Walp E. R., Lee R. A. and May C. L. (2013). Pancreatic α-cell specific deletion of mouse Arx leads to α-cell identity loss. PLoS ONE 8, e66214 10.1371/journal.pone.0066214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Treweek J. B., Kulkarni R. P., Deverman B. E., Chen C.-K., Lubeck E., Shah S., Cai L. and Gradinaru V. (2014). Single-cell phenotyping within transparent intact tissue through whole-body clearing. Cell 158, 945-958. 10.1016/j.cell.2014.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Malo C., Boyle C. R. and Buddington R. K. (1998). Diet influences development of the pig (Sus scrofa) intestine during the first 6 hours after birth. J. Nutr. 128, 1302-1310. [DOI] [PubMed] [Google Scholar]